Open Journal of Asthma
Inhalation Devices: Various forms of administration for Therapeutic Optimization
Renata Cristina de Angelo Calsaverini Leal*
Cite this as
de Angelo Calsaverini Leal RC (2017) Inhalation Devices: Various forms of administration for Therapeutic Optimization. Open J Asthma 1(1): 037-044. DOI: 10.17352/oja.000006Introduction: Aerosol therapy consists of spraying liquid particles suspended for therapeutic purposes in the respiratory tract. With direct absorption and deposition at the lung level, avoiding side effects and presenting fast response time. Several factors influence the drug action, such as size, particle movement, ventilatory flow, pulmonary expansion, anatomy, respiratory mechanics and the nebulizer and patient interface. The therapeutic optimization depends on the type of nebulizer differentiating itself by the physical principle that generates the mist.
Objectives: Check advantages and disadvantages of different inhalation devices. Methodology. This is a review of the PubMed database using descriptors: ultrasonic and jet nebulizer, aerosol deposition in the lung, metered dose inhaler and dry, inhaler therapy.
Results: Different devices are mentioned in the literature: pneumatic and ultrasonic nebulizers (administering solutions), metered pressurized inhalers - pMDI used with or without expander chamber (administering suspensions) and dry powder inhalers - DPI (administering powder).
Discussion and Conclusion: The US has advantages: quiet, does not require coordinating abilities, without propellant gases and quick nebulization with small amount of solution. Disadvantages: change in the active principle of thermosensitive drugs, deposition in the oropharynx and VAI of 2% of inhaled particles. The NJ has 10% VAI drug deposition. Disadvantages: longer use time, need energy source, hinders molecular transport and has variable flow rate. DPI has advantages: portable, simple, activated by inspiration and the dose deposited varies from 15 to 40%. Disadvantage: requires respiratory coordination. The pMDI offers advantages: deposition in VAI of 37%, practical, allows a micropulverization. Disadvantage: Required high inspiratory flow. Aerosol therapy aims at deposition of particles with a diameter of less than 5 μm in VAI. To achieve a concentration similar to the inhaled route in VA tissues, a higher dose of oral medication is required. For each patient there is a suitable inhaler.
Introduction
Aerosol therapy consists of spraying liquid particles suspended for therapeutic purposes in the respiratory tract, especially bronchodilator drugs and corticosteroids, which will act on the site itself. Absorption is direct and aims at particle deposition at the lung level, avoiding undesirable secondary systemic effects and presenting a faster response time than other routes of administration. According to international consensus, the inhalation route is preferentially chosen in cases of bronchial disease, since it allows the direct application of the medication in the lower airways. The classification involves modality of application, generation of particles and mainly deposition in the conduction pathways. In this context, several factors influence the action of the drug, such as particle size and movement, ventilation flow during inhalation, pulmonary expansion during aerosol administration, airway anatomy, respiratory mechanics and the interface between the nebulizer and the patient. Therapeutic optimization also depends on the type of nebulizer that is driven by jet (NJ), ultrasonic waves and dosed by propellant gas, differentiated by the physical principle that generates the mist.
Minor particles are deposited by sedimentation in less calibrous and more distal airways, by mixed flow, both swirling and laminar in more distal levels, or even by the molecular movement, called Brownian agitation or diffusion, in areas where there is no flow and Deposition may occur on patches of less than 1μ. It is also considered the characteristic of breathing as an influencing factor for the optimization of respirable particles. This is decisive for the application of jet nebulizers, since rapid breathing with a 1: 1 rhythm and even narrowing of the airways creates a turbulent flow with great deposition in the upper airway and oropharynx. Inversely proportional to powder inhalers, when the aerosol is generated by the patient in a flow greater than 60L / min.
In US nebulizers, the piezoelectric effect is responsible for the formation of the aerosol, where a mechanical force (expansion and compression) is applied to the quartz crystal causing a vibration with a frequency of 1 to 3 MHz, transmitted to the surface of the drug solution, which is thus pulverized into small particles. Among the advantages attributed to the US are: they are silent, do not require patient coordination, do not use propellant gases, residual volume (dead) is small and nebulization is fast with small amount of solution. In contrast, there are disadvantages such as temperature increase, which may alter the active principle of some thermo sensitive drugs and mainly a large deposition of the drug in the oropharynx.
The advent of nebulization for therapeutic purposes allows several ways to optimize drug deposition with topical effect, sparking worldwide research, which since the 20th century shows the first reports of an undeniable advance in inhalation therapy, which addresses aerosol therapy by Metered dose inhalers (pMDI) as a recommended method at all consensuses for the administration of corticosteroids and bronchodilators. Because it consists of a closed and pressurized container, the active principle is dispersed by means of a metered jet with practicality, efficiency and safety, allowing a micro pulverization impossible to be obtained by common nebulization.
In metered-dose inhalers, the drug is contained in a suspended metal cylinder with a chlorofluorocarbon propellant (CFC), widely used over the years, which has been universally restricted because of its deleterious effects on the ozone layer. Today the market for propellants for therapeutic purposes has alternative water soluble alternatives such as hydrocarbons (HFCs), hydrofluoroalkane (HFA), tetrafluoroethanes and difluoretanes with significant decrease of volatile organic compounds, sustaining the advantages over other types of nebulization with 60% respirable particles. In this context, it is considered that the homogeneous release of the mist, effectively contributes to the pulmonary deposition of the drug, with the least impact in the oropharynx. Particles of approximately 5 microns fulfill their therapeutic role. It is also worth noting that the drug dissolved in a solution releases a greater number of respirable particles.
PMDI was developed in 1956 and although instituted for drug administration to control asthma, studies have found that 50% of patients still do not use the device correctly. This error is attributed, mainly, to insufficient inspiratory flow velocity during application, and respiratory function orientation and re-education must be included in the treatment plan.
Pulmonary deposition by traditional devices may be less than 15%, with the aggravation of inadequate inhalation technique. In this way, the adaptive and more accepted therapeutic form reproduces greater adherence to the treatment. It is worth considering that, according to some studies, the efficacy of the formulations administered by HFA and pMDI may be 60% higher than other forms of administration, but with a possible compromise attributed to the wrong respiratory technique, with a use of only 37%.
According to randomized studies of radioisotope measurement, drug deposition in the oropharynx and stomach is inferior to pulmonary deposition
Anatomical Review
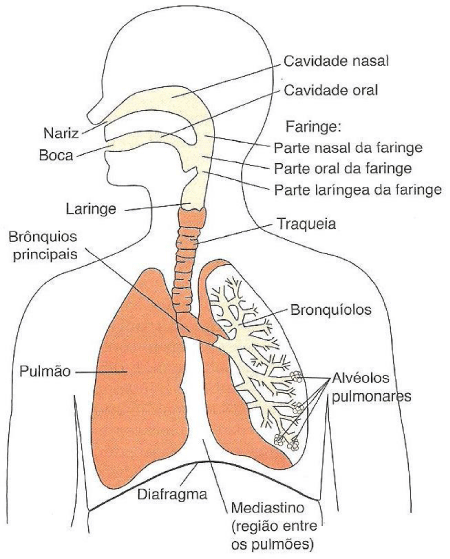
The respiratory system (Figure 1) is responsible for transporting oxygen (O2) to the lungs and eliminating carbon dioxide (CO2) from them, it is composed of upper and lower airways [1].
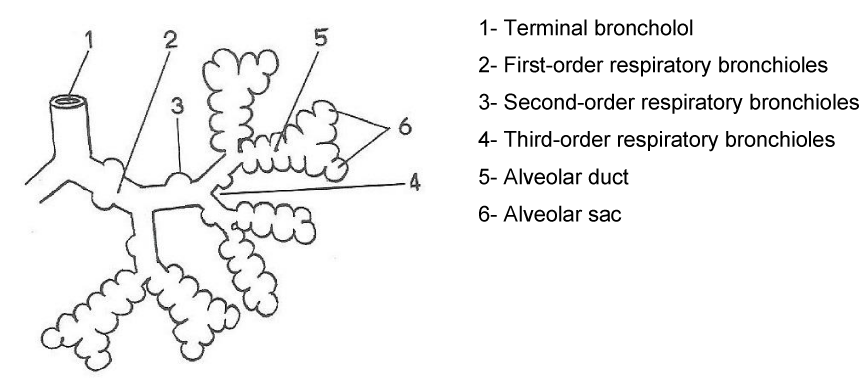
The upper airway (VAS) consists of the nose, nasal cavity, oral cavity, pharynx and larynx, whereas the inferior airway (VAI) is formed by the bronchial trachea and tree which is composed of the main bronchi and their subdivisions: terminal bronchioles, Respiratory bronchioles, alveolar ducts, alveolar sacs and pulmonary alveoli [1,2] (Figure 2).
Aerosol therapy
Aerosol therapy or inhalation therapy is the technique used to administer drugs directly into the respiratory system, which is quicker, more effective and produces fewer side effects than other therapeutic techniques [3].
The inhalation method, when used correctly, allows a better therapeutic use, since the drug action affects VAI avoiding systemic absorption [4].
Historic: In the 19th century, asthma cigarettes were produced containing anticholinergic substances. In the 1930s they developed the first nebulizers. In 1954, the dosimetric device appeared. Soon after (1970) the airbrush was developed (Spacer) to facilitate the use of the metered device and in 1980 constituted the dry powder devices [5].
Definition and physical characteristics: According to Aguiar, Ribeiro and Crispilho [3], “aerosol is the state of the intermediate matter between the liquid and the gaseous state, therefore, it is composed of particles neither as dispersed as the gas nor as concentrated as of the liquid “.
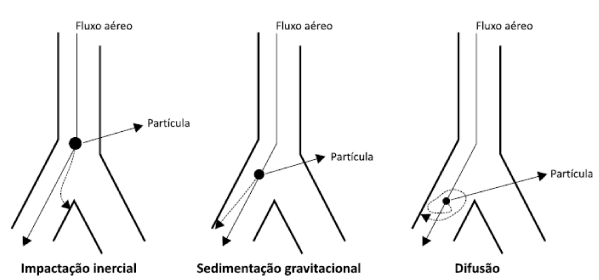
The particle size for VAI deposition should be between 1 and 5 μm (respirable particle) and occurs by sedimentation, particles larger than 5 μm deposited by inertial impaction, and those smaller than 1 μm are slowly deposited by diffusion. May be exhaled before reaching the proposed route as shown in figure 3 [5].
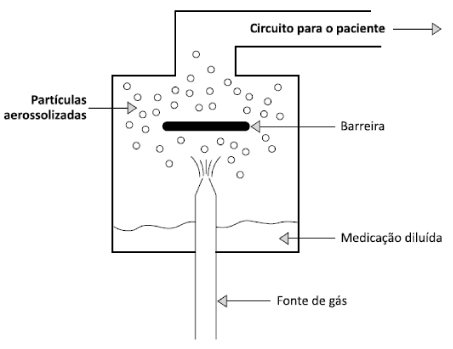
Inhalation devices Inhalatory devices are devices that produce aerosol [3]. Pryor and Webber [6], state that “the physiotherapist must be aware of the range of possibilities to enable the patient to derive maximum benefit from the prescribed drugs.” According to Aguiar, Ribeiro and Crispilho [3], the ideal device must be simple, portable, durable and mainly produce aerosol with respirable particles. The patient’s age, coordination and ability, respiratory condition severity and patient preference are recommended criteria when choosing the device [6]. Inadequate handling of inhalation devices results in lower drug deposition in the lungs and consequently the control of respiratory pathology will be compromised [7]. In the study by Lavorini et al. [8], apud Correia et al. [4], found that most of the patients did not use the inhalers correctly and that some patients did not receive it at the beginning of treatment. The research of Dalcin et al. [9], consists of 268 asthmatic individuals who used inhalation medication of which 187 handled correctly and 81 incorrectly. Those who presented an incorrect technique had associated some of the following factors: widowhood, family income of up to three minimum wages, low educational level, had two or more comorbidities and used a pressurized inhaler. Incorrect use of the inhaler device will lead to ineffective therapy in the studies of Muchão et al. [10], observed that the physicians participating in the research did not achieve good results regarding the correct techniques of device use and after theoretical-practical orientation the results were better, showing that guidelines and training are needed for professionals to use and teach patients the use Of inhaled devices. Types of inhalation devices Nebulizers In nebulizers the medication is in solution which is converted into aerosol by means of gas or sound wave, thus there are jet and ultrasonic nebulizers [6]. The jet nebulizer (NJ) uses oxygen or compressed air to produce the aerosol, illustrated in figures 4,5 [3].
Nebulizers have the advantages of use for all age groups, normal ventilatory pattern and low inspiratory flow, and present the following disadvantages: they are expensive, difficult transportation, requires oxygen and / or electrical power, higher dose, lower pulmonary deposition (However, In the study by Zuana et al. [11], composed of 40 patients with cystic fibrosis who used a PRONEB® compressor and nebulizer system (PARI Medical Holding GmbH, Starnberg, Germany) and inhalation with Dornase-α (Pulmozyme®; Roche, São Paulo, Brazil). The first collection was through samples of the reservoir cup and mouthpiece to perform the microbiological culture as well as sputum or smear samples from the patients’ oropharynx and resulted in the contamination of the nebulizers in 23 cases (57.5%) after education On hygiene and disinfection the number of contaminated nebulizers dropped to 10, indicating that when applying the standardized recommendations there is a decrease in the contamination of the nebulizers.
According to Zuana et al. [11], based on the Cystic Fibrosis Foundation (CFF) and the nebulizer manufacturer’s guidelines the steps comprise:
1. Hygiene (cleaning): after the use of the nebulizer, it must be dismantled, and its parts should be washed inside and out with neutral detergent and tap water (except the hose and its adapter, which must be dry, Leaving them connected for two minutes in the compressor or with both ends hanging down) and rinsed with tap water.
2. Disinfection: Put the still-dismantled parts in a container with water and boil for five minutes. If disinfected with boiling water, rinsing is not necessary. Do not boil the hose, its adapter or the mask. Perform this procedure once a day.
3. Drying: After the final rinse, let the water in the material drain and dry it preferably with paper towels or clean cloth.
4. Storage: Mount all parts of the nebulizer and store in a container for this sole purpose.
The breath-enhanced performance nebulizers allow the patient to inhale additional air in the inhalation and allow for drug recycling. The optimized particle inhalers use a vibrating network with piezoelectric element, which produces particles of adequate size, there is no recirculation of the drug decreasing the evaporation and cooling of it. Breath-activated nebulizers allow the medication to be released when the patient inhales, avoiding drug waste [12].
Dry powder inhalers: In the dry powder inhaler (IPo) the medication is powdered and micronized or contained within the device itself to provide breathable particles activated by the patient’s inspiratory flow which should be high (60L / min), aerosol deposition in the Lungs varies from 15 to 40% [3,12].
The advantages include: activated by inspiration, they are portable and simple and can contain several doses and present the following disadvantages: it needs high inspiratory flow and agglomeration of the powder due to the humidity [12].
There are several dry powder inhalers, including: Diskus® (Figure 6), Aerolizer®, Turbuhaler®, Pulvinal® and Handihaler® [12].
According to the Guidelines of the Brazilian Society of Pulmonology and Phthisiology for Asthma Management (2012) the IPo inhalation technique should be performed as follows:
A) Dose preparation
1. Capsule inhalers: remove or suspend the inhaler cap, place the capsule and drill it by pressing the side or front buttons;
2. Turbuhaler®: remove the lid, hold the IPo vertically, rotate the colored base counterclockwise and then clockwise until you hear a click;
3. Diskus®: turn the disk counterclockwise; Then pull the lever down until you hear a click;
4. Pulvinal®: remove the lid, hold the IPo vertically, push the brown button with one hand and with the other hand turn the IPo anticlockwise (a red mark will appear); Then release the brown button and rotate the device clockwise until you hear a click (a green mark will appear).
B) Use
1. Exhale and place the inhaler in the mouth;
2. Inspire fast and deeply;
3. Hold your breath for 10 seconds;
4. In capsule inhalers take fresh inspiration if dust remains in the capsule.
The study by Coelho et al. [13], consisted of a sample of 467 patients diagnosed with severe asthma and attending the Program for the Control of Asthma and Allergic Rhinitis in Bahia, whose objective was to evaluate the handling of inhalation devices such as the dosimetric inhaler with or without spacer and the Dry powder inhaler, the results obtained were good, since patients attending the program received guidelines to perform the correct mode of the inhaler, but some errors persisted.
At the Pulvinal®, carelessness occurred in the steps of leveling the powder from within the chamber, performing deep exhalation before using the device and inspiring rapid and deep. In the IPo Aerolizer® the failures consisted of not performing deep expiration before placing the device in the mouth, do not check for dust residues in the capsule and do not repeat the maneuver in case of dust residues [13].
According to Coelho et al. [13], in the subgroup of individuals using IPo Aerolizer® alone, all the essential steps were observed, a greater proportion of asthmatics with disease control were observed, demonstrating that adequate use of the devices is one of the predictors for the control of asthma symptoms.
In the subgroup of individuals using IPo Aerolizer® alone, all the essential steps were observed, a greater proportion of asthmatics with disease control were observed, demonstrating that adequate use of the devices is one of the predictors for the control of asthma symptoms (Figure 7).
They are portable devices, do not depend on the preparation of the medicine, have multiple doses and lower cost; Should be shaken prior to use to keep the drug in suspension; It is possible to use spacers (Figure 8), so that the drug does not deposit in the oropharynx, does not require respiratory coordination and that non-respirable particles are removed [3,5].
The disadvantages according to the Guidelines of the Brazilian Society of Pulmonology and Asthma Management (2012) include: requiring coordination between firing and inhalation if used without a spacer, oropharyngeal deposition of corticosteroids contributes to candidiasis, coughing and coughing, and lack of Dosages prevents knowledge of the number of doses remaining.
According to the Guidelines of the Brazilian Society of Pulmonology and Asthma Management (Asthma Management) (2012), the inhalation technique of IP without spacer occurs as follows:
1) Remove the cap and shake the inhaler;
2) Position the device vertically and its mouthpiece 3-5 cm from the mouth;
3) Keep the mouth open and normal expiration;
4) Trigger at the beginning of slow and deep inspiration;
5) Perform post-inspiratory pause of at least 10 seconds;
6) Repeat the technique if necessary.
According to the guidelines of the Brazilian Society of Pulmonology and Asthma Management for Asthma Management (2012), the IP inhalation technique with a spacer consists of:
1) Remove the cap and shake the inhaler;
2) Connect the inhaler to the spacer and position the nozzle outlet vertically;
3) Exhale and insert the nozzle of the spacer into the mouth;
4) Shoot the inhaler and inhale through the mouth, slowly and deeply;
5) Seal the nose to avoid nasal inspiration;
6) Perform post-inspiratory pause of at least 10 seconds;
7) Repeat the technique if necessary
Still on the study of Coelho et al. [13], evaluated the handling of the pressurized inhaler device. In the IP without a spacer, the failures were to not maintain the mouthpiece at the correct distance from the mouth, not to perform deep exhalation before using the device and not to maintain the inspiratory pause. In the IP with spacer the above mentioned errors remained in addition to not shaking the inhaler and in both IP modes the difficulty in coordinating device firing and inspiration.
Pryor and Webber [6], point out that it is necessary to rinse the mouth after inhalation to avoid oral candidiasis.
Aerosols in mechanical ventilation: Aerosol deposition in mechanically ventilated patients is hampered by deposition in the ventilator circuit and in the endotracheal tube (TET), so it is imperative that the technique be performed correctly to be effective [3].
Nebulizers and pressurized metered-dose inhalers with the spacer are the most commonly used devices in mechanically ventilated patients [14].
Vargas et al. [4], developed a study in order to verify if the technicians of nursing were performing the inhaler correctly, was composed of pre-test, training and post-test. According to [14,15], the inhalation technique consists of:
A. Metered pressurized inhaler with spacer:
(1) aspirate the tube, (2) adjust the current air volume> 500ml, (3) adjust an inspiratory flow <60L / min, (4) confirm the position of the device on the inspiratory loop, (5) Install it on the spacer, (6) trigger the device at the start of inspiration, and (7) repeat the next dose 20-30 seconds after the first jet. B) Nebulizer: (1) aspirate the tube, (2) adjust the current air volume> 500ml, (3) adjust an inspiratory flow <60L / min, (4) place the solution in the nebulizer device, (5) 6 ml of 0.9% saline, (6) install the nebulizer in the inspiratory loop 30 cm from the TET connection, (7) adjust a flow of 6L / min into the nebulizer, (8) tap the nebulizer lightly during operation and (9) disconnect the nebulizer from the circuit.
The technique errors were evident, but after the guidelines were minimized, establishing that continuing education is part of the hospital routine so that inhalation techniques are not forgotten and treatment is optimized [14].
Aguiar, Ribeiro and Crispilho [3], further describe that the humidifier should be switched off before initiating therapy, placing the nebulizer flow between 8-10 L / min, adjusting the respiratory rate by an average of 12 breaths per minute (rpm), Perform the inspiratory pause and use one-way valves so that the mist is released only in the inspiratory phase.
Drugs Used in Inhaler Therapy: According to Aguiar, Ribeiro and Crispilho [3] the main drugs used are:
A) short-acting inhaled β2-agonists: Salbutamol, fenoterol, and terbutaline are available for acute exacerbations, with the following adverse effects: tremors, arrhythmias, and hypokalemia.
B) Anticholinergics: Promoting bronchodilation in acute attacks, ipratropium bromide is available, adverse effects are: glaucoma, dry oral mucosa and urinary retention.
C) long-acting β-agonists: Longer-acting bronchodilator action, available on formoterol and salmeterol, adverse effects are: tremor, arrhythmia, and hypokalemia.
D) Corticosteroid: It has an anti-inflammatory effect, the adverse effects include: loss of bone mass, growth deficit, oral candidiasis, dysphonia and chronic cough.
E) Mucolytics: They are used to reduce viscoelasticity of the sputum, among them are: Dornase-α and N-acetylcysteine.
Advantages and disadvantages of each inhaler device: Table 1 based on the Guidelines of the Brazilian Society of Pulmonology and Asthma Management (2012) shows the main advantages and disadvantages of each inhaler device.
Therapeutic optimization
Therapeutic optimization is achieved when the patient correctly handles inhalation devices [16]. Pryor and Webber [6], announce that “for each inhalation device individual instructions should be carefully and followed up”.
It is worth noting that the pharmacokinetics of the drugs handled will be compromised by the following factors: age, respiratory pattern, the use of spacers and application of the inhalation technique [17,18].
In the present study, the results of the present study were similar to those reported by Oliveira et al. [19]. (1998) found that in using the inhalation technique, the treatment would not have an effect and show that when performing the inhalation technique, Guidelines for health professionals the benefits are visible in patients.
In the study by Alcofarado et al. [20-24], lung scintigraphy of 8 volunteers without pneumopathies was performed in order to verify the influence of lateral decubitus on the pulmonary deposition of radioaerosol. The analysis demonstrated that pulmonary aerosol deposition is greater in the dependent lung, in the transverse direction it was better deposited in the middle and lower segments and in the longitudinal direction in the intermediate and peripheral lung zones (Figure 9).
Objective
Check advantages and disadvantages of different inhaled devices.
Methodology
The present study is a bibliographical review, the searches were carried out in books of the FUNEC Municipal Library of Education and in databases of SciELO (Scientific Electronic Library Online) and Google Scholar using the following descriptors: Pulmonary aerosol deposition, inhalation devices, inhalers, inhalation, nebulizers and vaporizers and inhalation therapy, articles were published after the year 2010 and in Portuguese and English, the search period was from February 25, 2017 to March 24. April 2017.
Results
Different devices are mentioned in the literature: pneumatic and ultrasonic nebulizers (administering solutions), metered pressurized inhalers - pMDI used with or without expander chamber (administering suspensions) and dry powder inhalers - DPI (administering powder).
Discussion
The US has advantages: quiet, does not require coordinating abilities, without propellant gases and quick nebulization with small amount of solution. Disadvantages: change in the active principle of thermosensitive drugs, deposition in the oropharynx and VAI of 2% of inhaled particles. The NJ has 10% VAI drug deposition. Disadvantages: longer use time, need energy source, hinders molecular transport and has variable flow rate. DPI has advantages: portable, simple, activated by inspiration and the dose deposited varies from 15 to 40%. Disadvantage: requires respiratory coordination. The pMDI offers advantages: deposition in VAI of 37%, practical, allows a micropulverization. Disadvantage: Required high inspiratory flow.
Conclusion
Aerosol therapy aims at the deposition of particles at the lung level avoiding undesirable secondary systemic effects and presenting a faster response time than the other routes of administration. It is reasonable to state that the inhalation of particles with a diameter of less than 5 μm is necessary because the nasal cavities and the nasopharynx are a barrier to the upper diameter particles. To achieve an identical concentration in the airway tissues, the patient would need a much larger dose of oral medication than the subclassed inhalation route in fog production modalities. It has been observed that, over time, the progressive increase in inhaled drug administration tends to consider the factors of particle deposition in respiratory distal levels, which allows to conclude that the inhalation treatment is optimized.
- LIPPERT, Lynn S (2013) Cinesiologia clínica e anatomia. 5 ed. Rio de Janeiro: Guanabara Koogan p: 208-216. Link: https://goo.gl/sRyaAC
- TITTEL K (2014) Anatomia descritiva e funcional do corpo humano. 14 ed. São Paulo: Santos Editora p: 313-319. Link: https://goo.gl/RvxD6U
- AGUIAR S, RIBEIRO C, CRISPILHO F, SARMENTO JV (2015) O abc da fisioterapia respiratória. 2 ed. Barueri SP: Manole p: 214-225. Link: https://goo.gl/unQZ2X
- Correia S, Fábio L, Vanessa A, Adelino D, Telma M S (2015) Avaliação do conhecimento sobre a utilização de inaladores entre médicos e profissionais de farmácia dos Açores. Revista Portuguesa de Medicina Geral e Familiar. 31: 14-22. Link: https://goo.gl/bB9qgB
- PARENTE AAAI, Paula NM (2013) Aerossolterapia. Revista Pulmão 22: 14-19. Link: https://goo.gl/5fuviG
- Pryor A, Barbara W (2002) Fisioterapia para problemas respiratórios e cardíacos. Rio de Janeiro: Guanabara Koogan, 2ed, p: 125-132. Link: https://goo.gl/8cxeEa
- Vasconcelos MM. Herbeth SM, Arruda SS, Terehoff V, Torres S (2015) Prevalência do uso inadequado de dispositivos inalatórios por pacientes com asma e/ou dpoc atendidos em ambulatório especializado. Revista Saúde e Ciência Online 4: 6-18. Link: https://150.165.111.246/revistasaudeeciencia/index.php/RSC-UFCG/article/view/263/175
- Lavorini F, Magnan A, Dubus JC, Voshaar T, Corbetta L, et al. (2008) Effect of incorrect use of dry powder inhalers on management of patients with asthma and COPD. Respir Med 102: 593-604. Link: https://goo.gl/kxCYUE
- Tarso Roth Dalcin PD, Maltz Grutcki D, Paganella Laporte P, Borges de Lima P, Menegotto SM, et al. (2007) Fatores relacionados ao uso incorreto dos dispositivos inalatórios em pacientes asmáticos. J Bras Pneumol 40: 13-20. Link: https://goo.gl/VVqJer
- Pereira Muchão P, Ribeiro Ferreira da Silva Filho LV, Carlos Pastorino A, Carlos Rodrigues J (2011) Uso de inaladores dosimetrados em pacientes com asma: conhecimentos e efeitos de uma orientação teórico-prática para pediatras. Einstein 9: 337-342. Link: https://goo.gl/cE2CDN
- Della Zuana A, Oliveira Garcia DD, Turola Passos Juliani RC, Ribeiro Ferreira da Silva Filho LV (2014) Efeito de um programa de educação para cuidadores e pacientes com fibrose cística na contaminação de nebulizadores de uso domiciliar. J Bras Pneumol 40: 119-127. Link: https://goo.gl/yr5CAx
- Pereira Muchão F, Silva Filho LVRFD (2017) Avanços na inaloterapia em pediatria. Jornal de Pediatria 86: 367-376. Link: https://goo.gl/RtWvXH
- Carvalho Coelho AC, Souza-Machado A, Leite M, Almeida P, Castro L, et al. (2011) Manuseio de dispositivos inalatórios e controle da asma em asmáticos graves em um centro de referência em Salvador. Jornal Brasileiro de Pneumologia 37: 720-728. Link: https://goo.gl/okv7oA
- Oliveira Vargas MAD, Teixeira C, Zanchin F, Ghiot A, Pauli K, et al. (2012) Capacitação dos técnicos de enfermagem para as melhores práticas no uso de broncodilatadores em pacientes mecanicamente ventilados. Texto & Contexto 21: 505-512. Link: https://goo.gl/p33t63
- Oliveira Vargas MAD, Teixeira C, Zanchin F, Ghiot A, Pauli K, et al. (2012) Capacitação dos técnicos de enfermagem para as melhores práticas no uso de broncodilatadores em pacientes mecanicamente ventilados. Texto & Contexto Enfermagem 21: 505-512Link: https://goo.gl/aidZiw
- Oliveira Santos DD, Cleusa Martins M, Lucena Cipriano S, Carvalho Pinto RM, Cukier A, et al. (2017) Atenção farmacêutica ao portador de asma persistente: avaliação da aderência ao tratamento e da técnica de utilização dos medicamentos inalatórios. Jornal Brasileiro de Pneumologia 36: 14-22. Link: https://goo.gl/uz3rM2
- Velasco HF, Cabral CZ, Pinheiro PP, Azambuja Rde C, Vitola LS, et al. (2017) Use of digital media for the education of health professionals in the treatment of childhood asthma. J Pediatr (Rio J) 91: 183-188. Link: https://goo.gl/NFG5cy
- Velasco HF, Cabral CZ, Pinheiro PP, Azambuja Rde C, Vitola LS, et al. (2017) Use of digital media for the education of health professionals in the treatment of childhood asthma. J Pediatr (Rio J) 91: 183-188. Link: https://goo.gl/NFG5cy
- Paula Duarte DO, Baptista Menezes AM, Bertoldi AD, Wehrmeister FC, Cardozo Macedo SE (2017) Avaliação da técnica de utilização de dispositivos inalatórios no tratamento de doenças respiratórias no sul do Brasil: estudo de base populacional. Jornal Brasileiro de Pneumologia 40: 513-520. Link: https://goo.gl/yZPHhB
- Alcoforado L, Pessôa Filho LC, Brandão DC, Galvão AM, Reinaux CMA, et al. (2011) Influência da variação dos decúbitos laterais na deposição pulmonar de aerossol. Revista Brasileira de Fisioterapia 15: 278-283. Link: https://goo.gl/4tN2uY
- Maricoto T, Madanelo S, Rodrigues L, Teixeira G, Valente C, et al. (2016) Educação para a melhora da técnica inalatória e seu impacto no controle da asma e DPOC: um estudo piloto de efetividade-intervenção. J Bras Pneumol 42: 440-443. Link: https://goo.gl/ztXjYK
- Paula Duarte DO, Baptista Menezes AM, Bertoldi AD, Wehrmeister FC (2017) Uso de inaladores na população de adolescentes e adultos com diagnóstico médico autorreferido de asma, bronquite ou enfisema em Pelotas, RS. Jornal Brasileiro de Pneumologia 39: 287-295. Link: https://goo.gl/XNEU31
- Diretrizes da sociedade brasileira de pneumologia e tisiologia para o manejo da asma – 2012. Jornal Brasileiro de Pneumologia 38: S1-S46. Link: https://goo.gl/zTQQX1
- Simões LZ, Martins MC, Cunha Possari JND, Carvalho GD, Carvalho Coelho AC, et al. (2015) Validação de escores de uso de dispositivos para inalação: valoração dos erros cometidos. J Bras Pneumol 41: 313-322. Link: https://goo.gl/f9n8qX

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
