Journal of Vaccines and Immunology
Immunity against Pasteurella multocida in Animals Vaccinated with Inactivated Pasteurella multocida and Herbal Adjuvant ‘DIP-HIP’
Himanshi Tanwar, Anand Prakash Yadav, Brijbhushan, Shweta, Shashi Bala Singh and Lilly Ganju*
Cite this as
Tanwar H, Yadav AP, Brijbhushan, Shweta, Singh SB, et al. (2016) Immunity against Pasteurella multocida in Animals Vaccinated with Inactivated Pasteurella multocida and Herbal Adjuvant ‘DIP-HIP’. J Vaccines Immun 2(1): 010-014. DOI: 10.17352/jvi.000014Background: Haemorrhagic septicaemia (HS) is acute, highly contagious form of disease of water buffalo, cattle, and bison caused by Pasteurella multocida (PM). It is considered the most economically important bacterial disease of cattle and buffalo, .in tropical areas of Asia, particularly in Southeast Asia. PM is a gram-negative coccobacillus, belonging to Pasteurellaceae family. PM is transmitted either during direct contact or via fomites by ingestion or inhalation via contaminated feed and water. Currently, there are no broadly protective vaccines –adjuvant formulation with long-lasting immunity available against HS. Aluminium-based mineral salts such as alum are the only immunologic adjuvants. Herbal adjuvants are evaluated extensively to replace the classical adjuvants being safe and efficacious.
Result: In the present study, one herbal adjuvant DIP-HIP is evaluated with inactivated PM (iPM) antigen as vaccine in both small (mice) as well as big (cattle) animals for its efficacy. The results suggest that after single booster DIP-HIP + iPM emulsion gave maximum antibody titres in comparison to iPM alone or Alum + iPM in both mice as well as seronegative cattle. To further confirm the potential of DIP-HIP adjuvant formulation with iPM antigen in providing protection against HS, the median lethal dose (LD50) of PM was determined (1 X 107 CFU/ml), following which DIP-HIP + iPM emulsion immunized mice were challenged with this dose. The DIP-HIP containing formulation turned out to be broadly protective against HS with long-lasting immunity.
Conclusion: DIP-HIP is a potential adjuvant, which can be used in HS vaccines to provide extended immunity
Abbreviations
HS: Haemorrhagic Septicaemia; PM: Pasteurella multocida; iPM: Inactivated Pasteurella multocida; LD50: Median Lethal Dose; CFU: Colony-Forming Unit; RP-HPLC: Reversed-Phase High-Performance Liquid Chromatography
Introduction
Each year, more than fifty thousand cattle and buffaloes die in India because of Haemorrhagic septicaemia (HS) caused by a bacterium, Pasteurella multocida (PM). Classical HS as defined is caused by PM Asian serotypes B:2, identified in most areas where the disease is endemic, and African serotype E:2 found only in Africa (Carter and Heddleston classification system). The prevalence of disease is higher during monsoon when humidity and temperature are high and characterized by an acute, highly fatal septicemia with high morbidity and mortality [1-5]. In India, the frequency of disease outbreaks varies considerably in different states and from year to year in each state [6]. Previous study has demonstrated that India suffer an annual economic loss of approximately 230 million rupees due to HS infection, frequently prevalent in poor husbandry conditions [7]. A recent outbreak in 2013 in West Bengal, resulted in infection amongst more than 150 animals, and almost 87% of the infected buffaloes died [8].
The cattle are infected when they ingest or inhale the causative organism. Buffaloes have been found to be more vulnerable than cattle to HS with shorter course of disease and mortality reaching 100% if not treated at early stage [9]. The characteristic lesion of HS is swelling of the subcutis and muscle of the submandibular region, neck, and brisket by clear to blood-tinged edema fluid. Hemorrhages are often most prominent in the pharyngeal and cervical lymph nodes. The infected animals show typical symptoms of HS such as pyrexia, hypersalivation, nasal discharge, sudden drop of milk yield, abdominal pain, severe diarrhea and dysentry, rapid respiration and cyanotic mucus membrane before death [10]. Clinical diagnosis of HS in endemic areas is based on history, lapses in vaccination, environmental conditions, and the typical clinical signs while a definitive diagnosis of HS is based on isolation of PM serotype B:2 or E:2 (or other less common serotypes recognized by the OIE as causing HS) from the blood and tissues of an animal with above mentioned characteristic signs. The passive mouse protection test using specific B:2 and E:2 immune rabbit sera has been used in Asia and Africa to identify different serotypes of PM (The Merck Veterinary Manual).
If administered very early in the disease, antimicrobials drugs such as sulfonamides, tetracyclines, penicillin, gentamicin, kanamycin, enrofloxacin, tilmicosin, and chloramphenicol are effective against HS. However, due to rapid development of disease, therapy is often unsuccessful and hence vaccines against PM are better answer to prevent this disease (https://www.merckmanuals.com/vet/generalized_conditions/hemorrhagic_septicemia/overview_of_hemorrhagic_septicemia.html). Currently inactivated and live attenuated vaccines are available but they do not provide long term immunity. Some of the routinely used vaccine formulations include bacterins, alum precipitated oil adjuvant and multiple emulsion vaccines [11], with considerable variation in their duration of induced immunity [12] and side effects. Hence, there is a dire need of vaccines against HS which can provide ample and long lasting immune response, safety with minimum side effects, lesser or no booster dose and cost effectiveness.
Seabuckthorn (Hippophae rhamnoides L.) is a traditional medicinal plant and member of Elaeagnaceae family. The plant has been used extensively in oriental traditional system of medicine for treatment of asthma, skin diseases, gastric ulcers and lung disorders [13,14]. In the present study, we have explored adjuvant activity of alcoholic extract of seabuckthorn leaves, named as DIP-HIP, as adjuvant along with inactivated PM (iPM) as vaccine in Swiss albino mice, validating in seronegative calves and compared with classical adjuvant alum.
Methods
Preparation of DIP-HIP Adjuvant
Collection of plant material: Seabuckthorn leaves were collected from hilly regions of Western Himalayas, India, in the month of September. Leaves collected were washed thoroughly in distilled water to remove any impurity and shade dried in a clean dust free environment for extraction process. The voucher specimen of the plant material is preserved in the herbarium and ethnobotanical identification of the plant was carried out in Defense Institute of High Altitude Research (DIHAR), Leh, India (Ethanobotanical ID: 058/FRL/2002).
Preparation of the extract: Dried leaves of Seabuckthorn were powdered and extracted with 70% ethanol. Supernatant was collected and the residue was re-extracted with 70% ethanol. This process was repeated four times. The collected supernatant was pooled and vacuum dried using rotatory vacuum at 40°C and then lyophilized to obtain dried ‘DIP-HIP’ powder. The HPLC analysis of extract confirmed the presence of Quercetin, a potent flavonoid [15]. In another study, some of the bioactive phenolic constituents, such as quercetin-3-O-galactoside, quercetin-3-O-glucoside, kaempferol and isorhamnetin were quantified in aqueous and hydroalcoholic SBT leaf extracts by RP-HPLC [16].
Immunization in mice
Male/Female Swiss albino mice (6–8 weeks old) were obtained from IAEC approved animal house of the institute. The animals were housed under standard conditions at 25±2°C and fed with standard food and water ad libitum. The experiments were conducted under the surveillance of the Ethics Committee of Institute for CPCSEA. Healthy mice with average weight of 25g were selected for the study. The animals were divided into four groups viz. (i) Control (n=5); (ii) iPM+ DIP-HIP (1: 3:: ethanol: water) (n=7); (iii) iPM+2% Al(OH)3 (n=7) and (iv) iPM alone (n=7). The iPM was obtained from Biovet Pvt Ltd., Bengaluru, India, and the recommended dose of antigen, by the company, for immunization used was 3x109 CFU/dose. The animals were primarily immunized with emulsions on day 0 followed by booster on 21st day (intramuscular) and finally bled on 28th day after immunization as standardized for DIP-HIP containing emulsions by our lab. The formulations were prepared as indicated in Table 1.
Antibody response
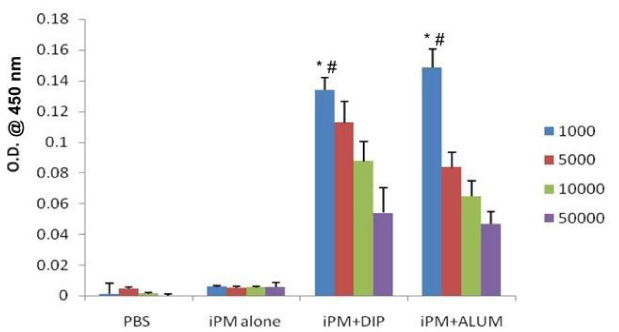
Indirect ELISA was performed using sonicated antigen (semi-purified soluble protoplasmic antigen) to measure antibody response against PM in mice. Briefly antigen was coated on microtitre plates in carbonate-bicarbonate buffer (pH- 9.6) and left overnight at room temperature. Plates were then washed three times with phosphate buffered saline containing 0.5% Tween 20 (PBST). 1% BSA in PBST was then added and incubated for 1 hour for blocking non-specific binding in plate followed by three times washing with PBST. The test sera, at different dilutions (1: 1000; 5000; 10000 and 50000), were added and incubated at 370C for 2 hours. Plates were washed five times and after addition of rabbit anti-mouse IgG peroxidase conjugate, incubated for 1 hour at 370C. After washing plates again, substrate (o- Phenylenediamine containing 0.01% hydrogen peroxide) was added and incubated in dark for color development. The reaction was stopped using 2 N sulphuric acid and plates were read at 450 nm in ELISA reader (Biotek, USA).
Immunization in cattle
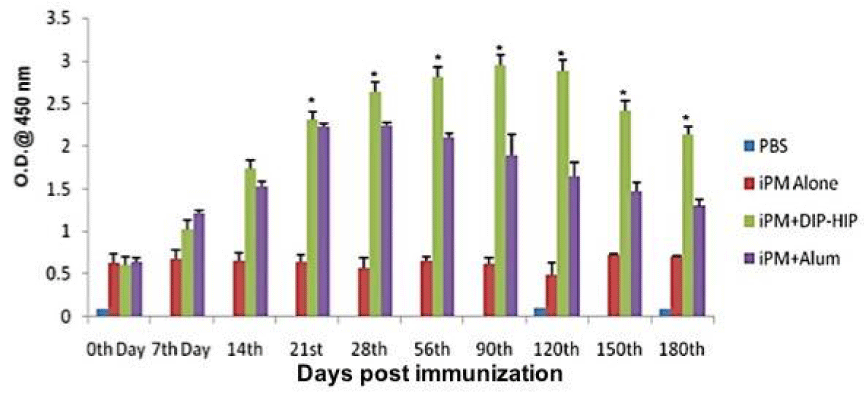
Seronegative male calves between the age group of 6 to 18 months were selected for the study. The animals were divided into four groups viz. (i) Control (n=4); (ii) iPM+DIP-HIP (n=6); (iii) iPM+2% Al (OH)3 (n=6) and (iv) iPM alone (n=4). For the immunization, formulations were prepared using the recommended dose of antigen which was 3x109 CFU/ml. Animals were bled for sera samples on 14th, 21st, 28th, 56th, 90th, 120th, and 180th days after the immunization. Formulations were prepared as indicated in Table 2.
Antibody response
Indirect ELISA was performed using sonicated antigen (semi-purified soluble protoplasmic antigen derived from PM) to measure antibody response against PM. Briefly antigen was coated on microtitre plates in carbonate-bicarbonate buffer (pH- 9.6) and left overnight at room temperature. Plates were then washed three times with phosphate buffered saline containing 0.5% Tween 20 (PBST). Test sera, at a dilution of 1: 100, were added and incubated at 370C for 2 hours. Plates were washed five times and after addition of rabbit anti-bovine IgG peroxidase conjugate, incubated for 2 hours at 370C. After washing plates again, substrate (o- Phenylenediamine containing 0.01% hydrogen peroxide) was added and incubated in dark for color development. The reaction was stopped using 2N sulphuric acid and plates were read at 450nm in ELISA reader.
Bacterial culture
Active PM bacterial culture (from BIOVET Pvt. Ltd. Bangalore) was inoculated in LB media in a conical flask and incubated for 16 to 18 hours on shaking incubator at 370C. CFU count was measured through spectrophotometer at 600 nm (Biotek, USA). The bacterial culture was spun down at 5000 rpm for 10 min, pellet was washed and re-suspended in 5ml of 1x PBS (sterile, pH 7.2). Stock of 1010 CFU/ml was serially diluted up to 101 CFU/ml for LD50 dose determination [17].
LD50
For calculation of LD50 dose of PM mice were divided in ten groups with 10 animals per group and intramuscularly administered with an 8-12 hour old broth culture of virulent strain of PM in the range of 101 to 1010 CFU/ml. The animals were then observed for 24, 48 and 72h for evaluation of mortality rate amongst the groups. The median lethal dose (LD50) was calculated as the amount of a given substance required to kill 50% of a test population after 48 hours.
Challenge experiment
For the challenge study, 20 Swiss albino mice with average weight of 25g were selected and divided into control and test groups (n=5). All the animals of test group were immunized intramuscularly with iPM+DIP-HIP formulation and a booster dose after 14 days. Both the vaccinated and control mice were challenged intramuscularly with the calculated LD50 dose of virulent strain of PM. The protection provided by the vaccine was calculated as:
Number of protection units= LD50 in control animals- LD50 in vaccinated animals.
Results
Antibody response in mice
To assess the antibody response, whole IgG antibody titer was evaluated against iPM antigen. There was a significant increase in antibody titre of iPM+DIP-HIP immunized animals as compared to antigen alone control group (Figure 1).
Antibody response in cattle
The herbal adjuvant DIP-HIP proved to be an effective adjuvant with iPM vaccine, hence the same formulation was finally used to check its efficacy in the natural host of PM i.e. cattle. Calves were vaccinated with DIP-HIP and compared with Alum to assess humoral response for long term duration. The antibody titer of animals vaccinated with iPM antigen and Algel increased steadily from 28th to 56th days. In case of iPM+DIP-HIP, the antibody titer increased from 90th to 120th days. Although there was a decrease in antibody titer in sera samples of iPM+DIP-HIP immunized animals after 120 days but the titer showed more protection till 180 days (Figure 2).
LD50 and Challenge study
The formulation of iPM+DIP-HIP proved to be highly effective in both mice as well cattle so it was interesting to determine the lethal dose of virulent PM. To calculate LD50, mice were administered with different dilutions of active PM from 1x101 cfu/ml to 1x1010 cfu/ml. The mortality rate of mice was monitored after 24, 48 and 72hrs. It was 1 x 107 cfu/ml group which showed 50% mortality after 48 hrs (Table 3).
To prove the protective efficacy of iPM+DIP-HIP emulsion against lethal infection of PM, mice were challenged with 1x107 CFU/ml after 1 week of booster immunization with iPM+DIP-HIP. Control mice started dying within 36hrs of challenge (80% mortality), which continued till 48hrs (85% mortality) followed by 72hrs (100% mortality). Interestingly in test groups the overall survival rate after 36hrs was 95%, whereas 90% in 48hrs, and 85% and 80% in 72hrs and 96hrs and the observation was continued for 20 days with no further mortality in the test group (Figure 3).
Discussion
In spite of the fact that HS is one of the major fatal diseases in cattle and buffaloes and leads to massive economic losses, the nature of immune response against its causative organism PM is inadequately comprehended. The acute disease can persist up to 3 -5 days, and is characterized by 104°–106°F (40°–41.1°C) fever, apathy or restlessness and reluctance to move, lacrimation, nasal discharge that begins as serous and progresses to mucopurulent, subcutaneous swelling in the pharyngeal region that can extend sometimes up to the forelegs, progressive respiratory difficulty, terminal recumbency, and possibly abdominal pain with diarrhea (The Merck Veterinary Manual). Despite treatment with antibiotics, the bacterium may persevere in the tonsils of carriers for several months and appear actively in the nasopharynx from where it may shed intermittently in the nasal secretions [18,19]. Hence, vaccination is the only approach to prevent HS at present [20]. Killed vaccines are most commonly used for prevention and include bacterins, alum-precipitated and aluminum hydroxide gel vaccines, and oil-adjuvant vaccines. A live avirulent vaccine prepared from a PM serotype B:3 (4) of fallow deer origin seems effective and is recommended for use by the Food and Agricultural Organization of the United Nations (FAO) in southeast Asia. Various modified-live and subunit vaccines made from either purified or recombinant bacterial components have also been investigated experimentally. The oil-adjuvant vaccine provides protection for 9–12 months and is given annually. Although it provides the best immunity, it is unpopular in the field because of its viscosity and difficulty of administration. Oil-based vaccines combined with tween 80 or saponin has also been used in attempts to increase the ease of administration or immune protection (The Merck Veterinary Manual). Hence, the currently available vaccines are inefficient as they fail to provide robust and long term immunity. Thus development of efficient vaccines is expected to confer more solid and long lasting immunity.
In the present study, we evaluated the efficacy of an herbal adjuvant DIP-HIP along with iPM as vaccine against HS, in mice and further confirmed in cattle which are the ultimate host. Our results showed that herbal adjuvant DIP-HIP along with iPM conferred robust immunity against the disease in mice when compared to signature adjuvant alum and since a significant amount of neutralizing antibodies was produced in iPM+DIP-HIP emulsions as confirmed by whole IgG titre, the natural host of PM i.e. cattle were immunized with iPM+DIP-HIP and they also showed significantly high neutralizing antibody level against iPM even after 180 days. In the final set of experiments, a LD50 dose was calculated and found to be 1x107 CFU/ml. The calculated dose of active PM was used to challenge iPM+DIP-HIP immunized mice to evaluate the efficacy of this vaccine in providing protection against HS. We found that, animals immunized with iPM+DIP-HIP survived the lethal dose of virulent PM.
Various vaccines against HS have been used so far but none has proved to provide long lasting immune response with minimum side effects, lesser or no booster dose and cost effectiveness. Emulsion vaccines (water–in-oil) developed using small quantities of mineral oil [21], provide protective immunity up to 1 year but are difficult to inject due to high viscosity, formation of abscess at the site of injection and post vaccination reactions [22]. While acellular vaccines with only antigenic part capsule, LPS, fimbriae [23], have also been used but these require frequent booster doses, inducing a less durable immune response. Various studies have shown that classical alum based vaccine do not provide immunity beyond 3 months [22,24,25]. In the present study, an herbal adjuvant DIP-HIP based vaccine was used to immunize mice and showed a significantly high antibody response against HS suggesting superior adjuvant activity of DIP-HIP. Our results showed that alum vaccinated cattle showed waned antibody titers after 150 days but DIP-HIP vaccinated animals showed significantly high level of antibodies even at 180 days post immunization. Therefore, it is evident from the data obtained from challenge study and humoral immune response that DIP-HIP is a potent adjuvant and after the infection protected animals from a deadly disease HS in cattle.
Funding
This study was funded by Defence Research and Development Organisation, Government of India, under the project DIP-251/C-7.
Authors thank Mr. Bhagwat Singh (Experimental Animals Facility) for providing animals for experimental work.
- Bain RVS, De Alwis MC, Carter GR, Gupta BK (1982) Haemorrhagic Septicaemia. FAO Animal Production and Health Paper No. 33. FAO, Rome, Italy.
- Carter GR, De Alwis MC (1989) Haemorrhagic septicaemia. In: Pasteurella and Pasteurellosis, Adlam C & Rutter JM, eds. Academic Press, London, UK 131–160.
- De Alwis MC (1992) Haemorrhagic septicaemia – a general review. Brit Vet J 148: 99–112.
- Mustafa AA, Ghalib HW, Shigidi MT (1978) Carrier rate of Pasteurella multocida in a cattle herd associated with an outbreak of haemorrhagic septicaemia in the Sudan. Brit Vet J 375–378.
- Singh VP, Kumar AA, Srivastava SK, Rathore BS (1996) Significance of HS in Asia: India. International Workshop on Diagnosis and Control of HS. Bali, Indonesia, Indonesian Department of Agriculture 16.
- Khera SS (1979) The incidence and distribution of epizootic diseases in India: haemorrhagic septicaemia. Bull off Int Epizoot 91: 331–347.
- Singh VP, Sinha DK, Gupta SK, Chauhan RS (2008) Prevalence of H.S in India. Proceedings of V Annual Scientist Meet on Haemorrhagic Septecaemia, Guwahati. Assam.
- Mitra J, Chowdhury M, Bhattacharya C (2013) Outbreak of hemorrhagic septicemia in free range buffalo and cattle grazing at riverside grassland in Murshidabad District, West Bengal, India. Exploratory animal and Medical Research 3: 178–182.
- Benkirane A, De Alwis MCL (2002) Haemorrhagic septicaemia, its significance, prevention and control in Asia. Vet Med Czech 47: 234-240.
- Buxton A, Fraser G (1977) Animal Microbiology (Vol. l). Blackwell Scientific Publication. Oxford. London. Edinburg 121-126.
- Verma R, Jaiswal TN (1997) Protection, humoral and cell mediated immune responses in calves immunized with multiple emulsion haemorrhagic septicaemia vaccine. Vaccine 15: 1254–1260.
- Chandrasekaran S1, Kennett L, Yeap PC, Muniandy N, Rani B, et al. (1994) Characterization of immune response and duration of protection in buffaloes immunized with haemorrhagic septicaemia vaccines. Vet Microbiol 41: 213–219.
- Bazaron EG, Tsybikova DTS (1978) Hippophae rhamnoides, a drug agent in Indian-Tibetian medicine. Rastitelʹnye resursy 14: 67-69.
- Beveridge T, Li TS, Oomah BD, Smith A (1999) Sea Buckthorn Products: Manufacture and Composition. J Agr Food Chem 47: 3480−3488.
- Jain M, Ganju L, Katiyal A, Padwad Y, Mishra KP, et al. (2008) Effect of Hippophae rhamnoides leaf extract against Dengue virus infection in human blood-derived macrophages. Phytomed 15: 793-799.
- Upadhyay NK, Kumar MSY, Gupta A (2010) Antioxidant, cytoprotective and anti-bacterial effects of Seabuckthorn (Hippophae rhamnoides L) leaves. Food Chem. Toxicol 48: 3443-3448.
- Roier S, Fenninger JC, Leitner DR, Rechberger GN, Reidl J, et al. (2013) Immunogenicity of Pasteurella multocida and Mannheimia haemolytica outer membrane vesicles. Int J Med Microbiol 303: 247–256.
- de Alwis MC1, Wijewardana TG, Gomis AI, Vipulasiri AA (1990) Persistence of the carrier status in haemorrhagic septicaemia (Pasteurella multocida serotype 6:B infection) in buffaloes. Trop. Anim. Health Pro 22: 185–194.
- Harper M1, Boyce JD, Adler B (2006) Pasteurella multocida pathogenesis: 125 years after Pasteur. FEMS Microbiol Lett 265: 1-10.
- OIE (2009) Haemorrhagic septicaemia, Chapter 2.4.12. Terrestrial Manual, Office International Des Epizooties (OIE), Paris, France 739–750.
- Bain, R.V.S., De Alwis, M.C.L., Carter, G.R., Gupta, B.K., (1982) Haemorrhagic Septicaemia. FAO Animal Production and Health Paper No. 33. FAO, Rome, Italy.
- Verma R, Jaiswal TN (1998) Haemorrhagic septicaemia vaccines. Vaccine 16: 1184–1192.
- Hatfaludi T, Al-Hasani K, Boyce JD, Adler B (2010) Outer membrane proteins of Pasteurella multocida. Vet Microbiol 144: 1–17.
- Muneer R, Hussain M, Zahoor AB (2005) Efficacy of Oil Based haemorrhagic Septicaemia Vaccine: A Field Trial. Int J of Agri Biol 7: 571-573.
- Qureshi, S., Saxena, H.M., (2014) Estimation of titers of antibody against Pasteurella multocida in cattle vaccinated with haemorrhagic septicemia alum precipitated vaccine. Vet World 7: 224-228.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
