Journal of Vaccines and Immunology
Purified Recombinant VP2 Protein Can Provide Complete Protection to very Virulent Infectious Bursal Disease Virus Challenge as a Subunit Vaccine
Pang Wenqiang1#, Zhao Kunkun1#, Xue Jingjing1, Geng Xiaolin1, Yuan Yue1, Huang Yuxin1, Yang Yujie1, Jin Yudan1, Tian Hui1, Wu Peng1, Li Xiangdong1, Liu Wujie1* and Tian Kegong1,2*
2College of Animal Science and Veterinary Medicine, Henan Agricultural University, Zhengzhou, Henan 450002, P.R. China
#These authors contributed equally to this work
Tian Kegong, National Research Center for Veterinary Medicine, Luoyang, China, Tel: +86-(0379) 60687971; Fax: +86-(0379) 60687090; E-mail: tiankg@263.net
Cite this as
Wenqiang P, Kunkun Z, Jingjing X, Xiaolin G, Yue Y, et al. (2015) Purified Recombinant VP2 Protein Can Provide Complete Protection to very Virulent Infectious Bursal Disease Virus Challenge as a Subunit Vaccine. J Vaccines Immun 1(1): 039-042. DOI: 10.17352/jvi.000009Background: The very virulent infectious bursal disease virus has become the dominant path type that damage lymphoid tissues with high mortality in young chickens in China. Current commercial vaccines are modified live vaccines originated from classic form of virulent virus and cannot provide complete protection as they cause bursal atrophy and immunosuppression. There is an urgent call to develop more effective and safer vaccines.
Methods: In this study, we successfully expressed the soluble VP2 protein in E.coli and purified recombinant protein by using ion-exchange chromatography. The recombinant protein was subsequently used on chickens as a subunit vaccine.
Result: The purified recombinant VP2 protein can generate high agar gel diffusion precipitation antibodies and provide complete protection to a very virulent filed infectious bursal disease virus challenge as shown by results of clinical manifestations and histopathological examination. By contrast, commercial vaccines can only provide 60% protection as compared with recombinant VP2 protein.
Conclusion: The subunit vaccine based on recombinant VP2 protein could be a promising vaccine candidate to be used on chickens.
Abbreviations
IBDV: Infectious Bursal Disease Virus; DPV: Days Post-Vaccination; AGP: Agar Gel diffusion Precipitation;
Introduction
Infectious bursal disease virus (IBDV) is the etiological agent of IBD, which affects lymphoid tissue of the cloacal bursa of young chickens and causes severe immune suppression with high mortality [1]. There are two serotypes of IBDV, and only serotype 1 viruses are virulent to chickens. Serotype 1 viruses can be further divided into different pathotypes according to their pathogenicity, namely the classic form of virulent IBDV (cvIBDV), antigenic variant strains (avIBDV), and very virulent strains (vvIBDV) [2]. Since 1990s, vvIBDV has become the dominant pathotype with increasing number of isolates obtained even from the vaccinated chicken flocks and caused huge amount of economic losses in China [3].
IBDV belongs to the genus Avibirnavirus of the family Birnaviridae. The genome of virus consists of two double-stranded RNA segments. Five viral proteins (VP1-VP5) have so far been identified. Among them, VP2 is the major host-protective antigen and anti-IBDV antibody induced by recombinant VP2 could protect chickens against virus infection [4]. Therefore, VP2 protein was previously expressed in prokaryocyte such as E.coli and eukaryotes such as yeast and baculovirus to work as subunit vaccines [4-6]. Compared to eukaryotes, prokaryocyte E.coli has several advantages over eukaryote expression system since it could be easily handled and is less expensive. However, several studies reported the VP2 expression products in E.coli were insoluble and useless in production of subunit vaccines against IBDV [7,8]. One study has successfully expressed the soluble recombinant VP2 protein in E.coli bacteria and the crude products were used directly on chickens [9]. Although results of the study showed crude subunit vaccines could provide protection against IBDV, biosafety concerns such as bacteria components and antibiotic gene in vaccines will jeopardize food consumers when they consume the chickens vaccinated with above vaccines. Also, pre-exist antibodies against E.coli in chickens will deteriorate the efficacy of vaccination if the recombinant vaccines contain bacterial components. Therefore, in this study, we expressed soluble recombinant VP2 protein in E.coli and removed the bacteria components by ion-exchange chromatography. The purified recombinant VP2 protein was then evaluated the efficacy on chickens as a subunit vaccine. Our results showed that the purified VP2 protein could induce high level of AGP antibodies and provide a complete protection after a vvIBDV strain challenge.
Materials and Methods
Virus
vvIBDV strain HuB was isolated from a poultry farm in Hubei province in China and was used for final challenge study. Briefly, bursae of sick chickens were collected and homogenised in PBS (1:10 ratio). The ELD50 was performed on chicken embryo and the titer was 104.3ELD50/0.2ml after Reed-Muench calculation. For virus challenge, 0.2ml of a 1/20 dilution in PBS of a bursal homogenate of vvIBDV stain was used [10]. The final challenge titer for one chicken was 1x103ELD50/0.2ml.
Preparation of recombinant protein
The code optimized full-length of VP2 gene (1356 bp) was synthesized and inserted into the expression plasmid pET32a (Supplementary Figure 1). IPTG was used to induce the expression of recombinant protein with a final concentration of 0.4mM at 28 °C for 4 hours. Cells were harvested and were broken by sonication. The supernatant containing soluble recombinant VP2 proteins were purified by ion-exchange chromatography to remove cell proteins. Briefly, Q Sepharose Fast Flow was packed in an XK26/20 column (2.5 ml) and was equilibrated by three column volumes of 50 mM Tris-HCl (pH8.0). The resuspended protein solution was loaded into the column. The column was washed by four column volumes of 50 mM Tris-HCl (pH8.0) and the protein was eluted by 0.5 M NaCl with 50 mM Tris-HCl (pH8.0). The crude and purified proteins were subjected to SDS-PAGE and western-blot. A polyclonal anti-IBDV antibody was used to test the specificity of recombinant protein in western-blotting.
Animal trial
Forty 21-day old chickens were randomly divided into four groups. Recombinant VP2 protein at 1:16 AGP antigen titer was mixed with commercial Marcol 52 oil (ExonMobil, France) emulsion at 1:2 (v/v) to make water-in-oil emulsion vaccine. Chickens in group VP2 were intramuscularly injected with recombinant VP2 vaccine at a dose of 0.2 ml per chicken. Chickens in group B87 received one dose of commercial live vaccines of IBDV B87 strain (HLJ Animal-use Biological Products Co., Ltd., China). Chickens in groups CC and NC received placebo PBS. After 21 days post-vaccination (dpv), chickens in first three groups were challenge with vvIBDV HuB. Chickens in group NC were not received viral challenge and worked as sterile control throughout the study. After 10 days post-challenge, the survived chickens were euthanized. Bursa of fabricius were weighed and the ratio of bursa of Fabricius (BF) and body weight (BW) was calculated using the formula: (BF weight (in g)/BW (in g)) ×1000. The levels of protection were evaluated based on the mortality and histopathological bursal lesions as previously described [11]. The animal trial in this study was approved by the Animal Care and Ethics Committee of China National Research Center for Veterinary Medicine, and conventional animal welfare regulations and standards were taken into account.
Statistical analysis
All data were expressed as mean + SD and were analyzed by one-way ANOVA followed by post-hoc Tukey’s test in Graph Pad Prism5.0 Software (San Diego, CA). P values of less 0.05 were considered significant.
Results
Expression of recombinant of VP2 protein
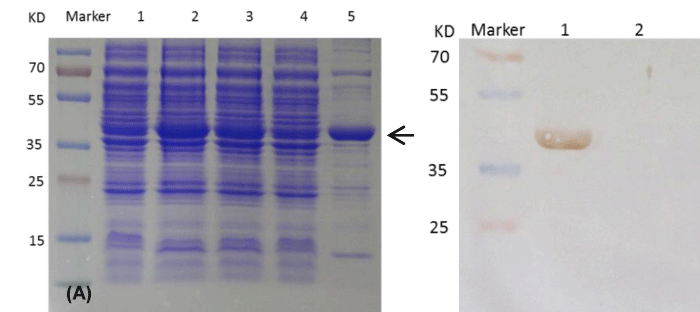
After induced by IPTG in E.coli BL21DE3 at 28 °C for 4 hours, cells were disrupted by sonication. The recombinant VP2 protein was found in the supernatant of cell lysis by SDS-PAGE result (Figure 1A). The MW of recombinant VP2 protein in this study is 37kd, which is consistent with one early report [9], but lower than 52 kd reported by Yu [8]. After purification by ion-exchange chromatography, the purified protein was subjected to western blot. The recombinant protein reacted specifically with IBDV VP2 antibody as shown by Figure 1B.
AGP-antibody response after vaccination
Every 7 days post-vaccination, IBDV-specific antibodies were monitored by AGP. As shown by Table 1, antibodies could be detected at 14dpv, and all chickens vaccinated with recombinant VP2 subunit vaccine developed positive antibodies. As compared with subunit vaccine, 8 out 10 chickens vaccinated with commercial vaccines showed positive antibodies. At 21 dpv, all chickens in vaccinated groups showed positive antibody response. Chickens in CC and NC groups did not show IBDV-specific antibody immune response as expected.
Protection after vvIBDV challenge
After challenge, typical clinical signs of IBD including anorexia and ruffled feathers began to appear in CC group. Chickens in this group died at 3-6 dpc with 60% mortality. No clinical signs were observed in other three groups. At necropsy, bursa of fabricius of chickens in CC groups showed severe hemorrhages, lypmphod necrosis, and lymphocyte depletion during histopathological examination. Several chickens vaccinated with B87 showed slight fibroplasias in the inter follicular connective tissues and infiltration of recticular epithelial cells. No hemorrhages or necrosis were observed. By contrast, the integrity of bursal follicles of VP2-vaccinated chickens was similar to the chickens in NC group and no hemorrhages or necrosis was observed. The histopathological bursal follicle lesion scores were summarized in Table 2. Therefore, the above results showed that recombinant VP2 subunit vaccine could provide complete protection to the vvIBDV HuB challenge.
Discussion
vvIBDV has become the predominant pathotype and led to huge economic losses to avian industry in China [2,3]. Current commercial vaccines are mainly live attenuated IBDV and cannot provide complete protection since they cause bursal atrophy and immunosuppression [9]. Therefore, there is an urgent call for more effective and safer vaccines to tackle the current problem. By using modern molecular technologies, IBDV recombinant live vaccines based on reverse genetics, DNA vaccines, and subunit vaccines were extensively studied to explore the potential as vaccine candidates [9,12,13]. Among of them, subunit vaccines seem more promising since they showed better protection as compared with traditional live vaccines and DNA vaccines. The recombinant VP2-based subunit vaccines induce both viral neutralizing antibodies and cellular immunity which confers good protection to the field virus reinfection [4].
Several studies reported the failure of expressing VP2 protein in E.coli or inefficacy of working as a subunit vaccine [7,8]. One group successfully expressed the soluble form of recombinant VP2 protein and proved the crude recombinant bacterial product could provide good protection [9]. However, the crude bacterial products have a huge biosafety issues and could not be certified to production under current regulation of veterinary biological administration. To make up for it, we purified the recombinant VP2 protein in the supernatant of lysed cells and tested the efficacy of it as a vaccine candidate. The purity and correct protein folding of recombinant protein are critical for protein biological properties since VP2 contains at least three independent epitopes responsible for the induction of protective neutralizing epitopes [14]. The inefficacy of insoluble recombinant VP2 protein in previous study could be due to the incorrect folding of protein after renaturation of inclusion body protein.
As compared with commercial live vaccines, VP2 subunit vaccine in this study could elicit higher titer of AGP antibodies (Table 1). Previous study showed a high correlation between the presence percentage of AGP anti-IBDV antibodies and protection percentage of chickens after viral challenge [9], which indicates the subunit vaccine in this study could be a decent vaccine candidate to control infection. Therefore, the vaccinated chickens were challenged with a vvIBDV strain on 21 days-post vaccination. The challenge results showed that VP2 subunit vaccine could provide better protection than commercial live vaccines based on clinical manifestations and histopathological examinations. Moreover, VP2 subunit vaccine in this study did not lead to bursal atrophy but provide 100% protection to the vvIBDV HuB strain challenge.
Conclusion
To conclude, we expressed and purified the recombinant IBDV VP2 protein in E.coli. The chicken study results showed that the recombinant VP2 protein could be used as a good subunit vaccine since it provided a complete protection to the challenge of a current circulating strain of IBDV.
- Wyeth PJ, Cullen GA (1976) maternally derived antibody--effect on susceptibility of chicks to infectious bursal disease. Avian pathology: journal of the WVPA 5: 253-260.
- He X, Wei P, Yang X, Guan D, Wang G, (2012) Molecular epidemiology of infectious bursal disease viruses isolated from Southern China during the years 2000-2010. Virus genes 45: 246-255 .
- Xu MY, Lin SY, Zhao Y, Jin JH, Tang N, et al. (2015) Characteristics of very virulent infectious bursal disease viruses isolated from Chinese broiler chickens (2012-2013). Acta tropica 141: 128-134 .
- Pradhan SN, Prince PR, Madhumathi J, Roy P, Narayanan RB, et al. (2012) Protective immune responses of recombinant VP2 subunit antigen of infectious bursal disease virus in chickens. Veterinary immunology and immunopathology 148: 293-301 .
- Pitcovski J, Gutter B, Gallili G, Goldway M, Perelman B, et al. (2003) Development and large-scale use of recombinant VP2 vaccine for the prevention of infectious bursal disease of chickens. Vaccine 21: 4736-4743.
- Xu XG, Tong DW, Wang ZS, Zhang Q, Li ZC, et al. (2011) Baculovirus virions displaying infectious bursal disease virus VP2 protein protect chickens against infectious bursal disease virus infection. Avian diseases 55: 223-229.
- Azad AA, McKern NM, Macreadie IG, Failla P, Heine HG, et al. (1991) Physicochemical and immunological characterization of recombinant host-protective antigen (VP2) of infectious bursal disease virus. Vaccine 9: 715-722.
- Yu L, Song AK, Zhang AB, Deng R (2000) Cloning and expression of the VP2 gene of an infectious bursal disease virus. Avian diseases 44: 170-178 .
- Rong J, Cheng T, Liu X, Jiang T, Gu H, et al. (2005) Development of recombinant VP2 vaccine for the prevention of infectious bursal disease of chickens. Vaccine 23: 4844-4851.
- Martinez-Torrecuadrada JL, Saubi N, Pagès-Manté A, Castón JR, Espuña E, et al. (2003) Structure-dependent efficacy of infectious bursal disease virus (IBDV) recombinant vaccines. Vaccine 23: 3342-3350 .
- Shaw I, Davison TF (2000) Protection from IBDV-induced bursal damage by a recombinant fowlpox vaccine, fpIBD1, is dependent on the titre of challenge virus and chicken genotype. Vaccine 18: 3230-3241.
- Gao L, Qi X, Li K, Gao H, Gao Y, et al. (2011) Development of a tailored vaccine against challenge with very virulent infectious bursal disease virus of chickens using reverse genetics. Vaccine 29: 5550-5557 .
- Pradhan SN, Prince PR, Madhumathi J, Arunkumar C, Roy P, et al. (2014) DNA vaccination with VP2 gene fragment confers protection against Infectious Bursal Disease Virus in chickens. Veterinary microbiology 171: 13-22.
- Brandt M, Yao K, Liu M, Heckert RA, Vakharia VN (2001) Molecular determinants of virulence, cell tropism, and pathogenic phenotype of infectious bursal disease virus. Journal of virology 75: 11974-11982.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
