Journal of Surgery and Surgical Research
The burden of aerobic bacterial nosocomial infections, associated risk factors and antibiotic susceptibility patterns in a surgical site in Ethiopia: A systematic review
Tsegaye Alemayehu*
Cite this as
Alemayehu T (2020) The burden of aerobic bacterial nosocomial infections, associated risk factors and antibiotic susceptibility patterns in a surgical site in Ethiopia: A systematic review. J Surg Surgical Res 6(2): 126-132. DOI: 10.17352/2455-2968.000112Nosocomial infection is an infection that acquired after exposure of patients to hospital for 48-72 hrs, but not present during admission. Surgical site infection is among the leading nosocomial infection that acquired after operation or admission. This review aims to determine the burden of NI in surgical site infection in Ethiopia systematically. Among a 167 mean of clinically suspected patients samples 9-92% were culture-confirmed with a mean of 70.125 and median 67. Seven studies identified 49.3-100% of culture-confirmed infection as SSI; two studies reported BSI 2.2 & 20.8 percent and one study declare UTI as 29.8 percent among 77 cultures confirmed and one study not reported about infection identified. Ward type, type of operation, wound type, being a male, site of a wound, age ≥ 51, diabetes mellitus, anaemia, antibiotic usage after surgery, 11-15 days preoperative hospital stay, postoperative hospital stay; surgical procedure, urinary catheter, mechanical vent, IV catheter, longer duration from admission to discharge, longer duration of preoperative and preoperative prophylaxis identified as potential risk factors. S. aureus and CoNS is among the leading gram-positive bacterial isolate and E. coli, Klebsiella spp, Proteus spp and P. aeruginosa are among gram-negative organisms that isolated from eight studies in Ethiopia.
Introduction
Nosocomial Infection (NI) is an infection that acquired after the admission of patients to a hospital within 48-72 hrs, but not present during admission[1,2]. It is a major public health problem that causes morbidity, mortality and increased health care cost for hospitalization worldwide and remains significant now a day’s [3,4]. Surgical Site Infections (SSI) are among these nosocomial an infection that occurs after an operation in the part of the body where the surgery took place. Most SSIs only involves the skin surrounding the incision; others may be deeper and more serious [5].
During the 1950s, a severe epidemic of NI occurred in surgical units in Europe and America, but thanks to the beginning of antiseptic surgery and antibiotics as prophylaxis, the problem reduced a lot [6]. Despite the technological advances that have been made in surgery and wound management, wound infection has been regarded as the most common NI currently [7]. Surgical site infections are a major problem in developed countries, where it affects from 0.5% to 15% of hospitalized patients and as many as 50% or more of patients in Intensive Care Units (ICUs). The prevalence of NI in some developed countries, in the USA (15%), German and France (<3%) [8,9] and Brazil (16.9%) [10] were reported. In developing countries, the magnitude of the problem remains underestimated or even unknown largely because NI diagnosis is complex and surveillance activities to guide interventions require expertise and resources. A study conducted in Tanzania among patients undergoing major surgery reported 26% SSI [11]. In Ethiopia, different studies reported that the prevalence of post-surgical wound infection ranges from 14.8-60% [12-15]. The variation in prevalence might be due to the difference in the type of operation (obstetrics & gynaecology, general surgery and orthopaedic) and the underlying status of patients as well as the infection control practices of the hospital environment and the type of etiologic agent [9,16,17].
The most predominant bacteria in SSIs are S. aureus, Enterococcus spp, P. aeruginosa, E. coli, and other Enterobacterales. Even though isolation of single bacterial species is common, 9-27% of polymicrobial, isolation from different surgical sites were reported [10,11,16,18]. These infections pose therapeutic challenges and are associated with substantially longer duration of hospital stay, increased hospital cost, higher morbidity and mortality[8,19], particularly when the agents are Methicillin-Resistant S. aureus (MRSA), Extended-Spectrum Beta-Lactamase (ESBL) producing Enterobacterales and/or other agents collectively referred to as Multidrug-Resistant (MDR) [18,20,21].
Studies from developing countries have shown high rates of resistance (ranging from 50 -100%) to the commonly used antibiotics like ampicillin, trimethoprim-sulphamethoxazole, gentamicin, chloramphenicol and third-generation cephalosporins among S. aureus, E. coli, and P. aeruginosa [11,22] as opposed to low rates of resistance ranging from 0-50% in developed countries [23]. However, in both settings, substantial rates of resistance to oxacillin, erythromycin and clindamycin to S. aureus, (ranged from 10-60%) were reported [11,16,22,24] whereas, vancomycin resistance (for S. aureus and other Gram-positive bacteria), amikacin, piperacillin, tazobactam and Imipenem resistance (for E. coli, P. aeruginosa and other Gram-negative bacteria) showed resistant rates less than 25% [23,25].
Factors underlying for NI after surgery are multiple and include the type of surgical procedure, the skills of the surgeon, the duration of surgery and the underlying disease of the host[26]. Both infection and wound healing are adversely influenced by poorly controlled diabetes mellitus. Age is considered an important factor, neonates and the elderly are particularly at risk of infection. Lifestyle can also impinge on immuno-competency especially stress, alcohol and drug abuse, smoking and lack of exercise or sleep [27].
In Ethiopia, there are few studies conducted on SSI [15,22,28-33]. This review provides a general overview of the burden of aerobic bacterial nosocomial infections, associated risk factors and antibiotic susceptibility patterns in a surgical site in Ethiopia based on the information available in the scientific literature.
Literature search strategy
A literature search was performed with English language restriction but without time restriction to retrieve publications on the burden of NI in the surgical site in 2017. PubMed was searched using a combination of the following keywords, including “nosocomial infection in the surgical site, postoperative infection,” together with the country name “Ethiopia”. Reference lists of retrieved articles were hand-searched for additional studies that are not on PubMed sited but its Portable Document Format (PDF) is freely accessible from Google. Studies that are done on either prevalence and antibiotics susceptibility or prevalence and risk factors were selected and included in the review. There is no communication, which made and no permission found from authors.
Result
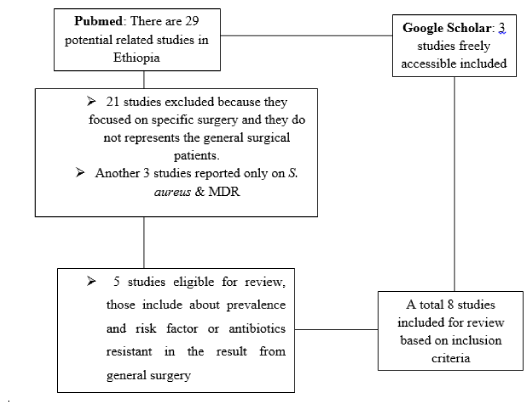
The PubMed search yielded 29 papers. Of these, 5 met the illegibility criteria and 3 studies from Google scholar added (Figure 1).
Characteristics of studies and prevalence of SSI
Eight studies from five different regions of Ethiopia with prospective cross-sectional study design were included: Four studies from Amhara region; Two, from Southern Nation, Nationalities and People Representatives (SNNPR); one, from Addis Ababa and one from Tigray [15,22,28-33]. Authors name, year of publication/ study period and study area; study design, sample size, number and percent of clinically suspected SSI; number, percent and type of specimen taken; number and percent of culture-confirmed positive; number, percent and type of infection; significant risk factors p<0.05 and total, list and percent of isolate manipulated from the result part of all studies.
All studies based on prospective cross-sectional studies. Almost all studies reported both clinical and microbiological data except one study from Bahir Dar not reported isolated organisms [30]. The mean of sample size is 435 with a range of 100-854 [15,22,28-33]. Six studies reported the mean age of patients with a mean of mean 20.7 yrs with a range of 26-38.2 years [15,22,28,30,32]. Age range reported in five studies which vary from 6 month- 100 years [15,28,30-32] but not in the other three studies [22,29,33]. The mean is 169 for clinically suspected NI in SSI with a median of 166 cases with (8.2-100%) sampled patients [15,22,28-33]. All researchers used a swabbed sample from the wound site [15,22,28-33] however, three studies also used blood for Bloodstream Infection (BSI) [15,29,30] and one study used urine for Urinary Tract Infection (UTI) [15]. Among clinically suspected patients was confirmed culture positive with a mean of 70.125 and median 67 with 9-92 percent [15,22,28-33]. Seven studies identified 49.3-100 percent of culture-confirmed infection as SSI; two studies reported BSI 2.2 & 20.8 percent and one study declare UTI as 29.8 percent among 77 cultures confirmed and 1 study not reported about infection identified (Table1).
Risk factors and potential bacteria
Potential risk factors for acquiring NI in surgical site infections presented in table 2. S. aureus, E. coli, CoNS, Klebsiella spp and Proteus spp and P. aeruginosa are the most frequently isolated bacteria in descending order after calculated the average of the isolates from seven studies. Isolated organism not reported in a study from Bahir Dar which only focused on risk factors [30] (Table 2).
Antibiotic resistance rate
Antibiotics resistance among gram positive and gram-negative bacteria occurred with different rates of resistance. Total percent of antibiotics resistance for gram positive and negative bacteria that isolated from each sample for specific drug calculated and rates of resistance determined. Bacteria that showed resistance < 60% are low rates of resistance, 60-80% intermediate rates resistance, 81-99 % high rates of resistance and 100% fully resistance (Table 3).
Discussion
The small number of papers retrieved is evidence that little information is available on the burden of NI in the surgical site in Ethiopia. The review has shown that published studies were conducted in certain regions of the country and the majority of them are from the same hospitals. Besides, the scope of the studies is limited, since all were conducted in single hospitals. This review tried to include the prevalence SSI, types of bacteria, risk factors and antibiotics resistance is evaluated which is the strength of our study. The limitation of our review is only based on limited databases that may have an impact on the interpretation of the result.
Our review showed great variability in the reported prevalence of NI in the surgical site that ranges from 7.25 - 92%. Taking the average percentage of SSI in our study is 28.3 %, this finding is consistent with the prevalence reported from Vietnam (8.3-43.3%) [34], systematic review from Africa (2.5-30.9%) [35], Uganda 28.4% [36] and higher than reported from Nigeria (20.3%) [7], Saudi Arabia (11.4%) [37], Turkey (2.18%) [38]. The high prevalence of NI in surgical sites in Ethiopia may be due to lack of proper infection prevention, insufficient health personnel, overcrowded beds and a longer hospital stay before and after surgery.
In this review, different risk factors were identified age ≥ 51 is one factor for acquiring of SSI [30] in one study, but a prospective study from China showed that patients aged over 75 years (5.6%) were more likely to develop SSI than those under the age of 75 years (3.0%), but the association is not significant with P>0.05. There was no relationship observed between gender and development of SSI in the study from Nigeria with (P<0.98, OR 0.99, 95% Cl 0.47–2.09) [7]. Ward type is among significant factors for the development of SSI with p = 0.001, 0.012 & 0.05 in studies from Gonder, Tigray & Hawassa respectively [22,32,33]. A study from Saudi Arabia assessed acquiring infection is significant in different wards with p< 0.0001 [39].
In these review type of operation is identified as a significant risk factor with p<0.001 in emergency surgery only [22] and p<0.038 in all type of operation [32]. Type of operation also was assessed a study from China with the significant association in emergency surgery with p<0.000 which agreed with this review. Lower rate of SSI reported in emergency operation compared to elective from Vietnam as opposed to the finding of this review [34]. Acquiring infection in emergency operation is due to low preoperative operation and use of antiseptics before surgery [40].
The indwelling device is considered as associated risk factors for different NI. Among 8 reviewed articles one study identified this device as risk factors: Urinary catheter (p < 0.000), Mechanical vent (p<0.000) and IV catheter (p<0.000) for the formation of infection other than surgical site infection such as BSI, UTI and Respiratory infection [15]. Similarly, a study from Vojvodina agreed as significantly associated risk factors for the formation of NI [3]. Preoperative hospital stay for 11-15 Days (p<0.03) & Hospital stay after operation for 5-10 days (p<0.004), 11-15 days (p<0.000) and > 15days (p<0.001) was one of the main risk factors for acquiring infection after surgery from a study from Bahir Dar [30]. A study from Saudi Arabia showed the length of hospital stay had a significant association for the development of NI between days of 8-13 and above with p<0.0001 [39], but not good predictor from Vietnam [34]. Diabetics Mellitus (DM) and anaemia among the risks factors that assessed in one study from Bahir Dar by Wondimagegn, et al. with a p-value less than 0.018 & 0.007, respectively. Patients who were diabetic had six times increased risk of surgical site infection than those who were not diabetic. Anaemia patients have three and half times the risk for postoperative infection when compared with those without anaemia in a study from Nigeria [7], which support the study from Bahir Dar but the other 7 studies failed to assess about DM and anaemia. Antibiotic used before and after surgery determined as risk factors; from Mekelle and Bahir Dar with p<0.012 & 0.02 respectively. According to a study from China by Cheng k, et al. only giving prophylaxis not a guaranty for prevention of SSI infection rather coordinated infection prevention needed from management to all personal that involved in the surgical ward. Also in one systematic review, a statistically significant association between antibiotic prophylaxis and increased risk of SSI observed in one study lacks biologic plausibility as a causal relationship given well-documented evidence regarding a protective effect of antibiotics for SSI [41].
According to this review, S. aureus [18.5%] and E. coli [17.8%] were the predominant bacterial isolate followed by Klebsiella spp [16.4%], CoNS [13.5%], Proteus spp [10.8%], P. Aeruginosa [10.2%],
For this review antibiotics, resistance classified as those < 60% resistance for a specific drug as low-rates resistant, 60-80 % intermediate rate of resistant, 81%-99% high rates of resistant and 100% completely resistant. A drug that has a low rate of resistant in one area may have a high level of resistant in another area for both grams positive and gram-negative bacteria as tried to show Table 3.
Conclusion
Nosocomial infection in SSI due to the resistant organism is the most serious challenge for both physicians and patients to cure on time and spending more money on treatments. According to this review, the resulted infection rate or prevalence, which ranges from 7.25-92% was comparatively the highest report from Africa. A wide range of risk factors identified for SSI in this review (age, sex, indwelling device, anaemia, diabetes mellitus, wound type, type of operation, sites of the wound, longer pre- and post-operative hospital stay, pre and post-operative antibiotics usage and ward type. S. aureus and CoNS are the most frequently isolated organism among gram positive and E. coli, Klebsiella spp, Proteus spp is frequently isolated gram-negative bacteria followed by Enterobacter spp and Citrobacter spp. In this review, different rates of resistance observed, a drug that is resistant to one area have low rates of resistance in another area, so empirical treatment should be avoided to reduce further cost and life loss. High rates of resistance (100%) occurred among gram-positive bacteria for P, AMP, TAT, SXT, CAF and SXT, AML and TAT among gram-negative bacteria, so ordering this drugs has no value in those areas.
Author contribution
TA conceived the idea, reviewed available data and prepared the manuscript.
Availability of data and material
Can be obtained from the corresponding author.
- Soni N, Wadhwa V, Ohri K (2013) Recent Evidence in the Management of Nosocomial Infections. Link: https://bit.ly/39VnYpz
- Bereket W, Hemalatha K, Getenet B, Wondwossen T, Solomon A, et al. (2012) Update on bacterial nosocomial infections. Eur Rev Med Pharmacol Sci 16: 1039-1044. Link: https://bit.ly/31fGhC7
- Ćosić G, Đekić J, Petrović M, Krtinić G, Karać T, et al. (2014) The most frequent hospital-acquired infections related to medical interventions in hospitals in Vojvodina province. Archives of Biological Sciences 66: 523-535. Link: https://bit.ly/30pVdyj
- Lourda G, Gerard M, Mark M (2012) Northern Ireland point prevalence survey of hospital-acquired infections and antimicrobial use 2012. In.: Norther Ireland: Public Health Agency. Link: https://bit.ly/2PmxYyS
- New York State DoH, Albany (2012) Hospital-acquired infections.
- Nyamogoba H, Obala A (2002) Nosocomial infections in developing countries: cost-effective control and prevention. East Afr Med J 79: 435-441. Link: https://bit.ly/2Poxmsq
- Nwankwo E, Ibeh I, Enabulele O (2012) Incidence and risk factors of surgical site infection in a tertiary health institution in Kano, Northwestern Nigeria. Int J Infect Control 8: 8-13. Link: https://bit.ly/3hZST6Y
- Astagneau P, Rioux C, Golliot F, Brücker G (2001) Morbidity and mortality associated with surgical site infections: results from the 1997–1999 INCISO surveillance. J Hosp Infect 48: 267-274. Link: https://bit.ly/2PpVsTy
- Bärwolff S, Sohr D, Geffers C, Brandt C, Vonberg RP, et al. (2006) Reduction of surgical site infections after Caesarean delivery using surveillance. J Hosp Infect 64: 156-161. Link: https://bit.ly/2BWEj0X
- Santos K, Fonseca L, Neto GB, Gontijo Filho P (1997) Surgical site infection: rates, aetiology and resistance patterns to antimicrobials among strains isolated at Rio de Janeiro University Hospital. Infection 25: 217-220. Link: https://bit.ly/3k5FHzs
- Mawalla B, Mshana SE, Chalya PL, Imirzalioglu C, Mahalu W (2011) Predictors of surgical site infections among patients undergoing major surgery at Bugando Medical Centre in Northwestern Tanzania. BMC Surg 11: 21. Link: https://bit.ly/31fcObr
- Godebo G, Kibru G, Tassew H (2013) Multidrug-resistant bacterial isolates in infected wounds at Jimma University Specialized Hospital, Ethiopia. Ann Clin Microbiol Antimicrob 12: 17. Link: https://bit.ly/3frpCAL
- Mulu A, Moges F, Tessema B, Kassu A (2006) Pattern and multiple drug resistance of bacterial pathogens isolated from wound infection at University of Gondar Teaching Hospital, Northwest Ethiopia. Ethiop Med J 244: 125-131. Link: https://bit.ly/33B0dCr
- Zeamanuel T, Asrat D, Yimtubezinash W, Kidanu E (2009) Bacteriology of surgical site and catheter-related urinary tract infections among patients admitted in Mekelle Hospital, Mekelle, Tigray, Ethiopia. Ethiop Med J 47: 117-127. Link: https://bit.ly/3gr4NGP
- Endalafer N (2008) Bacterial nosocomial infections and their antimicrobial susceptibility patterns in surgical wards and surgical intensive care unit of Tikur Anbessa university hospital. Addis Ababa: Addis Ababa University.
- Anguzu J, Olila D (2007) Drug sensitivity patterns of bacterial isolates from septic post-operative wounds in a regional referral hospital in Uganda. Afr Health Sci 7: 148-154. Link: https://bit.ly/3frpSzJ
- Whitehouse JD, Friedman ND, Kirkland KB, Richardson WJ, Sexton DJ (2002) The impact of surgical-site infections following orthopaedic surgery at a community hospital and a university hospital adverse quality of life, excess length of stay, and extra cost. Infect Control Hosp Epidemiol 23: 183-189. Link: https://bit.ly/31fhVZl
- Fehr J, Hatz C, Soka I, Kibatala P, Urassa H, et al. (2006) Risk factors for surgical site infection in a Tanzanian district hospital: a challenge for the traditional National Nosocomial Infections Surveillance system index. Infect Control Hosp Epidemiol 27: 1401-1404. Link: https://bit.ly/2XrhQ3r
- Kirkland KB, Briggs JP, Trivette SL, Wilkinson WE, Sexton DJ (1999) The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol 20: 725-730. Link: https://bit.ly/3gs5yiW
- Engemann JJ, Carmeli Y, Cosgrove SE, Fowler VG, Bronstein MZ, et al. (2003) Adverse clinical and economic outcomes attributable to methicillin resistance among patients with Staphylococcus aureus surgical site infection. Clin Infect Dis 36: 592-598. Link: https://bit.ly/2DAhQY4
- Wassef M, Hussein A, Em AR, El-Sherif R (2012) A prospective surveillance of surgical site infections: Study for the efficacy of preoperative antibiotic prophylaxis. African Journal of Microbiology Research 6: 3072-3078. Link: https://bit.ly/2DwIHEa
- Amare B, Abdurrahman Z, Moges B, Ali J, Muluken L, et al. (2011) Postoperative surgical site bacterial infections and drug susceptibility patterns at Gondar University Teaching Hospital, Northwest Ethiopia. J Bacteriol Parasitol 2: 126. Link: https://bit.ly/2EOmPow
- Hawser SP, Bouchillon SK, Hoban DJ, Badal RE (2010) Epidemiologic trends, occurrence of extended-spectrum β-lactamase production, and performance of ertapenem and comparators in patients with intra-abdominal infections: Analysis of global trend data from 2002–2007 from the SMART study. Surg Infect 11: 371-378. Link: https://bit.ly/2EIr2db
- Throckmorton AD, Baddour LM, Hoskin TL, Boughey JC, Degnim AC (2010) Microbiology of surgical site infections complicating breast surgery. Surg Infect 11: 355-359. Link: https://bit.ly/2BUgp63
- Kownhar H, Shankar EM, Vignesh R, Sekar R, Velu V, et al. (2008) High isolation rate of Staphylococcus aureus from surgical site infections in an Indian hospital. J Antimicrob Chemother 61: 758-760. Link: https://bit.ly/3i2Q4Ci
- Mustafa A, Bukhari I, Kakru D, Tabish S, Qadri G (2004) Incidence of nosocomial wound infection in post-operative patients at a teaching hospital In Kashmir. Jk Practitioner 11: 38-40. Link: https://bit.ly/2XqGJwf
- Cooper R (2005) Understanding wound infection. European Wound Management Association (EWMA) Position Document: Identifying Criteria for Wound Infection. Link: https://bit.ly/31bqhky
- Dessalegn L, Shimelis T, Tadesse E, Gebre-Selassie S (2014) Aerobic bacterial isolates from the post-surgical wound and their antimicrobial susceptibility pattern: a hospital-based cross-sectional study. J Med Res 3: 18-23. Link: https://bit.ly/3ftaLpj
- Mulu W, Kibru G, Beyene G, Damtie M (2012) Postoperative nosocomial infections and antimicrobial resistance pattern of bacteria isolates among patients admitted at Felege Hiwot Referral Hospital, Bahirdar, Ethiopia. Ethiop J Health Sci 22: 7-18. Link: https://bit.ly/3i6tNDK
- Mulu W, Kibru G, Beyene G, Damtie H (2013) Associated risk factors for postoperative nosocomial infections among patients admitted at Felege Hiwot Referral Hospital, Bahir Dar, Northwest Ethiopia. Clin Med Res 2: 140-147. Link: https://bit.ly/39RQ2du
- Gelaw A, Gebre-Selassie S, Tiruneh M, Mathios E, Yifru S (2014) Isolation of bacterial pathogens from patients with postoperative surgical site infections and possible sources of infections at the University of Gondar Hospital, Northwest Ethiopia. Journal of Environmental and Occupational Health 3: 103-108. Link: https://bit.ly/30nSiWY
- Mengesha RE, Kasa BG-S, Saravanan M, Berhe DF, Wasihun AG (2014) Aerobic bacteria in post-surgical wound infections and pattern of their antimicrobial susceptibility in Ayder Teaching and Referral Hospital, Mekelle, Ethiopia. BMC Res Notes 7: 575. Link: https://bit.ly/3hZUE44
- Guta M, Aragaw K, Merid Y (2014) Bacteria from infected surgical wounds and their antimicrobial resistance in Hawassa University Referral Teaching Hospital, Southern Ethiopia. African Journal of Microbiology Research 8: 1118-1124. Link: https://bit.ly/30nHWGC
- Nguyen D, MacLeod WB, Phung DC, Cong QT, Nguyen VH, et al. (2001) Incidence and predictors of surgical-site infections in Vietnam. Infect Control Hosp Epidemiol 22: 485-492. Link: https://bit.ly/39TzvFX
- Nejad SB, Allegranzi B, Syed SB, Ellis B, Pittet D (2011) Health-care-associated infection in Africa: a systematic review. Bull World Health Organ 89: 757-765. Link: https://bit.ly/2D9e9Zz
- Moses M (2016) Prevalence of surgical site infections in non-diabetic patients undergoing major surgery. St. Francis Hospital Nsambya. Uganda J Med Implants Surg 1: 2. Link: https://bit.ly/3i6fY8o
- Rawabdeh AAA, Al Mulhim ARS, Khan ZU (2016) Surgical Site Infections Incidence, their Predictors and Causative Organisms in a Teaching Hospital. International Journal of Community & Family Medicine. Link: https://bit.ly/3k7Dl32
- Ceken S, Yavuz S, Sensoy A, Imamoglu O (2016) Epidemiology and risk factors for surgical site infections following thoracic surgery. Int J Clin Exp Med 9: 12018-12024. Link: https://bit.ly/30nT1aE
- Balkhy HH, Cunningham G, Chew FK, Francis C, Al Nakhli DJ, et al. (2016) Hospital-and community-acquired infections: a point prevalence and risk factors survey in a tertiary care centre in Saudi Arabia. Int J Infect Dis 10: 326-333. Link: https://bit.ly/2EOKJ3d
- Cheng K, Li J, Kong Q, Wang C, Ye N, et al. (2015) Risk factors for surgical site infection in a teaching hospital: a prospective study of 1,138 patients. Patient Prefer Adherence 9: 1171-1177. Link: https://bit.ly/2DBs4XO
- Korol E, Johnston K, Waser N, Sifakis F, Jafri HS, et al. (2013) A systematic review of risk factors associated with surgical site infections among surgical patients. PloS one 8: e83743. Link: https://bit.ly/33pXR9k
- Raza MS, Chander A, Ranabhat A (2013) Antimicrobial susceptibility patterns of the bacterial isolates in post-operative wound infections in a tertiary care hospital, Kathmandu, Nepal. Open J Med Microbiol 3: 159-163. Link: https://bit.ly/2Xnj7II
- Seni J, Najjuka CF, Kateete DP, Makobore P, Joloba ML, et al. (2013) Antimicrobial resistance in hospitalized surgical patients: a silently emerging public health concern in Uganda. BMC Res Notes 6: 298. Link: https://bit.ly/2XpDHIN
- Scherbaum M, Kösters K, Mürbeth RE, Ngoa UA, Kremsner PG, et al. (2014) Incidence, pathogens and resistance patterns of nosocomial infections at a rural hospital in Gabon. BMC Infect Dis 14: 124. Link: https://bit.ly/3fq6l2C
- Amoran O, Sogebi A, Fatugase O (2013) Rates and Risk Factors Associated with Surgical Site Infections in a Tertiary Care Center in South-Western Nigeria. Link: https://bit.ly/3k7FuvJ
- Mirsepasi H, Persson S, Struve C, Andersen LO, Petersen AM, et al. (2014) Microbial diversity in faecal samples depends on DNA extraction method: easyMag DNA extraction compared to QIAamp DNA stool mini kit extraction. BMC Res Notes 7: 50. Link: https://bit.ly/2XrNwFX
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
