Journal of Surgery and Surgical Research
OHSU 3D Printed CRISIS Ventilator
Albert Chi1*, Whitney Menzel1, Evan Fontaine1, Dennis Child2 and Stephanie Nonas3
2Respiratory Therapy Oregon Health and Science University Portland, OR, USA
3Division of Pulmonary Critical Care, Department of Medicine Oregon Health and Science University Portland, OR, USA
Cite this as
Chi A, Menzel W, Fontaine E, Child D, Nonas S (2020) OHSU 3D Printed CRISIS Ventilator. J Surg Surgical Res 6(1): 051-055. DOI: 10.17352/2455-2968.000097Overview
In response to national and international shortages in the availability of medical ventilators, our team has developed and tested a novel low-cost (~10 USD) 3D-printed flow-driven pressure-regulated mechanical ventilator capable of basic and complex ventilation needs. This device requires no electronics and can be sustained with either O2 tanks or standard hospital 50psi O2 wall supply. The design can be printed rapidly, mass-produced and immediately deliverable to any crisis areas in need. The CRISIS Ventilator has the potential to be produced on site depending on 3D printing capabilities. Our team includes physicians, scientists, a respiratory therapist, and engineers in healthcare dedicated to developing a rapidly deliverable ventilator solution to the global COVID-19 pandemic.
The Problem
The worldwide pandemic of COVID-19, a respiratory disease caused by the novel coronavirus (SARS-CoV-2019), is a monumental public health threat that has led to significant impacts on healthcare systems worldwide. Based on current data, somewhere between 10% to 25% of patients sick with COVID-19 eventually require assistance breathing [1]. Roughly 5% of patients will develop acute respiratory distress syndrome (ARDS), at which point only mechanical ventilation can give them a chance of defeating COVID-19. The United States has approximately 173,000 ventilators scattered across the country, according to the Center for Health Security at Johns Hopkins University [2], but experts from Harvard University predict that there could be 1.4 to 31 times as many patients who need one [3]. The U.S. Food and Drug Administration (FDA) Emergency Use of Authorizations (EUAs) have expanded to mechanical ventilators to drive private and public industry to develop innovative solutions to this dire problem [4]. Globally the need for mechanical ventilation is even more dire in low- and middle-income countries, where resources are limited and hospitals are hindered by equipment and infrastructure challenges [5].
Our proposed solution
Here we propose the CRISIS VENT (CV) system as a solution, a multiple-use device that can be created with a standard 3D printer in a sterile environment and rapidly produced and deployed at minimal cost of approximately 10 US dollars in materials. This new device design has the capacity to treat patients requiring standard mechanical ventilatory support but can also effectively treat the sickest patients with progressive ARDS. We have included design features that allow lung protective ventilator strategies employed for ARDS treatment with low tidal volumes and high Positive End Expiratory Pressure (PEEP). Our device also has the advantage of using various oxygen sources and can be easily altered to meet patient demands regardless of the ventilator support required.
Key features of the CRISIS VENT System
1. Can be built entirely with off-the-shelf components and a basic 3D printer.
2. Diminishes supply chain issues, is rapidly scalable, and low cost.
3. Does not require specialty manufacturing of parts.
4. Is capable of numerous ventilatory settings to treat varying degrees of respiratory failure, including basic ventilation and ARDS.
5. Can be replicated anywhere in the world.
CRISIS VENT System: Device prototype and testing
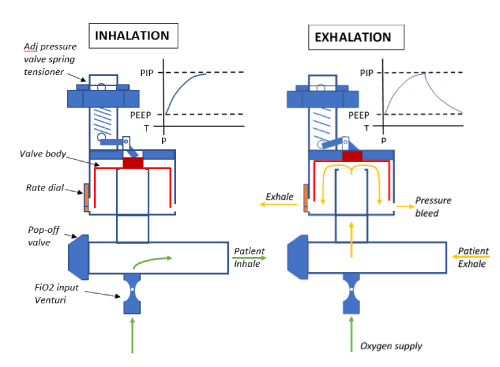
How it works: The CV system is dependent on three parameters: 1) inlet flow, 2) Peak Inspiratory Pressure (PIP), and 3) expiratory resistance, which can be set and or adjusted. PEEP values for the CV are dependent and determined by the set PIP and flow, which can both be manually adjusted. The system functions with the use of an adjustable pressure valve, which opens at peak pressure, and then closes after exhalation pressure is met. The respiration rate is determined by changing the size of the output orifice using the rate valve, which allows accurate sizing of the orifice. Currently the device works with an adjustable PIP ranging from 15 to 50 cmH2O and a PEEP value between 5 and 20 cm H2O. The PIP and the PEEP values are related as the PEEP is determined by the geometry of the valve which is still being tuned. These two values are directly correlated by this design-driven ratio. These settings could easily be applied to infants to adults.
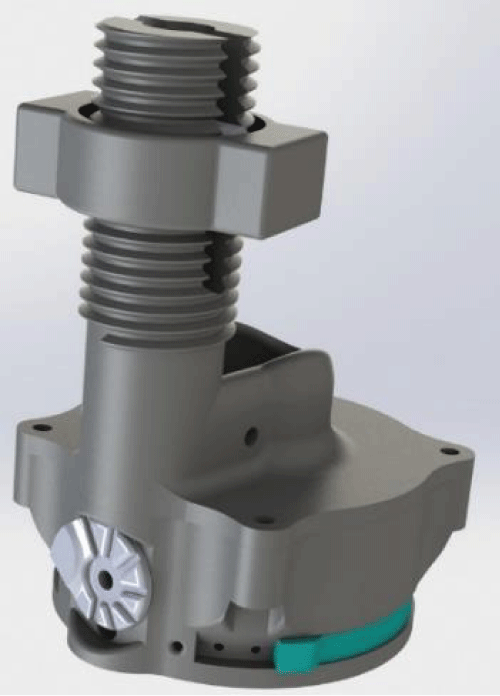
Prototype testing: The CV System has been successfully manufactured and undergone preliminary testing in the OHSU Ventilator Simulation Laboratory. A complete print of the device can be completed in under 3 hours depending on the printing method and assembled with easily obtainable hardware supplies of an extension spring, M3 X 16 fastener, M3 X 12 fastener and a post-production addition of SmothOn Ecoflex silicone for the valve seal. The device has been printed in materials that are biocompatible and have a melting point that allows it to be autoclaved with pressurized saturated steam at 121 °C (250 °F) for up to 30 minutes. We are currently prototyping a second generation design which has completely 3D printed parts minus the silicone seal Figures 1,2.
Methods
Test Lung
The Michigan Instruments’ Training and Test Lung (TTL) is a dynamic lung simulator used to evaluate and demonstrate mechanical ventilation and lung pathologies. The TTL is a unique, versatile instrument that provides simulation of the structures and mechanics of the human pulmonary system. Through the use of elastomer lung compartments, it accurately reflects typical adult and infant residual lung capacity. A simple but comprehensive set of controls provides the user with the ability to adjust the airway resistance and lung compliance of the TTL with ease. These adjustments allow for the simulation of both healthy and diseased lungs.
Gas flow/Ventilator analyzer
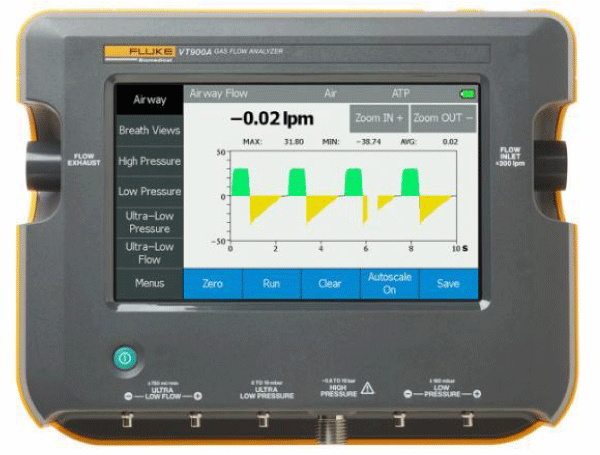
The VT650/VT900A Gas Flow Analyzer is a general purpose gas flow analyzer with special features for testing mechanical patient ventilators. The Analyzer measures bi-directional air flow, high and differential low pressure, barometric pressure, Oxygen concentration and airway pressure, and airway temperature and airway humidity. The Analyzer can be controlled externally using USB commands or automated with available software. The Analyzer operates on a rechargeable Li-Ion battery or external power supply for stationary or portable use Figures 3,4.
Ventilator
The Avea is a fourth generation, servo-controlled, software-driven ventilator from Vyaire Medical. It is a comprehensive ventilation system that has a dynamic range of breathing gas delivery that provides for neonatal through adult patients. This is a FDA approved ventilator used in Intensive Care Units and currently available in the simulation lab.
OHSU 3D crisis ventilator
Flow driven, pressure regulated 3D ventilator which is printed at OHSU with commercially available Fused Deposition Modeling (FDM) 3D printers from Nylon material and assembled with readily available materials as detailed in design section able to be printed and assembled rapidly Figures 5,6.
Two test case scenarios were simulated using the Michigan TTL to represent normal lung function and poor lung function. Normal lung function was assigned a compliance value of 0.1 L/cmH2O and poor lung function was assigned a compliance value of 0.01 L/cmH2O on the Michigan TTL.
The Avea was connected to the TTL in a Volume Assist Control MODE with a set PEEP, Tidal Volume and Respiratory Rate (RR) for both POOR lung and NORMAL lung case scenarios. Pressure, flow and volume curves were observed and recorded with the FLUKE VT900A.
The CRISIS Vent by design is dependent on 3 parameters of inlet flow, peak inspiratory pressure and expiratory resistance which can be set and or adjusted. PEEP values for the Crisis Vent is dependent and determined by the set PIP and flow which both can be manually adjusted. Changes in RR can be made by manual adjustments of the rate valve.
Once the performance curves were recorded, the AVEA was substituted with the CRISIS Vent and adjustments made to match the PEEP, PIP and RR for AVEA performance for both NORMAL and POOR test case scenarios Figures 7,8.
Preliminary results
Rate and tidal volume comparison
Normal lung
TTL Settings: Compliance value of 0.01 L/cmH2O
AVEA Vol AC Setting: PEEP 10 TV 500cc RR 20
Measured PIP: 20 cm H2O Set Peak Flow Rates: 30L /min
CRISIS Vent Performance: PEEP: 10 TV 500-525cc RR 20
Set PIP: 20 cm H20 Set Flow Rate: 30L/min
Poor lung
TTL Settings: Compliance value of 0.1 L/cmH2O
AVEA Volume AC Setting: PEEP 10 TV 300cc RR 20
Measured PIP: 49 cm H2O Set Flow 30L/min
CRISIS Vent Performance: PEEP: 13 TV 250cc RR 24
Set PIP: 49 cm H20 Set Flow Rate: 30L/min
Methodology for CV vent changes
Step 1: Start Flow 30L/min (measures up to 60L/min)
Step 2: Set Rate Valve to middle setting in order to insure cycling
Step 2: Set PIP to desired TV (Max PIP 55 cc H2O)
Step 3: Adjust Rate Valve to desired RR
Step 4: Observe PEEP if PEEP is below desired value decrease Flow Rate to increase PEEP
Step 5: RR may increase and rate valve may need to be adjusted
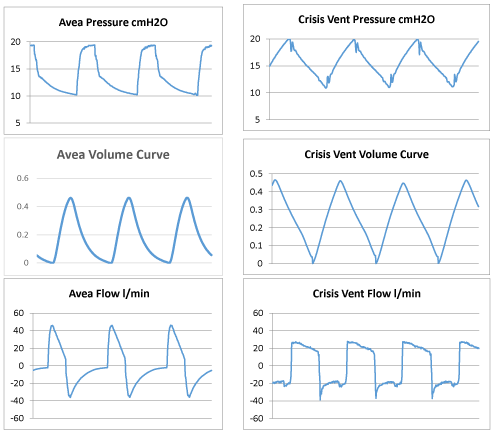
Pressure, volume and flow curves of AVEA (Pressure Control Mode) vs CRISIS Vent
Normal lung
TTL Settings: Compliance value of 0.01 L/cmH2O
Avea Settings – Pressure Control Mode
Crisis vent
Poor lung
TTL Settings: Compliance value of 0.1 L/cmH2O
Avea Settings – Pressure Control Mode
Crisis vent
Medical and technical considerations
1. Our ventilator device diminishes supply chain issues, is rapidly scalable, and low cost. Depending on the printing method and industry collaboration, rapid print times could be mass produced within hours. In this currently pandemic, multiple potential collaborators which the ability for print quality assurance and production capabilities have been identified which include Stratasys, 3D Systems and NIKE. The footprint of these collaborators is represented both nationally and internationally with locations potentially near areas in critical need of ventilators.
2. Our ventilator is capable of numerous ventilatory settings to treat varying degrees of respiratory failure, including basic ventilation and ARDS. See methods for initial preliminary results of AVEA and CV comparisons for NORMAL and POOR lung simulations. CV range limitation test will be performed this week for both NORMAL and POOR and released once available.
3. Our team has built a promising regulatory approval pathway. Regarding our regulatory plan, the FDA issued an EUA on ventilatory devices on March 24, 2020. As a result, our device has the capability of rapid testing, approval, and delivery in a shortened timeframe. We are currently working with with the FDA Center for Devices and Radiological Health to provide information regarding the devices design, labeling, testing, and quality management under which it was developed to determine the devices eligibility for an EUA criteria for safety, performance, and labeling set forth in the recent FDA EUA ventilator guidance documents.
The path forward
We have brought on partners for developing, manufacturing, and distributing our device. All partners have volunteered their manufacturing capabilities and gifted materials for rapid prototyping and manufacturing scalability of our device during the pandemic. The device could be rapidly produced and shipped to hospital systems in need worldwide at no cost. Locally we have the capability to produce up to 100 devices per week. Our partnerships with these large U.S.-based companies have the potential of a worldwide footprint that will allow us to maintain and control the quality processes and regulatory requirements and to keep pace with production needs. Additionally, we plan to leverage their extensive global distribution channels to ensure that we can widely disperse our device to regions in greatest need. This device has the promise of broad global application beyond the COVID pandemic.
Summary
We are currently in the process of FDA Emergency Use Authorization Approval for emergency use during the COVID-19 pandemic with the goal of providing this technology for anyone in need for free.
- Italian Society of Anesthesia, Analgesia, Resuscitation and Intensive Care (Società Italiana di Anestesia Analgesia Rianimazione e Terapia Intensiva [SIAARTI]). Clinical ethics recommendations for the allocation of intensive care treatments, in exceptional, resource-limited circumstances. Link: https://bit.ly/3ccHaPc
- Ventilator stockpiling and availability in the US. Baltimore: Johns Hopkins Bloomberg School of Public Health, Center for Health Security. Link: https://bit.ly/36AxH2Y
- Truog RD, Mitchell C, Daley GQ (2020) The Toughest Triage - Allocating Ventilators in a Pandemic. N Engl J Med Link: https://bit.ly/3dbRCaX
- US Food & Drug Administration (2020). Coronavirus Disease 2019 (COVID-19) Emergency Use Authorizations for Medical Devices: Ventilators and Other Medical Device EUAs. US Food & Drug Administration. Link: https://bit.ly/36zeQWe
- Inglis R, Ayebale E, Schultz, M (2019) Optimizing respiratory management in resource-limited settings. Curr Opin Crit Care 25: 45–53. Link: https://bit.ly/2M1WuDI
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.





 Save to Mendeley
Save to Mendeley
