Journal of Surgery and Surgical Research
Cutaneous syncytial myoepithelioma: A potential pitfall in the differential diagnosis of superficial dermal tumors
Erdem Comut* and Nese Calli Demirkan
Cite this as
Comut E, Demirkan NC (2019) Cutaneous syncytial myoepithelioma: A potential pitfall in the differential diagnosis of superficial dermal tumors. J Surg Surgical Res 5(2): 074-077. DOI: 10.17352/2455-2968.000077In the spectrum of myoepithelial tumors of the skin, cutaneous myoepithelioma is composed solely of myoepithelial cells. Cutaneous syncytial myoepithelioma as a rare histological variant of cutaneous myoepithelioma has been first described in the last decade. This tumor is benign and rarely shows recurrence when incompletely resected. In addition to its distinctive common histological and immunohistochemical features, cutaneous syncytial myoepithelioma shares the same changes for most cases in molecular testing (EWS RNA binding protein 1 gene rearrangement). The differential diagnosis of other superficial dermal tumors is one of the major difficulties in the diagnosis of this tumor. In the current study, we present our findings of a 56 year-old woman who was diagnosed as cutaneous syncytial myoepithelioma.
Abbreviation
CK: Cytokeratins; CM: Cutaneous Myoepithelioma; CSM: Cutaneous Syncytial Myoepithelioma; EBFH: Epithelioid Benign Fibrous Histiocytoma; EMA: Epithelial Membrane Antigen; EWSR: EWS RNA Binding Protein 1; FISH: Fluorescence in Situ Hybridization; JXG: Juvenile Xantogranuloma
Introduction
Myoepithelial cell neoplasms represent a histologically broad spectrum and well defined tumor group, especially for salivary glands [1]. Extra-salivary gland representatives of these neoplasms are rare and include bone, respiratory tract, breast and retroperitoneum [1-4]. CM and soft tissue myepitheliomas have relatively newly been defined [5-7].
CM generally affects male adults (with peak incidence in the 3. and 5. decades, and a male to female ratio of 3:1) and extremities as anatomical localization [1,6-8]. It is composed solely of myoepithelial cells and regarded as a subgroup of cutaneous mixed tumor. These tumors usually present as a solitary, slow growing papule and histologically demonstrate a wide spectrum of features including epithelioid, spindled, histiocytoid or plasmocytoid cells showing solid, lobulated or reticular growth patterns in a myxoid or hyalinized stroma [7,9]. Immunohistochemically, neoplastic cells express EMA (epithelial membrane antigen) and S100 protein. Other markers like cytokeratins (CK), GFAP, SMA and p63 show variable expression [6,7,9]. As a rare histologic variant, CSM has been lately described and to the best of our knowledge, there are only 42 cases reported in the literature [8]. This variant demonstrates tightly packed histiocytoid-spindle cells growing in a sheet like pattern within a limited stroma. These tumors are negative for CK by immunohistochemistry unlike CM and have distinctive molecular characteristics [8]. These tumors are benign such as CM and rarely recur when incompletely resected [7,8]. At this point, we present our findings of a 56 year-old woman represented clinically by a papule on her left thigh and diagnosed as CSM in our department.
In November 2018, a 56 year-old woman with no significant clinical history presented at an external hospital for a papule on her left thigh. Complete excision of this lesion was performed by a plastic surgeon. The case was refered for a second opinion by the primary pathologist with the initial diagnosis of neurofibroma to the Pathology Department of Pamukkale University Hospital.
Results
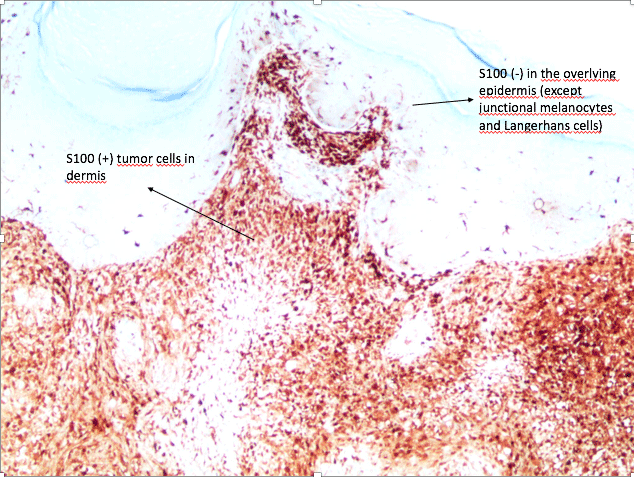
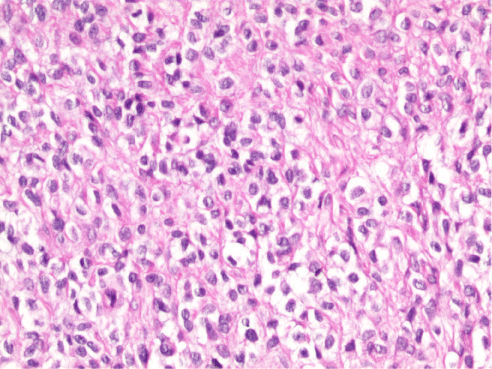
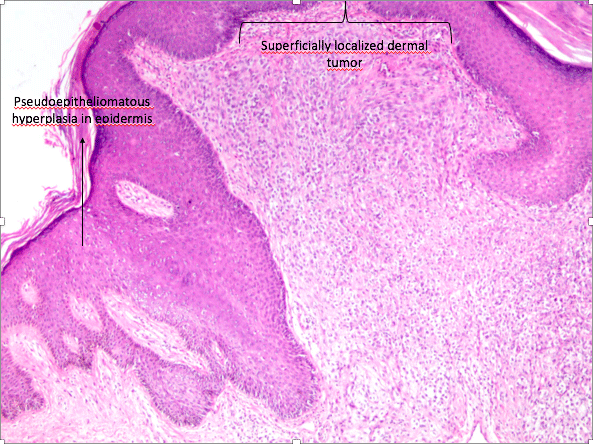
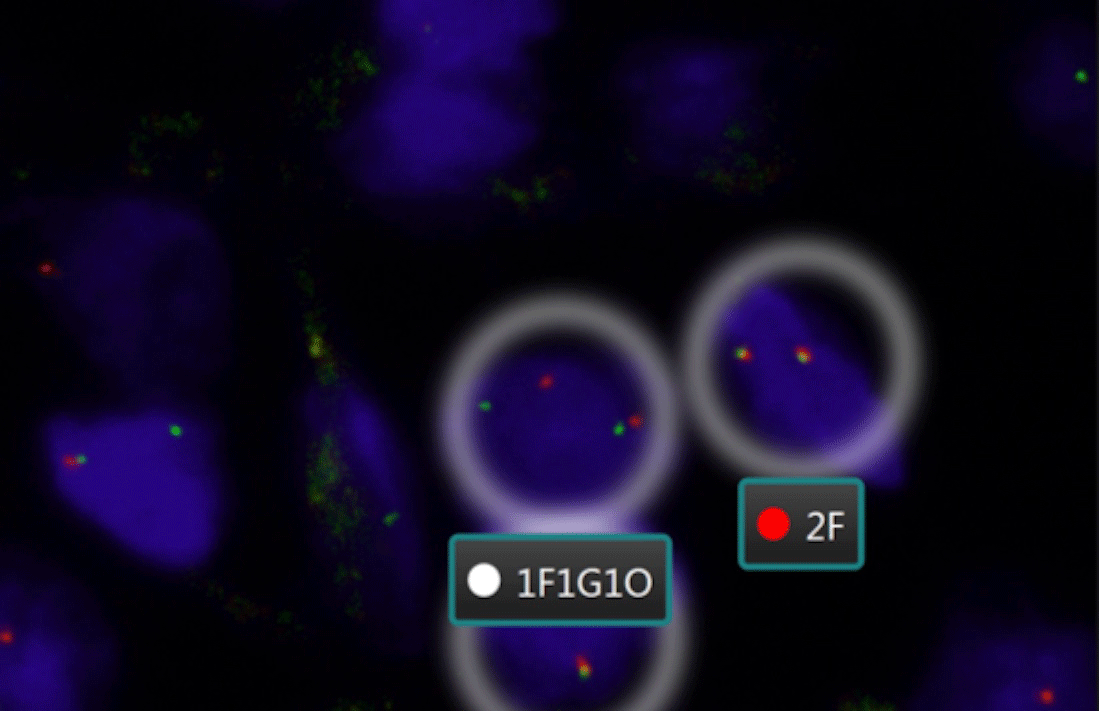
The lesion was described as a solitary, well-circumscribed, 0.6×0.4cm white papule in the primary pathology report. The slides obtained from two paraffin blocks were evaluated. On histopathological examination, hypergranulosis and irregular acanthosis were observed in the epidermis covered with compact keratin layer. The tumor was well-circumscribed, localized in the superficial dermis and the overlying epidermis showed pseudoepitheliomatous hyperplasia (Figure 1). The tumor consisted of ovoid to histiocytoid and spindle shaped cells with indistinct cell borders and focally clear/pale eosinophilic cytoplasm. Nuclei were monomorphic with vesicular chromatin. No significant atypia or necrosis were observed (Figure 2). Surgical margins were intact. Immunohistochemically, the tumor cells were positive for EMA, SMA and Vimentin. S100 protein was also diffusely positive in neoplastic cells (Figure 3). Melanocytic markers (HMB45 and SOX-10), CEA, CD34 and TFE-3 were all negative. Desmin couldn’t be assessed due to the technical problems. Ki-67 proliferation index was 7%. In molecular testing, a FISH (fluorescence in situ hybridization) analysis showed EWS RNA binding protein 1 (EWSR1) gene rearrangement with a break-apart probe with 90.2% positive cells (Figure 4). The final diagnosis was CSM in the current case. Since the surgical margins were clear, no further treatment was recommended.
Discussion
CSM represents a lately described entity and a histologic variant of CM. Fletcher published a case series with 38 individuals describing the clinicopathologic features of CSM in 2013 [8]. 27 men and 11 women were involved in the study with a 2.5:1 ratio. The age range of the patients was between 2 months and 74 years (median, 39 years). In terms of tumor location, there was a predilection for the extremities compared to the other affected sites like back, chest, buttock, nose and cheek. Most of the initially proposed diagnoses by the primary pathologists were epithelioid sarcoma, neurofibroma, perineuroma/sclerosing perineuroma, cellular neurothekoma, granular cell tumor, myofibroma and smooth muscle neoplasms [8]. Median tumor size was 0.8cm (range 0.3cm to 2.7cm). Local reccurence was observed in only one patient with positive surgical margin after local excision [8]. On histopathologic examination, CSM has limited/no stromal component and consists of ovoid to spindled or histiocytic cells with pale eosinophilic cytoplasm arranged in a syncytial fashion [10,11]. Tumors display minimal or no mitotic activity (range 0-4 per 10 high power fields). Necrosis or lymphovascular invasion is not observed in any cases. Adipocytic metaplasia or chondro-osseous differentiation may be seen [8]. All tumors show EMA and S100 positivity. Despite most tumors are negative for cytokeratins (CK), rare tumors can show focal positivity. Variable positive reactivity is seen with myoepithelial markers as GFAP, SMA and p63 [8,10-12].
In the current study, we also reported a CSM case with dermis-located and well-cirumscribed tumor displays a syncytial/sheet-like growth pattern. A wide spectrum of superficial dermal tumors is included in the differential diagnosis of this rare entity.
Epithelioid sarcoma is one of the clinically important mimickers of CSM. The presence of dermal epithelioid cell proliferation and the immunohistochemical expression of EMA protein are common features for both tumors. However, epithelioid sarcoma usually affects distal extremities of young adults and shows granuloma-like pattern of epithelioid cells around areas of necrosis on histopathologic examination. Epithelioid sarcoma shows lack of expression of myoepithelial markers and positive expression of CD34 and CK, whilst CSM is positive for myoepithelial markers and negative for the latter [8,10,11].
Dermal melanocytic neoplasms such as Spitz nevi should be considered in the differential diagnosis of CSM. Although Spitz nevi show dermal proliferation of epithelioid cells with abundant eosinophilic cytoplasm, these cells lack the typical syncytial growth pattern of CSM. Both tumors are S100 (+), whereas CSM lacks of expression of MelanA and HMB45 [10,11].
Epithelioid benign fibrous histiocytoma (EBFH) is a well-circumscribed dermal neoplasm with uniform, medium to large angulated epithelioid cells. The epithelioid cells with indistinctive cell borders and eosinophilic cytoplasm represent the same histological pattern as CSM. Compared to CSM, EBFH has a more hyalinized stroma with marked vascular structures. Different from CSM, binucleated cells may be seen in EBFH. Immunohistochemically, both neoplasms are EMA (+), on the other hand, EBFH lacks reactivity for myoepithelial markers including S100 [8,10].
Juvenile xantogranuloma (JXG) is a neoplasm that usually affects infants and young children. This neoplasm is composed of histiocytic cells and lacks the characteristic multinucleated giant cells especially in the early-stage lesions, so it should be considered in the differential diagnosis of CSM. JXG is negative for EMA and S100 protein, but shows CD68 and CD163 positivity [10,12].
As a histologic variant of neurothekoma, cellular neurothekoma may also mimic CSM due to the composition of this tumor with spindled and epithelioid cells with abundant eosinophilic cytoplasm and indistinctive cell borders. Immunohistochemically, cellular neurothekoma exhibits no positivity for EMA or S100 [11].
EWSR1 gene is located on the long arm of chromosome 22 at position 12.2 [13]. Although its function is not fully understood, EWSR1 protein takes part in cellular processes, cell signaling and RNA transport [14]. Mutations of EWSR1 gene are seen in a wide range of tumors including Ewing sarcoma, desmoplastic small round cell tumor, extraskeletal myxoid chondrosarcoma, clear cell sarcoma of soft tissue, myxoid liposarcoma and soft tissue myoepithelial tumors [15]. Rearrangement of this gene has been detected nearly half of CM cases [16]. Fletcher reported EWSR1 gene rearrangement was found in most CSM cases. Pre-B-Cell Leukemia Homeobox 3 (PBX3) has been determined as a fusion partner of EWSR1 in one study [17].
In conclusion, CSM is a lately described entity and represents a diagnostic challenge in terms of superficial dermal tumors. Because of its rarity and diagnostic difficulties, most CSM cases are referred to experienced pathologists with various initial diagnoses. Since EWSR1 gene rearrangement is seen in most cases, molecular testing can help to confirm the diagnosis of this tumor in addition to some prominent histopathological and immunohistochemical features. CSM rarely shows local recurrence when incompletely resected, otherwise it presents as a benign tumor.
- Kutzner H, Mentzel T, Kaddu S, Soares LM, Sangueza OP, et al. (2001) Cutaneous myoepithelioma: An under-recognized cutaneous neoplasm composed of myoepithelial cells. Am J Surg Pathol 25: 348-355. Link: http://bit.ly/2kfiQHA
- Bigotti G, Giorgio CG Di (1986) Myoepithelioma of the breast: Histologic, immunologic, and electromicroscopic appearance. J Surg Oncol 32: 58-64. Link: http://bit.ly/2mgj6H5
- Chand M, Mann JM, Sabayev V, Luo JJ, Cohen PR, et al. (2011) Endotracheal myoepithelioma. Chest 140: 242-244. Link: http://bit.ly/2lLK3SO
- Song W, Flucke U, Suurmeijer AJH (2017) Myoepithelial Tumors of Bone. Surg Pathol Clin 10: 657-674. Link: http://bit.ly/2ky3j62
- Kilpatrick SE, Hitchcock MG, Kraus MD, Calonje E, Fletcher CDM (1997) Mixed tumors and myoepitheliomas of soft tissue: A clinicopathologic study of 19 cases with a unifying concept. Am J Surg Pathol 21: 13-22. Link: http://bit.ly/2lLr2zV
- Michal M, Miettinen M (1999) Myoepitheliomas of the skin and soft tissues. Report of 12 cases. Virchows Arch 434: 393-400. Link: http://bit.ly/2kvfH6N
- Hornick JL, Fletcher CDM (2004) Cutaneous Myoepithelioma: A Clinicopathologic and Immunohistochemical Study of 14 Cases. Hum Pathol 35: 14-24. Link: http://bit.ly/2kirBkg
- Jo VY, Antonescu CR, Zhang L, Cin PD, Hornick JL, et al. (2013) Cutaneous syncytial myoepithelioma: Clinicopathologic characterization in a series of 38 cases. Am J Surg Pathol 37: 710-718. Link: http://bit.ly/2lOf8oK
- Mentzel T, Requena L, Kaddu S, Soares De Aleida LM, et al. (2003) Cutaneous myoepithelial neoplasms: Clinicopathologic and immunohistochemical study of 20 cases suggesting a continuous spectrum ranging from benign mixed tumor of the skin to cutaneous myoepithelioma and myoepithelial carcinoma. J Cutan Pathol 30: 294-302. Link: http://bit.ly/2kHtlnh
- Lee JH, Huang HY, Lan J, Hwang CC, Liu CY (2015) Cutaneous syncytial myoepithelioma: A case report with emphasis on the differential diagnosis of problematic dermal tumors. Oncol Lett 9: 2275-2277. Link: http://bit.ly/2kd8NTm
- Alomari AK, Brown N, Andea AA, Betz BL, Patel RM (2017) Cutaneous syncytial myoepithelioma: A recently described neoplasm which may mimic nevoid melanoma and epithelioid sarcoma. J Cutan Pathol 44: 892-897. Link: http://bit.ly/2kxBpXT
- Pizzi M, Facchin F, Kohlscheen E, Sartore L, Salmaso R, et al. (2016) Cutaneous syncytial myoepithelioma: Clinico-pathological features and differential diagnosis. Pathol Res Pract 212: 954-956. Link: http://bit.ly/2kiqYao
- Romeo S, Dei Tos AP (2010) Soft tissue tumors associated with EWSR1 translocation. Virchows Archi 456: 219-234. Link: http://bit.ly/2kIQzJM
- Lee J, Nguyen PT, Shim HS, Hyeon SJ, Im H, et al. (2018) EWSR1, a multifunctional protein, regulates cellular function and aging via genetic and epigenetic pathways. Biochim Biophys Acta Mol Basis Dis 1865: 1938-1945. Link: http://bit.ly/2lS7qd8
- Thway K, Fisher C (2019) Mesenchymal Tumors with EWSR1 Gene Rearrangements. Surg Pathol Clin 12: 165-1690. Link: http://bit.ly/2lOeHe6
- Flucke U, Palmedo G, Blankenhorn N, Slootweg PJ, Kutzner H, et al. (2011) EWSR1 gene rearrangement occurs in a subset of cutaneous myoepithelial tumors: A study of 18 cases. Mod Pathol 24: 1444-1450. Link: http://bit.ly/2mgaBvL
- MacKinnon WF, Carter MD, Bridge JA, Tremaine RD, Walsh NM (2019) EWSR1-PBX3 Gene Fusion in Cutaneous Syncytial Myoepithelioma . J Cutan Pathol 46: 421-424. Link: http://bit.ly/2lOevLU
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
