Journal of Novel Physiotherapy and Physical Rehabilitation
Walk to the Beat: A Case Report of the Use of a Novel Haptic Device to Improve Walking after Stroke
Rachel C Stockley1*, Glenis Donaldson1, Theo Georgiou2, Simon Holland2, Janet Van der Linden2, Josie Tetley1, Linda Garbutt1 and Ornella Pinzone1
2Open University, Centre for Research in Computing, Milton Keynes, UK
Cite this as
Stockley RC, Donaldson G, Georgiou T, Holland S, der Linden JV, et al.(2017) Walk to the Beat: A Case Report of the Use of a Novel Haptic Device to Improve Walking after Stroke. J Nov Physiother Phys Rehabil 4(2): 056-059. DOI: 10.17352/2455-5487.000047Background: Stroke affects 15 million people worldwide every year and leaves two-thirds of survivors with significant mobility deficits including reduced walking speed, increased unevenness of step length and asymmetry. Haptic cues, which utilise sensory stimulation and so are unaffected by visual or auditory interference could discreetly improve the gait of people after stroke. Therefore, the objective of this single mixed methods case study was to evaluate the use of a novel haptic device in a single participant after stroke.
Context and purpose: After initial familiarisation, gait symmetry, walking speed and cadence of a 69 year old male stroke survivor were recorded using a Qualisys Motion Capture system whilst he walked on a ten metre walkway, firstly without and then with a haptic device on each leg, which provided a metronomic rhythmical vibratory cue. The participant then provided a user evaluation of the devices using a semi structured interview.
Results: The haptic device was evaluated positively by the participant although he noted it needed to be refined to increase its wear-ability and acceptability for everyday use. Whilst gait speed and cadence remained unaltered, there was a 14% improvement in temporal gait symmetry when wearing the haptic device, suggesting it improved this aspect of gait.
Conclusion: Whilst limited by its design, the findings of this single case study indicate that the haptic device could be a novel technology-based therapeutic adjunct to improve gait symmetry after stroke. It also provides key understanding of user needs which can be used to guide the development of a new prototype device for stroke survivors.
Brief summary: Many stroke survivors have residual mobility problems. Haptic cueing may improve walking by providing a tactile cue that the participant follows to improve symmetry. This single case study suggests that gait symmetry could be improved by haptic cueing and indicates factors affecting the wear ability of such a device.
Abbreviations
GPS: Gait Profile Score; RC: Rhythmic Cue
Background
Each year, over 15 million people have a stroke and it is second largest cause of death worldwide [1,2]. In many countries, the majority of stroke survivors will be older people and the number of stroke survivors over the age of 75 has increased by over 113% just in the last two decades [3]. Age is the single most important risk factor for stroke and the incidence of stroke doubles with each decade over 55 years [2]. 1 in 5 women and 1 in 6 men will have had a stroke by the age of 75 [4], and in high income countries, 50% of strokes occurred in people over the age of 75 years [3]. Many stroke survivors are left with marked mobility deficits including reduced balance and significant gait asymmetry [5]. Consequently many older people who have had a stroke report reduced community mobility, which can lead to low activity levels, social isolation, a loss of sense of self and can precipitate a range of health problems [5-8], including an increased risk of falls [9]. In turn, these can reduce the ability of older individuals to remain independent in the community, increasing care burden and reducing their quality of life.
Rehabilitation of gait after stroke often includes forms of rhythmic cueing which harnesses entrainment processes in the central nervous system [10]. Entrainment is the mechanism by which an individual can follow and reproduce rhythmical stimuli, for example, tapping along to a beat [11]. Rhythmic auditory cueing has been shown to be effective in improving walking symmetry after stroke, although its effectiveness is limited when there is environmental noise [12,13]. Haptics, in which a sensory stimulus is provided by touch rather than sound, have been used in people to compensate for sensory deficits and have been recognized to provide a useful training cue in groups with minimal sensory impairments [14]. Haptic cueing, in which a rhythmic cue (RC) is provided by vibrotactiles worn next to the skin, is unaffected by environmental noise but is likely to utilize entrainment processes and so could provide an effective alternative cueing device for community ambulation. If this device can be developed to be acceptable to people after stroke, it may provide a technology-based solution to improve mobility and wider quality of life, particularly for older people after stroke. Therefore, this single case study aimed to evaluate a novel, prototype haptic device that deliver RC in a participant after stroke.
Materials and Methods
A repeated measure design was utilized. The participant (D) was a right handed, 69 year old male who had sustained a single unilateral, haemorrhagic stroke 43 years ago. He was able to walk for at least 20 metres and for 5 minutes without the use of a walking aid but reported persistent problems with balance, mild sensory loss and an uneven walking pattern. He was independent in all activities of daily living (ADL), scored 53 from 56 on the Berg Balance scale and 14 from 15 on the Rivermead Mobility Index (he could not run) [15,16].
D attended for testing over two days after giving informed consent. His gait was evaluated using the Qualisys Motion Capture system, comprising eight optoelectronic cameras, sampling at a frequency of 100 Hz. The trajectories of 20 markers placed on anatomical lower limb landmarks, and 4 additional tracking clusters placed on the right and left shank and thigh, using a modified Calibrated Anatomical Systems Technique model (CAST), were collected and smoothed using a fourth order zero lag Butterworth low-pass filters, with a 6 Hz cut off frequency [17].
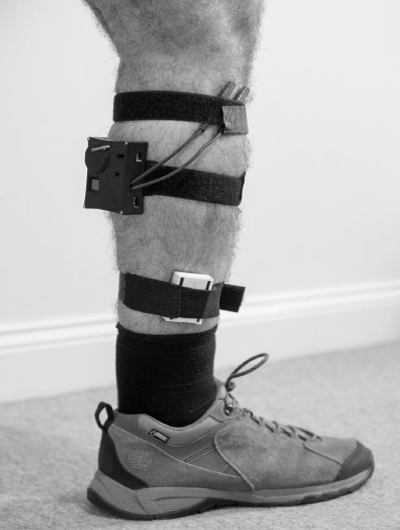
Each haptic device contained a computer, Wi-Fi chip, accelerometer and powerful low-latency vibrotactiles. These devices, designed by The Open University, produce a stimulus similar to a mobile phone’s vibration setting and can be programmed to provide a RC based upon an individual’s usual walking speed [11], the haptic device was fitted to both lower limbs (Figure 1). The location of the haptic device was chosen based upon the patient preference and to avoid blocking the movement analysis markers.
The participant completed a minimum of five walks on a ten metre walkway whilst wearing the devices, but with no haptic stimulation (familiarisation). He was instructed to walk at his own pace.
On the second testing visit, kinematic data was recorded again, firstly with the haptic devices switched off (test 1) and then with them turned on (test 2).
The haptic rhythms ‘cues’, transmitted by a host computer, were calculated from the participants step cycle at their usual walking pace from the first assessment using:
This indicated that a stimulus would be delivered every 505 milliseconds on alternating legs. The intensity of the haptic devices were increased so that the participant could feel the vibration but it was not too strong to be uncomfortable. After additional familiarisation (either walking on the spot or sitting and marking time to the rhythm) and a short rest, the participant was asked to ‘follow the rhythm’ as he performed a minimum of five walks whilst motion data were captured.
The participant was then interviewed by one researcher (JT) and asked about wearing the haptic device, the sensation of the device, any effects, and whether he felt that it could be useful for people after a stroke.
Ethical approval for the study was gained from MMU ethics committee (Reference Number: 1263).
Analysis
Visual 3D software(C-Motion inc, Germantown, USA) was used for processing the data. Overall temporal asymmetry was calculated using: [18].
Normative values are between 0.9-1.1; a score of greater than 1 indicates a tendency to rely on the non-paretic limb [18].
Spatial symmetry was calculated using: [18].
Results
During the non-haptic condition (test 1), D demonstrated marked temporal asymmetry (Table 1) with over use of the non-paretic leg during gait. When wearing the haptic device (test 2), there was a 14% decrease in asymmetry with an associated small decrease in the GPS, as shown in Table 1. There were no changes in spatial symmetry, velocity or cadence between tests 1 and 2.
User evaluation
On interview, DH stated that he found the device to be “very good for the brief time it was operating”. He felt that it worked as it: “acts in its own way as a bit of a reminder to get or to help me with rhythm”, and it “helped with the rhythm, you know of walking, rather than anything else really”.
Whilst finding the devices comfortable to wear, he did note some limitations, highlighting their size as a barrier to use in the long term: “…it’s obviously a prototype so there is a lot of refinement to be done with regards to size….. you don’t want to feel that someone will stare at you because you’ve got these things on”. Similarly, the investigators noted that battery life and reliable wireless capability required development in order to increase the portability of the device.
Discussion
This was a single case study evaluating the use of novel haptic devices that delivered RC on the gait of an older stroke survivor who had a stroke over 40 years ago. Whilst the findings of this study are limited by its design, they provide indications of the acceptability of these novel devices, their potential effects and highlight areas for development.
The participant demonstrated problems with his walking despite being several decades after his stroke. The findings that his walking speed was 86% of ‘normal’ [18], and he was unable to run suggests that D was typical of many people who do not regain their previous level of mobility after stroke. He had also developed several compensatory strategies when walking by overusing his non-paretic limb so that he demonstrated notable gait asymmetry [18], which is common in many older people [21]. Whilst these compensations do allow continued functioning at some level, they can also predispose individuals to reductions in bone mineral density on the paretic side, the potential for overuse of the non-paretic limb and increases the risk of falls [9,22]. These problems can necessitate hospital admission and reduce the ability to live and function in the community.
Whilst wearing the haptic device seemed to have no effect upon spatial symmetry, cadence or gait velocity, D exhibited improvements in the temporal symmetry of gait after only a brief familiarisation period, despite being several decades after his stroke. As the cueing frequency of the haptic devices was set at the frequency of D’s usual walking pace, it is not surprising that they had little effect upon his cadence and gait velocity. However, the changes seen in temporal symmetry after only a brief familiarisation period indicates that the RC provided by the haptic devices could improve walking efficiency and quality whilst they are being worn.
As others have shown that gait asymmetry can contribute to an increased risk of falls, these findings could indicate that the use of haptic devices on an everyday basis and in the community may potentially reduce the risk of falls with associated benefits to the individual and cost savings for healthcare services [9]. Furthermore, the haptic devices could also have a role in improving the walking of older people who have not had a stroke but who commonly demonstrate reduced gait symmetry [21].
Based on the participant’s feedback, the rhythmical cues provided by the haptic devices were well tolerated. In the device used in the current study, the intensity of the cue could be altered so that it was effective but comfortable for the participant which was a useful feature.
The use of RC utilises entrainment models in which rhythmic vibrations synchronize motor responses into stable time relationships [23]. Entrainment is the distinctly human ability to follow or reproduce a rhythm and differs from stimulus response in that it does not exhibit any delay [24]. The ability to follow a beat involves activity in supplementary motor and premotor areas, the basal ganglia and cerebellum [25]. In healthy adults, entrainment to RC has been demonstrated after only a few repetitions of a rhythm [24]. However, this ability has not been fully investigated in older people nor in people who have sustained damage to their brain, for example, after stroke. A novel finding of this study is that the participant appeared to achieve entrainment in a similar time period to healthy people. This finding is confirmed by the responses to cueing seen in other patient groups [14], and supports the development and future evaluation of haptic devices for people with gait deficits after stroke. Based on the use evaluation provided in this study, key refinements should include reduction in the size of the device so that it can be worn more discreetly and greater portability to facilitate its use in the community.
Conclusion
This study is the first to evaluate the effects of haptic devices on the symmetry of gait in a person after stroke with consideration of the views of a user of the device. Whilst the results of the study are limited by the single case design, the key findings suggest that the haptic device could alter the temporal symmetry of walking after only a brief familiarisation period and so, if appropriately developed, could provide a method of improving walking in stroke survivors at home.
The current prototype is limited as it is not able to be used for longer periods of time in the community nor is it easy to don and doff. Further refinement of the haptic device, based on the user’s evaluation participant’s feedback from this study, is now required to improve its portability and acceptability to potential users. Once the prototype is further developed, larger scale trials are necessary to evaluate their effectiveness and efficacy, in both the short and long term, for people after stroke. Future research could also focus upon using the device as a training adjunct to physical therapy to maintain improvements between treatment sessions [14].
This study was funded by the Research Institute for Health and Social Care, Manchester Metropolitan University. The researchers would like to thank the participant who gave his time to participate in this study.
Consent for publication
The participant gave his consent for his data to be published.
- MacKay J, Menash G (2004) The Atlas of Heart Disease and Stroke. Link: https://goo.gl/eZtZu3
- Association S (2005) State of the Nation - Stroke Statistics.
- Feigin VL, Mohammad HF, Rita K, George AM, Myles C, et al. (2014) Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010 Lancet 383: 245-254. Link: https://goo.gl/3HTzF6
- Seshadri, S, Philip AW (2006) The lifetime risk of stroke: estimates from the Framingham Study. Stroke 37: 345-350. Link: https://goo.gl/FWOXNa
- Bohannon, Richard W, Morton, Melissa G, Wikholm, et al. (1991) Importance of four variables of walking to patients with stroke. Int J Rehabil Res 14: 246-250. Link: https://goo.gl/UYyIBr
- Hammarlund CS, Andersson K, Andersson M, Nilsson MH, Hagell P (2014) The significance of walking from the perspective of people with Parkinson's disease. J Parkinsons Dis 4: 657-663. Link: https://goo.gl/Vlyv4A
- Milene SF, Therezinha RC, Carolina NF, Ayrton RM (2015) Non-motor Factors Associated with the Attainment of Community Ambulation after Stroke. Clin Med Res 13: 58-64. Link: https://goo.gl/qyyTT8
- Jones D, GradDip BA, Rochester L, Birleson A, Hetherington V, et al. (2008) Everyday walking with Parkinson's disease: understanding personal challenges and strategies. Disabil Rehabil 30: 1213-1221. Link: https://goo.gl/MpHMf5
- Myint, PK, Poole KE, Warburton EA (2007) Hip fractures after stroke and their prevention. QJM 100: 539-545. Link: https://goo.gl/m2yM33
- States RA, Salem Y, Pappas E (2009) Overground gait training for individuals with chronic stroke: a Cochrane systematic review. J Neurol Phys Ther 33: 179-186. Link: https://goo.gl/xyGYQ7
- Georgiou T, Simon H, Janet van DL (2015) A blended user-centred design study for wearable haptic gait rehabilitation following hemiplegic stroke, in 9th International Conference on Pervasive Computing Technologies for Healthcare. Link: https://goo.gl/EXCZcW
- Giancarlo C, Luisa R, Antony S, Hugh W, Martin B, et al. (2002) A randomised controlled trial of intravenous immunoglobulin in IgM paraprotein associated demyelinating neuropathy. J.Neurol 249: 1370-1377. Link: https://goo.gl/fcyPZ2
- Lucas RN, Camila Quel DO, Louise A, Stella MM, Luci F TS, et al. (2015) Walking training with cueing of cadence improves walking speed and stride length after stroke more than walking training alone: a systematic review. J Physiother 61: 10-15. Link: https://goo.gl/aHN2w0
- Shull PB, Damian DD (2015) haptic wearables as sensory replacement, sensory augmentation and trainer - a review. J Neuroeng Rehabil. Link: https://goo.gl/Bk7aRd
- Rossier P, Wade DT, (2004) Validity and reliability comparison of 4 mobility measures in patients presenting with neurologic impairment. Arch Phys Med Rehabil 82: 9-13. Link: https://goo.gl/TgE2ai
- Blum L, Korner NB (2008) Usefulness of the Berg Balance Scale in stroke rehabilitation: a systematic review. Phys Ther 88: 559-566. Link: https://goo.gl/A1skqv
- Bing Yu, David G, Larry N, Kai Nan A, (1999) Estimate of the Optimum Cutoff Frequency for the Butterworth Low-Pass Digital Filter Journal of Applied Biomechanics 15: 318-329. Link: https://goo.gl/kn0sAS
- Kara KP, Iwona P, Cynthia JD, Valerie C, Mary CV, et al. (2008) Gait asymmetry in community-ambulating stroke survivors. Arch Phys Med Rehabil 89: 304-310. Link: https://goo.gl/CTJhwG
- Richard B, Jennifer LM, Mike S, Pam T, Jill R, et al. (2012) The minimal clinically important difference for the Gait Profile Score. Gait Posture 35: 612-615. Link: https://goo.gl/vbN3NP
- Richard B, Jennifer LM, Michael HS, Sarah B, Adam R, et al. (2009) The gait profile score and movement analysis profile. Gait Posture 30: 265-269. Link: https://goo.gl/TWxnIk
- Atefeh A, Mokhtar A, Mahmood B, Stephen WH, Reza F (2016) The effect of aging on gait parameters in able-bodied older subjects: a literature review. Aging Clin Exp Res 28: 393-405. Link: https://goo.gl/9QOuEc
- Beaupre GS, Lew HL (2006) Bone-density changes after stroke. Am J Phys Med Rehabil 85: 464-472. Link: https://goo.gl/76QQS2
- Ervin S, Yingying Fu, Alison P, Jillian AF, Tom C (2012) The effects of rhythmic sensory cues on the temporal dynamics of human gait. PLoS One. Link: https://goo.gl/YmcXTp
- Thaut, MH, (2003) Neural basis of rhythmic timing networks in the human brain. Ann N Y Acad Sci 999: 364-373. Link: https://goo.gl/jnA4vy
- Schaefer RS (2014) Auditory rhythmic cueing in movement rehabilitation: findings and possible mechanisms. Philos Trans R Soc Lond B Biol Sci. Link: https://goo.gl/nEhBmV
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
