Journal of Novel Physiotherapy and Physical Rehabilitation
Effects of Motor-level Electrical Stimulations on Postprandial Glucose Levels in Non-Diabetic Young Individuals
Han-Hung Huang1*, Shelly D Weise1, Man-Soo Ko2, Trevor Hansen1, Annika Johnson1 and Charity McCluskey1
2Department of Physical Therapy, University of Texas Medical Branch, Galveston, TX, USA
Cite this as
Huang HH, Weise SD, Ko MS, Hansen T, Johnson A, et al. (2017) Effects of Motor-level Electrical Stimulations on Postprandial Glucose Levels in Non-Diabetic Young Individuals. J Nov Physiother Phys Rehabil 4(1): 027-032. DOI: 10.17352/2455-5487.000042Background and objectives: Motor-level electrical stimulation (MES) has been shown to improve glucose tolerance and glucose uptake in both animals and humans. Recently, MES has been shown to improve the blood glucose control in people with Type 2 Diabetes (T2D). There are several types of MES applied in physical therapy clinics. However, it is unknown what types of MES optimally decrease postprandial glucose level. The purpose of this study was to compare the effects of three different types of MES on postprandial glucose levels in healthy non-diabetic subjects.
Methods: Twenty-eight subjects were randomly assigned to four groups: MES 1, MES 2, MES 3 and the control group. All subjects participated in an overnight fast of at least 8 hours and had their fasting blood glucose measured. Subjects were given a glucose supplement to drink within 10 minutes, rested in supine for 30 minutes then the second glucose level was measured. Subjects received a 30-minute MES treatment (except for the control group) followed by the third blood glucose level test. Subjects then rested an additional 30 minutes followed by obtaining the final blood glucose measurement. VO2 levels were monitored every 30 seconds, and heart rate was monitored every 3 minutes throughout the 90 minute study.
Results: There were no significant differences between groups on glucose levels and heart rate throughout the study. The MES 2, Russian Current, demonstrated a statistically significant increase of 10% in VO2 toward the end of treatment.
Conclusions: In this preliminary study, MES seems to have no effects on lowering postprandial glucose levels in healthy non-diabetic subjects. However, Russian Current may have a potential for optimally simulating physical activity. Future research is required with a more extended sampling method, a larger sample size, more intensive MES experimental protocols and a continuous glucose monitoring technique.
Abbreviations
MES: Motor-level Electrical Stimulation; T2D: Type 2 Diabetes; VO2: Oxygen Consumption
Introduction
Motor-level electrical stimulation (MES) is one of the modalities used in physical therapy clinics for different strengthening and rehabilitation purposes. By applying MES, the electrical current induces involuntary contraction of skeletal muscle simultaneously. MES has been suggested to mimic voluntary exercise in patients with neuromuscular disorders. The benefits include increasing heart rate, blood pressure, oxygen uptake, ventilatory capacity, muscle bulk, muscle oxidative processes, and muscle glycogen depletion [1-4]. More specifically, MES improves glucose tolerance and glucose uptake in both animals [5-8] and humans [9-13]. Recently, it has been reported that a 30-minute single bout of MES significantly attenuated postprandial glucose levels in middle-aged men with metabolic syndrome [14] and patients with Type 2 Diabetes (T2D) [15, 16]. One recent study also suggests that a 10-week MES treatment (twice weekly for 20 minutes) can improve glucose metabolism and functional performance in patients with T2D [17]. In summary, all these findings indicate that MES might emerge as a novel intervention to simulate exercise for controlling glucose levels.
Several types of MES are available in the clinics based on different parameter settings. For example, pulsed biphasic waveform, Russian Current, and low-rate transcutaneous electric nerve stimulation (TENS) are the three of the most common MES that simultaneously produce skeletal muscle contractions or twitches. The pulsed biphasic waveform has been recommended for different therapeutic purposes including muscle strengthening, muscle reeducation, muscle spasm reduction and edema reduction [18]. The Russian protocol was originally developed for muscle strengthening of Russian Olympic athletes [19]. The low-rate (acupuncture-like) TENS is typically applied for pain modulation by repetitive stimulation of motor nerves to produce muscle contractions or twitches, or of nociceptive A-delta nerves to produce brief sharp pain, to stimulate endogenous opioid production and release [18]. However, it is unclear which MES parameter settings best mimic exercise for controlling glucose levels.
In this study, we compared the postprandial glucose levels, heart rates and oxygen consumption (VO2) by applying three types of MES as described above on healthy non-diabetic subjects. The subjects were randomly assigned into four groups: MES 1 (pulsed biphasic waveform), MES 2 (Russian Current), MES 3 (low-rate TENS) and the control group. We hypothesized that the subjects who received a 30-minute single bout of MES would have a better outcome in decreasing postprandial glucose levels when compared to the controls. The objective was to determine the optimal type of MES to simulate an effective exercise for postprandial skeletal muscle glucose uptake.
Methods
Subjects
Twenty-eight healthy non-diabetic subjects were recruited from Angelo State University in San Angelo, Texas. After completing the informed consent, each subject filled out a health intake form. The research investigators reviewed the forms to screen general health conditions on each subject. The inclusion criteria were: currently healthy, able to follow verbal commands or instructions, able to read and fill out a subject intake form, able to fast for 8 hours, and weigh more than 94 lbs. The exclusion criteria were: diabetes diagnosis, infectious diseases, currently taking any blood thinning medications, presence of a blood clotting disorder, fear/aversion of finger pricked, fear/aversion of electrical stimulation, and presence of cardiac pacemaker, pregnancy, venous/arterial thrombosis, cardiac disease, malignant tumor, impaired mentation, and impaired sensation. The 28 subjects were randomly assigned by drawing a concealed envelope into four groups: MES 1, MES 2, MES 3 and the control group. The physical characteristics of the subjects in each group were summarized in Table 1. No significant differences were found among subjects except for height. The study protocol was approved by the IRB of Angelo State University (HUA-040115).
Postprandial glucose levels measurement
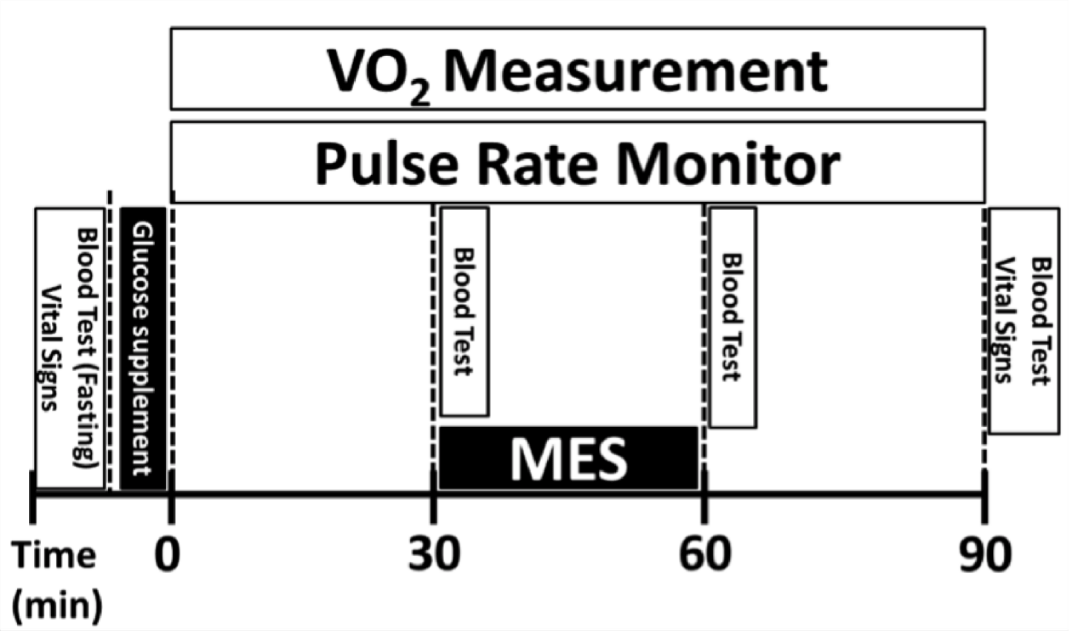
The entire procedure of this study was summarized in Figure 1. Briefly, each subject arrived at the Human Performance Lab in the Department of Physical Therapy at Angelo State University the morning after having completed an overnight fast for 8 hours. They verbally confirmed criteria guidelines, signed consent forms and completed the health intake form. The subjects had their vital signs (heart rate and blood pressure; Carescape V100 Monitor, GE Healthcare) and their first fasting blood glucose level measured by the equipment manufacturer’s directions (Glucose meter: ACCU-CHEK® Nano; Glucose test strips: ACCU-CHEK® SmartView; Lancets: ACCU-CHEK® Softclix). After measuring the baseline glucose level, a glucose supplement, Trutol™ 75g (Thermo Fisher Scientific, Inc.) was given to each subject to drink within 10 minutes. After the allotted drinking period, the subjects were positioned supine on a treatment table. Thirty minutes after finishing the supplement, the second blood glucose level was measured (as the first postprandial glucose level) followed by the onset of electrical stimulation. The subjects in the control group received the identical MES setup, but they did not receive any output of electrical currents. After the 30-minute MES treatment, the subjects had their third blood glucose level measured (as the second postprandial glucose level). The subjects remained in a supine position and rested for an additional 30 minutes on the treatment table. Following the final rest period, the subjects had their final blood glucose level measured (as the third postprandial glucose level). Finally, the subjects were dismissed after checking their vital signs.
Electrical stimulation protocol
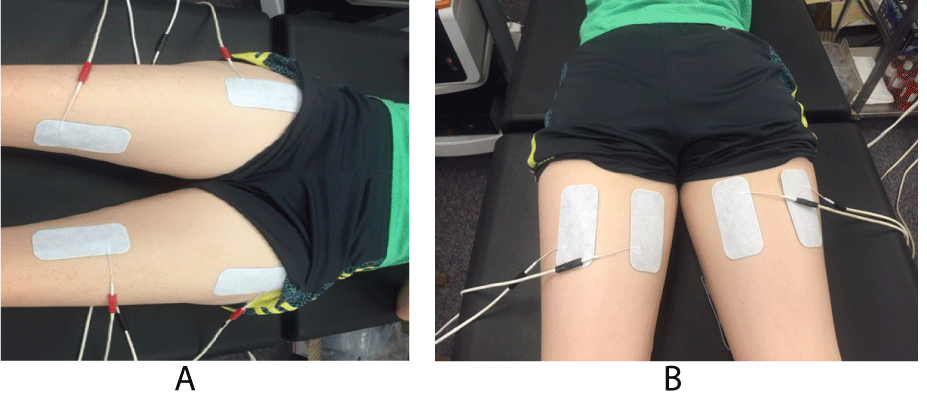
Thirty minutes after drinking the glucose supplement, electrical stimulation (Dynatron Solaris® 709) was applied to the subjects’ thighs for 30 minutes, excluding the control group. The control group had the identical electrode setup as the treatment groups, but they did not receive electrical currents. Four pairs of 2 in x 4 in rectangular electrodes (Dynatronics™, 7B0284-CS Ultra Polys™) were applied to each subject’s bilateral thighs (Figure 2). The target muscles on the thighs were the bilateral quadriceps and bilateral hamstrings. The electrode placement for the quadriceps was: proximal electrode- 15cm distal from ASIS and lateral to sartorius; distal electrode- medial to sartorius and 10 cm proximal from femoral epicondyle. The electrode placement for the hamstrings was: medial and lateral hamstrings; 5 cm distal to ischial tuberosity; 5 cm between the two electrodes. Alternating current (AC) was selected as the electrical current waveform in all treatment groups. The parameters including pulse duration and frequency/rate for each group were summarized in Table 2. The on: off time ratio in all 3 MES treatment groups was 3 second on and 3 second off to reduce muscle fatigue during the treatment. All these aforementioned parameters were selected based upon average values and with an effort to ensure significantly different parameters from one setting to another [18]. The 30-minute time period was chosen based on the literature [15, 16]. MES was administered for 30 minutes at the participant’s tolerance level. The current intensity was adjusted every 10 minutes to the maximal level without discomfort for each subject.
Oxygen consumption measurement and heart rate monitor
The entirety of this study was completed in a noise-controlled, comfortable, and low light environment. Maximal relaxation was targeted in order to obtain the most accurate measurement of resting oxygen consumption. Subjects were instructed to relax with limited movement and prohibited from other activities such as reading, talking, listening to music or other stimulating/engaging tasks. The oxygen consumption and heart rate were measured throughout the study for 90 minutes (Figure 3). The VO2 was calculated every 30 seconds and securely stored using COSMED Quark CPET software. Heart rate was monitored by a fingertip pulse oximeter (Nonin Onyx 9590 Oximeter) and recorded every 3 minutes.
Statistical analysis
Differences in the time-course changes of the lab parameters were analyzed by two-way repeated measure ANOVA. If the interaction between groups and time was significant, one-way ANOVA with Fisher’s Least Significant Difference (LSD) post hoc test was used to assess differences between groups at each time point. P values of less than 0.05 were considered to be statistically significant.
Results
Glucose Levels
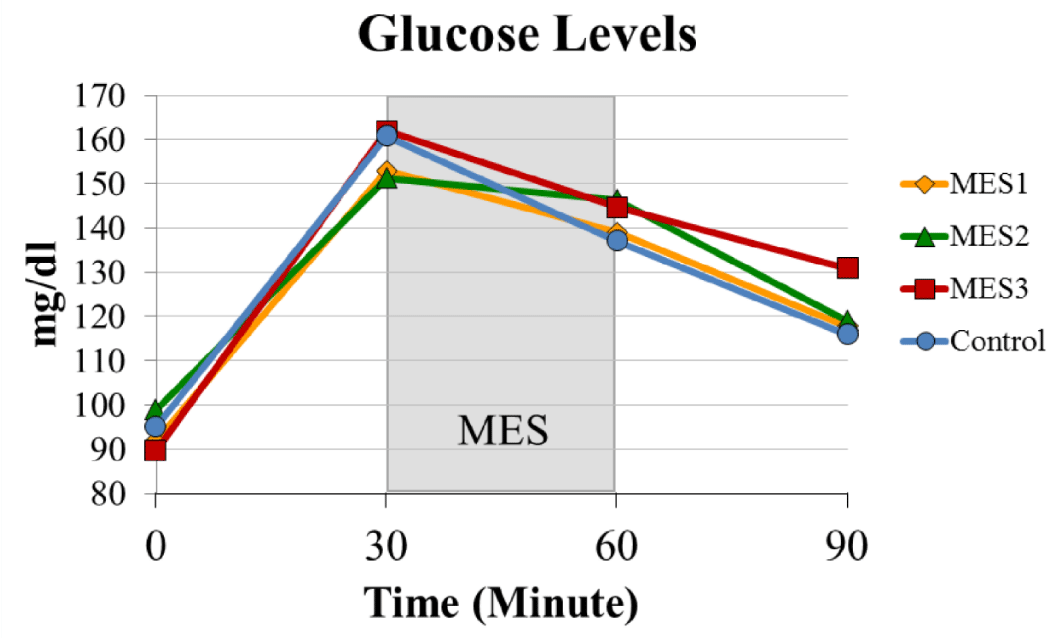
All three treatment groups showed a similar trend in the changes of glucose levels to the controls (Figure 4). There was no statistically significant difference between groups. The fasting blood glucose levels were between 89-99 mg/dL among groups. Approximately 30 minutes after completion of the glucose supplement, the postprandial glucose levels were between 151-162 mg/dL among groups. The glucose levels started to decrease once the treatment section began.
By the end of the study, the glucose levels decreased to 119-130 mg/dL in treatment groups and 116 mg/dL in the control group. In addition, MES 2 group seemed to have a relatively lower decline rate on glucose levels during the treatment (between the 30 and 60-minute time points) when compared to the other groups.
Heart Rates
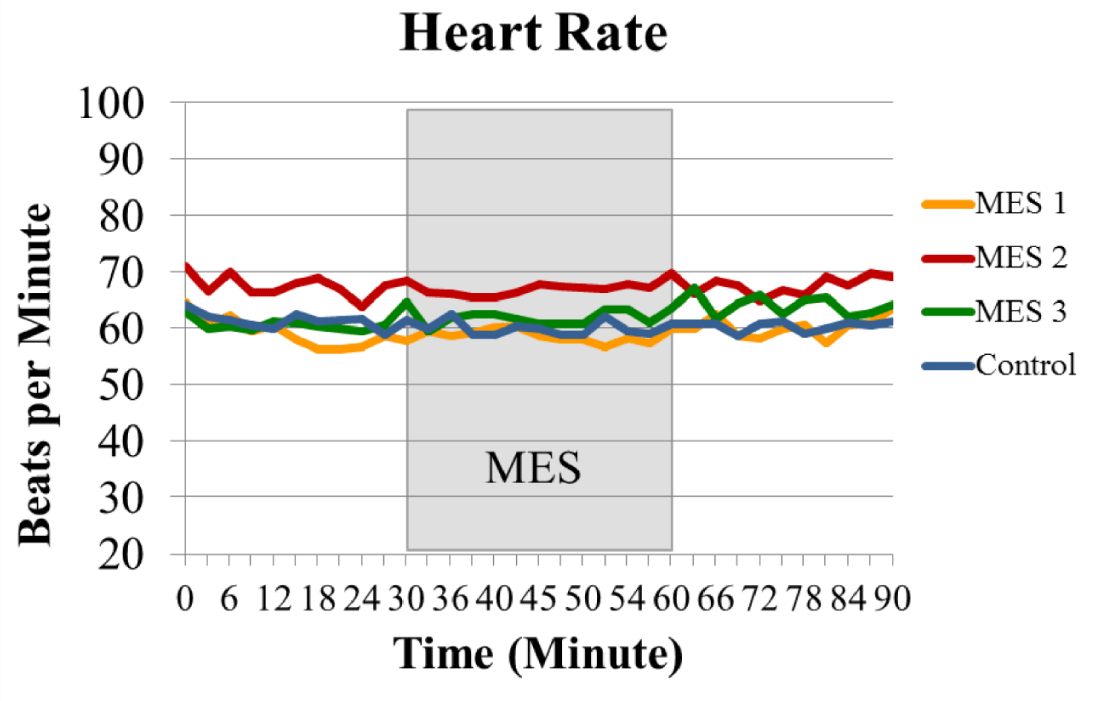
The heart rate was monitored every 3 minutes throughout the 90-minute study. All groups maintained an average within 55-71 beats per minute, and 30-minute of electrical stimulation did not significantly alter heart rate in all the treatment groups. There was no significant difference for heart rate between groups throughout the study (Figure 5).
Oxygen Consumption (VO2)
The VO2 was recorded every 30 seconds throughout the 90-minute study (Figure 6). During the first 30-minute of study, the resting VO2 was stable in each group. After MES treatment started, a general trend of increased VO2 was noted with all three treatment groups in comparison to the control group. In addition, both MES 2 and MES 3 prolonged the rise in VO2 after the 30-minute electrical stimulation treatment. There were statistically significant differences between MES 2 and Control (p < 0.001) as well as between MES 3 and Control (p < 0.05). On the MES 2, electrical stimulation significantly increased the VO2 (p < 0.05) between 51-60 minutes and between 61-70 minutes when compared to the baseline (0-10 minutes).
Discussion
Exercise has been suggested as an effective treatment to improve blood glucose control for people with T2D [20-24]. Research has shown that exercise can improve glucose uptake into skeletal muscle in patients with T2D [24]. However, many patients with T2D are physically restricted from the recommended exercise because of excessive obesity, orthopedic diseases, neurological diseases, chronic illness, or prolonged periods of being bedridden. Thus, these individuals who cannot receive the benefit of exercise remain exposed to the risk of diabetic complications such as stroke, heart disease, kidney failure, blindness, amputation, and premature death. MES might emerge as an innovative treatment to mimic exercise for controlling glucose levels in people with T2D, especially for those aforementioned populations who cannot perform adequate voluntary exercise. In order to identify the optimal use of MES in people with T2D, we suggested that it is necessary to determine which type of MES can best simulate exercise on healthy non-diabetic individuals first. Therefore, the objective of this study was to compare the effects of three types of MES on postprandial glucose levels in healthy subjects.
Previous studies have suggested that single-bout of MES increased whole body glucose uptake and attenuated postprandial hyperglycemia on non-diabetic subjects [12-14]. To our best knowledge, this is the first study comparing multiple parameters of electrical stimulation for postprandial glucose uptake in healthy subjects. However, our results show that there were no significant differences in postprandial glucose levels between treatment groups and the control group. The variations of research designs and treatment settings between previous studies and ours may explain the different responses in glucose levels to the MES.
In the two studies conducted by Hamada et al., the whole body glucose uptake was assessed on 8 male subjects who kept fasting [12, 13]. In our study, the postprandial glucose levels were monitored on each subject after taking the glucose supplement, Trutol™ 75g (300 kcal). In 2010, Kimura et al. recruited 14 male obese and pre-obese subjects to attend both an experimental trial (with MES) and a control trial (no MES) in random order with an interval of at least one week. After taking an approximate 800 kcal breakfast, 20-minute MES was applied on the subjects’ quadriceps, biceps femoris and gluteus maximus muscles with a 600 cm2 electrode area on each leg. The results showed that the postprandial glucose levels were lower in the MES trial than the control trial [14]. In our study, the MES potency did not appear to be high enough to increase glucose uptake in the skeletal muscle. To prevent the muscle fatigue during the 30-minute MES, we chose 50% duty cycle (3 second on and 3 second off) which equals to only 15 minutes of treatment in total. The total area of electrode placement in our study was only about 206 cm2 per leg on quadriceps and biceps femoris but not including gluteus maximus. In addition, the glucose challenge by Trutol™ 75g with only 300 kcal may not be enough to show the effect of MES, even though Trutol™ 75g is the protocol outlined by the Centers for Disease Control and Prevention (CDC) for 2 hour oral glucose tolerance test.
Gender differences in responses to MES could be a potential confounding factor in this study. A study investigating fiber type composition of the quadriceps muscle on young men and women suggested that males have relative larger area of type II muscle fibers than females [25]. Since MES primarily activates type II muscle fibers which have a larger capacity for glycogen utilization than type I fibers [12, 26], MES may promote greater skeletal muscle glycolysis and glucose uptake in males as compared to females. The subjects were all male in the aforementioned three studies showing positive responses to MES [12-14]. In our study, even with random assignment, MES 2 and MES 3 groups consisted solely of female, and MES 1 consisted majorly of male (86%). Our results did not show any significant effects of MES on the changes of glucose levels between groups. Recently, Miyamoto et al. also suggested that MES could have a greater metabolic response in males than in females with T2D [16]. However, there is limited research on the gender differences in responses to MES on healthy non-diabetic subjects or comparing the effects of different MES protocols within the same gender.
Heart rate and VO2 are two common methods to measure exercise intensity. Generally, the greater the intensity of the exercise being performed, the higher the heart rate and the amount of oxygen consumed by the body. Here, we monitored each subject’s heart rate and VO2 throughout the study to determine which group might receive the best simulation of exercise in terms of intensity. Although our results showed that 30-minute of MES did not significantly alter heart rates in each group, applying 30-minutes electrical stimulation with Russian Current (MES 2) or low-rate TENS (MES 3) significantly changed the VO2 levels. In particular, applying Russian Current significantly increased VO2 levels by 10 percent. The characteristics of parameters between each treatment group may explain this finding. The electrical pulse frequency of Russian Current was 2,500 Hz, whereas MES 1 and MES 3 were only 50 Hz and 2 Hz respectively. Russian Current delivers the most number of electrical pulses in every second to the skeletal muscle to induce contraction. Therefore, among the three types of MES tested in this study, we suggested that applying Russian Current on skeletal muscle might be the best to mimic physical activity. However, it needs to be acknowledged that the clinical meaning of 10% increase of VO2 might be limited. The protocol of Russian Current applied in this study can only be considered as a simulation of fairly low-intensity of exercise.
There are a few limitations in this study. First, the research volunteers were recruited via convenience sampling from the student population at Angelo State University in San Angelo, Texas with an average age of 24 to 25 plus small sample size in each group. Therefore, the generalizability and external validity of our findings are limited. Secondly, despite random assignment, two of treatment groups consisted solely of females. As mentioned previously, the gender differences of metabolic responses to MES might play a role in compromising our study results. In addition, while an euglycemic clamp is suggested the gold standard for continuous measurement of glucose metabolism [27], we measured glucose levels by handheld glucometer through finger-prick in every 30 minutes. Therefore, the detailed changes in glucose levels due to the effects of MES were not available.
Conclusions
Research already suggested that MES might emerge as an innovative therapeutic exercise for sedentary people with T2D. We compared the effects of MES between three different protocols on healthy non-diabetic subjects. Our results showed that 30-minute single-bout MES did not affect postprandial glucose levels. However, among three types of MES commonly used in the clinics, Russian Current may have a better potential to simulate physical activity. Future research is required with a more extended sampling method, a larger sample size, and more intensive MES experimental protocols to mimic exercise and a continuous monitoring technique by glucose clamp.
We wish to acknowledge Texas Physical Therapy Foundation for assistance in funding the project.
- Faghri PD, Glaser RM, Figoni SF (1992) Functional electrical stimulation leg cycle ergometer exercise: training effects on cardiorespiratory responses of spinal cord injured subjects at rest and during submaximal exercise. Arch Phys Med Rehabil 73: 10851093. Link: https://goo.gl/YBvwhz
- Hooker SP, Figoni SF, Glaser RM, Rodgers MM, Ezenwa BN, et al. (1990) Physiologic responses to prolonged electrically stimulated leg-cycle exercise in the spinal cord injured. Arch Phys Med Rehabil 71: 863-869. Link: https://goo.gl/iiWcnw
- Kim CK, Bangsbo J, Strange S, Karpakka J, Saltin B (1995) Metabolic response and muscle glycogen depletion pattern during prolonged electrically induced dynamic exercise in man. Scand J Rehabil Med 27: 51-58. Link: https://goo.gl/fzO3sy
- Scremin AM, Kurta L, Gentili A, Wiseman B, Perell K, et al. (1999) Increasing muscle mass in spinal cord injured persons with a functional electrical stimulation exercise program. Arch Phys Med Rehabil 80: 1531-1536. Link: https://goo.gl/LWUZ0A
- Benrick A, Maliqueo M, Johansson J, Sun M, Wu X, et al. (2014) Enhanced insulin sensitivity and acute regulation of metabolic genes and signaling pathways after a single electrical or manual acupuncture session in female insulin-resistant rats. Acta Diabetol 51: 963-972. Link: https://goo.gl/eqwRK8
- Sato D, Shinzawa G, Kusunoki M, Matsui T, Sasaki H, et al. (2013) Effects of electrical microstimulation of peripheral sympathetic nervous fascicle on glucose uptake in rats. J Artif Organs 16: 352-358. Link: https://goo.gl/VwCsLb
- Aslesen R, Engebretsen EM, Franch J, Jensen J (2001) Glucose uptake and metabolic stress in rat muscles stimulated electrically with different protocols. J Appl Physiol (1985) 91: 1237-1244. Link: https://goo.gl/H7u2wF
- Ryder JW, Kawano Y, Galuska D, Fahlman R, Wallberg-Henriksson H, et al. (1999) Postexercise glucose uptake and glycogen synthesis in skeletal muscle from GLUT4deficient mice. FASEB J 13: 2246-2256. Link: https://goo.gl/a0Olu3
- Poole RB, Harrold CP, Burridge JH, Byrne CD, Holt RI (2005) Electrical muscle stimulation acutely mimics exercise in neurologically intact individuals but has limited clinical benefits in patients with type 2 diabetes. Diabetes Obes Metab 7: 344-351. Link: https://goo.gl/2BHX8Q
- Arija-Blazquez A, Ceruelo-Abajo S, Diaz-Merino MS, Godino-Duran JA, MartinezDhier L, et al. (2014) Effects of electromyostimulation on muscle and bone in men with acute traumatic spinal cord injury: A randomized clinical trial. J Spinal Cord Med 37: 299-309. Link: https://goo.gl/AHTeBZ
- Hjeltnes N, Galuska D, Bjornholm M, Aksnes AK, Lannem A, et al. (1998) Exerciseinduced overexpression of key regulatory proteins involved in glucose uptake and metabolism in tetraplegic persons: molecular mechanism for improved glucose homeostasis. FASEB J 12: 1701-1712. Link: https://goo.gl/B16yDV
- Hamada T, Hayashi T, Kimura T, Nakao K, Moritani T (2004) Electrical stimulation of human lower extremities enhances energy consumption, carbohydrate oxidation, and whole body glucose uptake. J Appl Physiol (1985).96(3): 911-916. Link: https://goo.gl/lJfoll
- Hamada T, Sasaki H, Hayashi T, Moritani T, Nakao K (2003) Enhancement of whole body glucose uptake during and after human skeletal muscle low-frequency electrical stimulation. J Appl Physiol (1985) 94: 2107-2112. Link: https://goo.gl/SUFXXM
- Kimura T, Matsumoto K, Kameda N, Tanaka S, Hayashi T, et al. (2010) Percutaneous Electrical Muscle Stimulation Attenuates Postprandial Hyperglycemia in Obese and Preobese Japanese Men. International Journal of Sport and Health Science.8: 1-6. Link: https://goo.gl/gkayCx
- Miyamoto T, Fukuda K, Kimura T, Matsubara Y, Tsuda K, et al. (2012) Effect of percutaneous electrical muscle stimulation on postprandial hyperglycemia in type 2 diabetes. Diabetes Res Clin Pract 96: 306-312. Link: https://goo.gl/Z7Jvkc
- Miyamoto T, Fukuda K, Watanabe K, Hidaka M, Moritani T (2015) Gender difference in metabolic responses to surface electrical muscle stimulation in type 2 diabetes. J Electromyogr Kinesiol 25: 136-142. Link: https://goo.gl/RaLwuU
- van Buuren F, Horstkotte D, Mellwig KP, Frund A, Vlachojannis M, et al. (2015) Electrical Myostimulation (EMS) Improves Glucose Metabolism and Oxygen Uptake in Type 2 Diabetes Mellitus Patients--Results from the EMS Study. Diabetes Technol Ther 17: 413-419. Link: https://goo.gl/DHX5Ui
- Cameron MH (2013) Physical agents in rehabilitation: from research to practice. 4th edition,Saunders/Elsevier. Link: https://goo.gl/d4BQV8
- Ward AR, Shkuratova N (2002) Russian electrical stimulation: the early experiments. Phys Ther 82: 1019-1030. Link: https://goo.gl/lIOA0c
- O'Hagan C, Vito G De, Boreham CA (2013) Exercise prescription in the treatment of type 2 diabetes mellitus: current practices, existing guidelines and future directions. Sports Med 43: 39-49. Link: https://goo.gl/LvK40W
- van der Heijden MM, van Dooren FE, Pop VJ, Pouwer F (2013) Effects of exercise training on quality of life, symptoms of depression, symptoms of anxiety and emotional well-being in type 2 diabetes mellitus: a systematic review. Diabetologia 56: 12101225. Link: https://goo.gl/8pwygl
- Oliveira C, Simoes M, Carvalho J, Ribeiro J (2012) Combined exercise for people with type 2 diabetes mellitus: a systematic review. Diabetes Res Clin Pract 98: 187198. Link: https://goo.gl/c39Vlc
- Balducci S, Sacchetti M, Haxhi J, Orlando G, D'Errico V, et al. (2014) Physical exercise as therapy for type 2 diabetes mellitus. Diabetes Metab Res Rev.30 Suppl 1: 13-23. Link: https://goo.gl/N6VNPk
- Asano RY, Sales MM, Browne RA, Moraes JF, Coelho Junior HJ, et al. (2014) acute effects of physical exercise in type 2 diabetes: A review. World J Diabetes 5: 659-665. Link: https://goo.gl/JePRs6
- Staron RS, Hagerman FC, Hikida RS, Murray TF, Hostler DP, et al. (2000) Fiber type composition of the vastus lateralis muscle of young men and women. J Histochem Cytochem 48: 623-629. Link: https://goo.gl/J9yXn3
- Sinacore DR, Delitto A, King DS, Rose SJ (1990) Type II Fiber Activation with Electrical Stimulation: A Preliminary Report. Physical Therapy 70: 416-422. Link: https://goo.gl/bSrWAC
- Hompesch M, Rave K (2008) an analysis of how to measure glucose during glucose clamps: are glucose meters ready for research? J Diabetes Sci Technol 2: 896-898. Link: https://goo.gl/bCm5pe
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
