Journal of Neurology, Neurological Science and Disorders
Morphometry of the middle cerebral artery (sylvian artery) on MRI: Contribution to cerebral endovascular surgery
Racky Wade-Kane1*, Cheikh Seye2, Magaye Gaye1, Aïnina Ndiaye1, Ndeye Bigué Mar3, Sokhna Astou Gawane Thiam1, Karim Yacouba Garba1, Daouda Harouna Tireira1, Isseu Dior Seck1, Mamadou Ndiaye1, Philippe Manyacka Manyemb4, Sokhna Ba5, Abdoulaye Dione Diop5, Assane Ndiaye6, Mamadou Diop1, Jean Marc Ndoye1 and Abdoulaye Ndiaye1
2Department of Anatomy, Bambey University, Diourbel, Senegal
3Department of Anatomy, Iba Der Thiam University, Thies, Senegal
4Department of Anatomy, UFR 2S, Gaston Berger University, Saint-Louis, Senegal
5Diagnostic and Medical Imaging Center, Fann National University Hospital Center, Dakar, Senegal
6Department of Anatomy, Assane Seck University, Ziguinchor, Senegal
Cite this as
Wade-Kane R, Seye C, Gaye M, Ndiaye A, Mar NB, et al. (2022) Morphometry of the middle cerebral artery (sylvian artery) on MRI: Contribution to cerebral endovascular surgery. J Neurol Neurol Sci Disord 8(1): 001-006. DOI: 10.17352/jnnsd.000046Copyright
© 2022 Wade-Kane R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Introduction: The Middle Cerebral Artery (MCA), from its old nomenclature “sylvian artery”, is a terminal branch of the Internal Carotid Artery (ICA) of which it constitutes the main extension. It represents a fundamental branch of the brain vasculature. The objective of this work was to provide Magnetic Resonance Imaging (MRI) morphometric data of the MCA to inform the interventions of neurovascular specialists and to contribute to the advancement of microcatheter and stent technology.
Methodology: Morphometry was studied on 40 Cerebral Hemispheres (CH) of 20 right-handed subjects aged 18 to 55 years. We used a Philips MRI from the Achieva range at 1.5 Tesla with the T2-SE and TOF sequences.
After identification, the MCA morphometry consisted of measuring the luminal diameter at the origins of the four segments of the MCA. These were the M1 (sphenoidal), M2 (insular or sylvian), M3 (opercular) and M4 (cortical) segments.
Results: The diameter is greater at the level of the M1 segment then it decreases along the path of the MCA. Within the same HC, the average luminal diameters at the origin of segments M1, M2 and M3 decrease significantly and successively from front to back (from M1 to M3). However, there is no significant difference between the average luminal diameters of M3 and M4.
The M1, M2 and M3 segments show no significant mean difference between the right cerebral hemisphere (RCH) and the Left Cerebral Hemisphere (LCH). Only the M4 segment of the MCA presents a difference in the averages of the luminal diameter between the two CHs. Also, for this same segment M4, the distribution of RCH means is significantly higher than that of LCH.
Conclusion: These results can help in choosing the appropriate size (diameter) of the microcatheters. Also, they make it possible to determine new diameters of microcatheters in the neurovascular system, some distal artery segments of which have been inaccessible until now.
Introduction
The Middle Cerebral Artery (MCA), from its old nomenclature “Sylvian artery”, is a terminal branch of the Internal Carotid Artery (ICA) of which it constitutes the main extension. Thus, it receives 60 to 80% of the carotid blood flow and represents a fundamental branch of encephalic vascularization [1,2,3].
The MCA originates at the terminal bifurcation of the ICA [4]. It is located on the lateral aspect of the ICA, behind the Anterior Cerebral Artery (ACA), above the anterior choroidal and posterior communicating arteries, and at the external angle of the optic chiasm.
The trunk of the ACM is in the extension of the carotid siphon thus favoring the referral of thrombi or emboli in this artery during an ischemic stroke. It will reach the Lateral Fossa of the Brain (LFB) which is formerly called the “Sylvian valley” [5]. During this path, the MCA will present four segments M1, M2, M3, and M4, [6,7,8] which constitute its main trunk.
Authors like Serrador et al. were interested in the study of variations in the luminal diameter of the MCA. They evaluated the impact of increased CO2 and sympathetic tone on the luminal diameter of the MCA [9].
Today, MCA is the subject of therapeutic innovation with mechanical endovascular thrombectomy and endovascular treatment of MCA aneurysms [10]. These 2 treatments partly require the use of microcatheters. The choice of the diameters of these depends on the luminal diameter of the obstructed artery or carrier of the aneurysm. Hence, the objective of providing morphometric data of the MCA by MRI in order to contribute to the progress of the technology of microcatheters and stents.
Methodology
Subjects
MCA morphometry was studied on 40 Cerebral Hemispheres (CH) of 20 right-handed subjects aged 18 to 55 years. These subjects were recruited consecutively following their registration for a scheduled brain MRI examination at the Diagnostic and Medical Imaging Center (DMIC) of the Fann National University Hospital Center (FNUHC).
Materials
We used a Philips MRI from the Achieva range at 1.5 Tesla. The reconstruction of the images, their processing and exploitation were possible thanks to the integration of the Philips DICOM Viewer R1.2 and MILLENSYS Viewer 10.6 software in two of the console computers.
Method
The study was carried out on two basic sequences of a standard image acquisition protocol defined by the radiologist. These two sequences consisted of :
The T2-weighted spin echo (T2-SE) sequence with a RT equal to 5950 ms and an ET equal to 110 ms. The flip angle was 90° and the slices were frontal with a thickness of 5 mm and;
The flight time (TOF) angiography gradient echo sequence, with a RT equal to 23 ms and an ET equal to 6.9 ms. The flip angle was 23° and the slices were transverse with a thickness of 1.6 mm.
The frontal slices were strictly perpendicular to the anterior white commissure-posterior white commissure (AC-PC) axis.
The morphometry of the MCA consisted, after identification, in measuring the luminal diameter at the origins of the four segments of the MCA. These were the M1 (sphenoidal), M2 (insular or sylvian), M3 (opercular) and M4 (cortical) segments.
For origin identification: The origins of M1, M2 and M3 were identified on frontal slices in T2-SE sequence and that of M4 on a transverse slice in TOF Head Coil sequence. According to the radio-anatomical reading of the images:
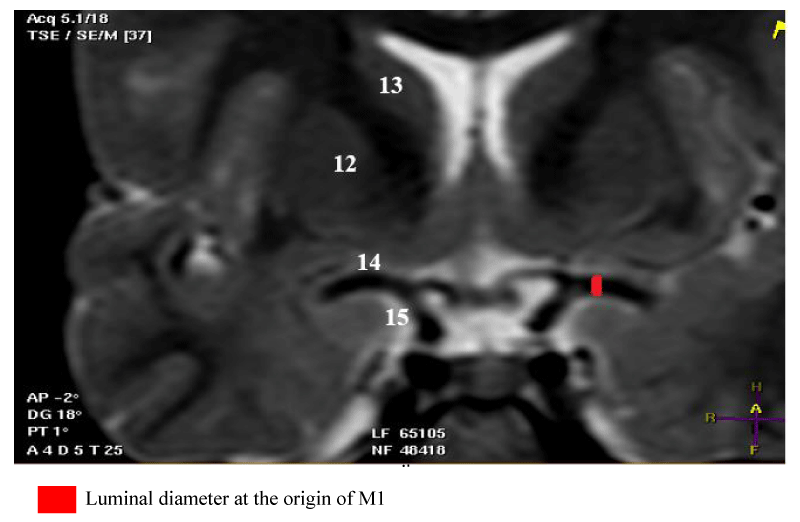
- The origin of M1 is located at the termination of the ICA. On a frontal slice passing through the M1 segment, it is well identified at the medial end of the ICA [Figure 1].
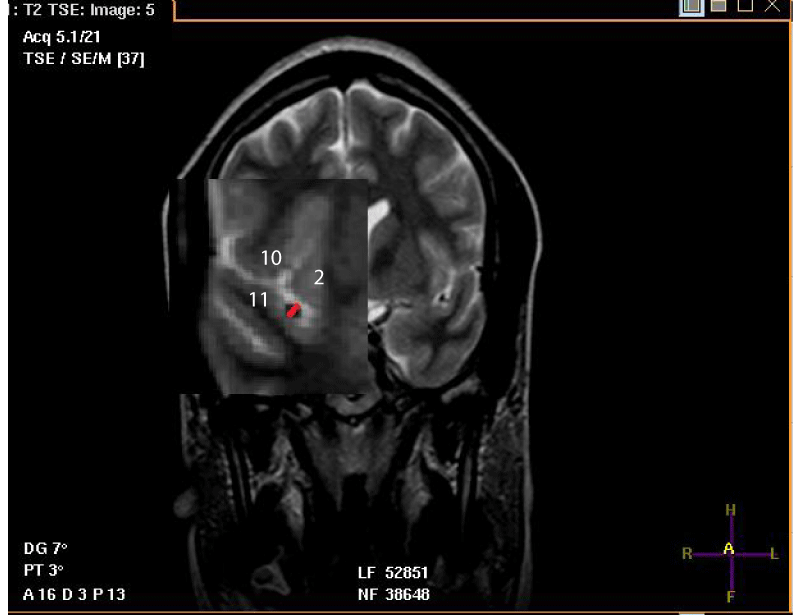
- The origin of M2 is located just after the knee of the MCA, at its exit from the falciform fold. The diameter is measured there at the initial portion of the segment and on the frontal slice. When M2 has two trunks, the measurement is performed on both trunks. The diameter of the largest trunk is reported, and this is the extension of the main trunk (MT) of the MCA [Figure 2].
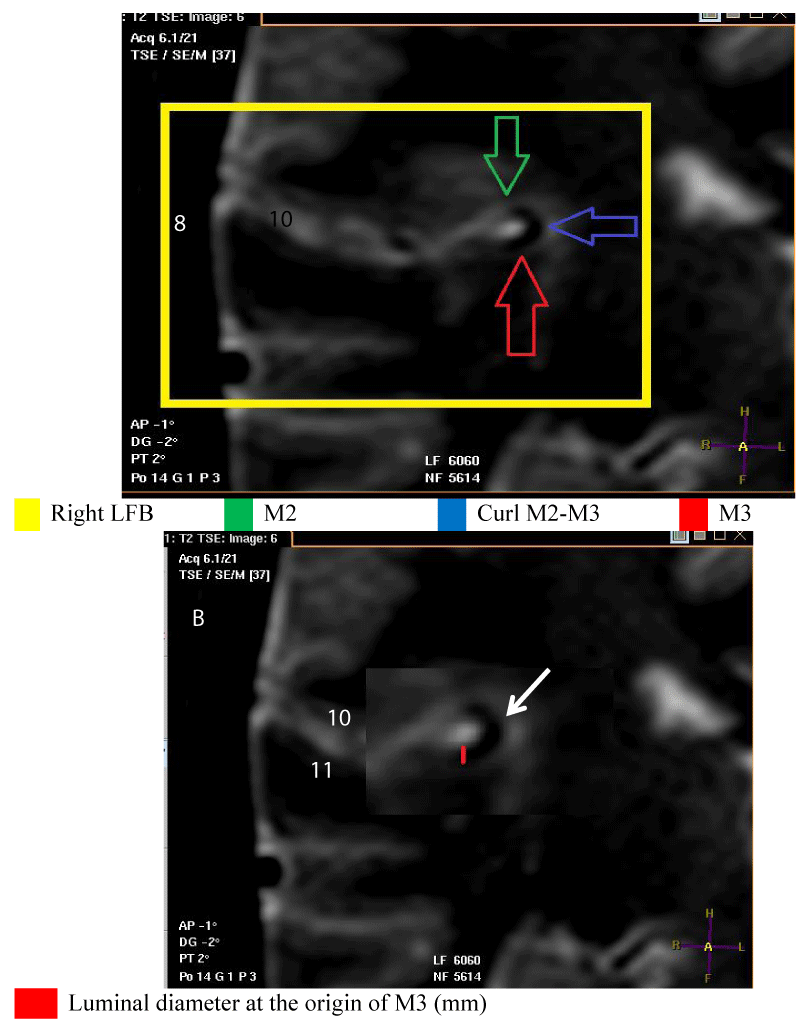
- The origin of M3 is located on a frontal slice, at the level of the medial end of this portion of the MT of the MCA which crosses the lateral sulcus (LS) horizontally and following M2. It is close to the postero-superior angle of the insula. Specifically, it is at the level of the posterosuperior recess (PSR) of the LFB [Figure 3].
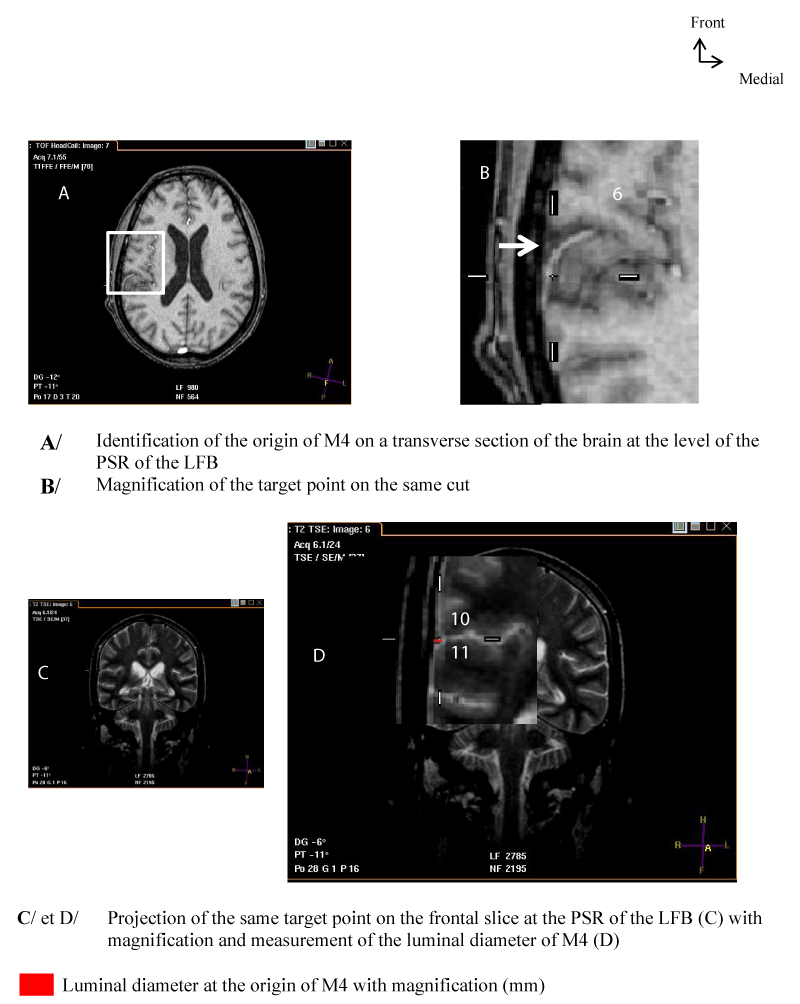
- The origin of M4 corresponds to the output of the MT of the MCA of the LS. On a frontal section, it is located at the posterior end of the lateral edge of the LS, thus extending M3 [Figure 4].
For the measurement of the luminal diameter: All measurements were performed on frontal slices in T2-SE sequence. On these slices, the luminal diameter of the trunk to be measured corresponded to that of a circle in hyposignal and to the clear circumference if the direction of the MT tended towards the horizontal. Also, the luminal diameter could correspond to that of tubing in hyposignal and with sharp edges if the direction of the MT tended upwards [Figures 1-4].
Data entry and processing
We used Excel and Epi info software to analyze and then compare the data. More precisely, we carried out a comparison (two by two) of mean values of series of quantitative variables. Given the size of the sample (20 subjects with a total of 40 cerebral hemispheres), a non-parametric Wilcoxon test on unpaired data is used.
Also, we compared the mean luminal diameters of the MCA segments of each hemisphere to the luminal diameter values of the NSK subject (theoretical value). The NSK reference subject is defined in the first part of this work which focused on LFB [5]. She had in each cerebral hemisphere, the most typical morphological characteristics of the LFB among the 20 right-handed adults [5]. Given the size of the sample (20 subjects with a total of 40 cerebral hemispheres) and assuming that the distributions of the variables are normal in the population from which the sample is taken, the T test for comparing an observed mean to a theoretical mean is used.
Ethical considerations
This study was approved by the Research Ethics Committee (REC) of Cheikh Anta Diop University (UCAD) in Dakar. The subjects consented to the study after reading the information sheet validated by the REC.
The data was collected taking into account compliance with the rules of medical ethics.
Results
1. Mean luminal diameters (mm) at the origin of each segment of the Main Trunk (MT) of the MCA according to the Cerebral Hemisphere (CH) [Table 1].
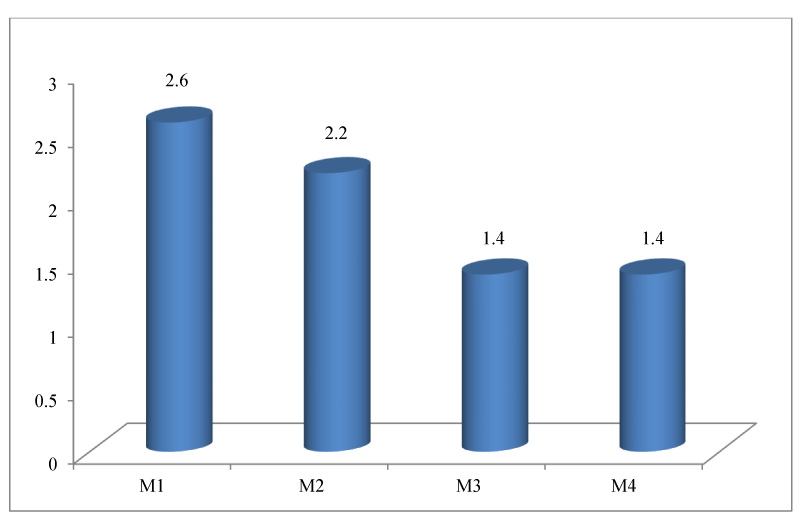
2. Representation of the means of the luminal diameters according to the segment of the main trunk of the MCA of the 40 cerebral hemispheres.
The diameter is greater at the level of the M1 segment then it decreases along the path of the MCA [Figure 5].
3. Comparison of the means of the luminal diameters between the four segments of the MCA of the same hemisphere.
Within the same hemisphere, the average luminal diameters at the origin of segments M1, M2 and M3 decrease significantly and successively from front to back (from M1 to M3). There is no significant difference between the mean luminal diameters of M3 and M4 [Tables 2,3].
4. Comparison of the means of the luminal diameters of segments between cerebral hemispheres.
The M1, M2 and M3 segments show no significant mean difference between RCH and LCH. Only the M4 segment of the MCA presents a difference in the averages of the luminal diameter between the two hemispheres. Also, for this same segment, the distribution of RCH means is significantly higher than that of LCH [Table 4].
5. Comparison of the means of the luminal diameter of the segments of the MCA of each CH with the values of the luminal diameter of the NSK subject (theoretical value).
Discussion
ACM Morphometry
The data for measuring the luminal diameter of the main trunk of the MCA found in the literature relate more to the M1 segment, after which comes the M4 segment. The M2 and M3 segments are often studied for their distribution. The result for the luminal diameter of the M1 segment is 2.6 mm on average. This number is identical to that reported by Maslehaty et al. in his study on the anatomical variations of the MCA [11]. Their results come from MRI data and angiographic computed tomography of subjects free of pathologies. The authors Serrador and Piepgras discuss an underestimation of their results estimated at 2.5 mm and 2.13 mm respectively, which they attribute to the methodology used in their work [12,9]. For example Piepgras et al. did not take into account in their study population atherosclerotic lesions which may be the cause of reduction in luminal diameter. Especially since more than a third of the sample were aged 65 or over. The results of Muller et al. obtained by angiography in 1991, show higher values than ours with 3.05 mm in women and 3.35 mm in men [9]. Also, the first reported dissection work showed large diameters of M1 of the order of 4 mm [1] and 3.9 mm. This overestimation could be explained by the extraluminal measurement of the diameter of M1 [1] and the perfusion of the MCA with latex before dissection [11]. All these data on the luminal diameter of M1 show that the rigorous selection criteria of patients and the choice of the type of imaging examination are decisive in the study of the luminal diameter of the cerebral arteries. Currently, standard angiography remains the gold standard for the luminal study of vessels and the calculation of catheter diameters. Nevertheless, angio-MRI is interesting because it is non-invasive, non-irradiating and requires no injection of iodinated contrast product. MRI angiography usually underestimates the diameters. MRI of the wall is on the other hand the gold standard for the study of the wall of vessels (vasculitis, etc.).
For the M4 segment, Chater et al. cited in the work of Gibo [7], found a diameter of 1.4 mm in 100% of CH. Waddington, also cited in the same work, found that 86% of angular arteries had a luminal diameter greater than 1 mm. Our results are close to those of these two authors, they showed a mean diameter of M4 of 1.4 mm. The M4 segment corresponding in our study to the angular artery or artery of the curved fold, has been described as one of the largest cortical arteries of the MCA. Those on the temporal pole and the frontal lobe were considered smaller [7]. The large diameter of M4 ensures long-term patency of its anastomosis. It is often used by neurosurgeons for bypass.
The MCA has hemispherical symmetry with a luminal diameter of M1 to M3. These results are like those of Piepgras who found no significant difference between the M1 segments of the two CH [12]. The asymmetry found at the level of M4 could be linked to the variability of its functional importance because in addition to the angular gyrus which it irrigates, it can supply the posterior parietal and temporal arteries [1].
The significant differences between the mean luminal diameters of the sample and those of the NSK reference subject could be due to inter-individual variations.
Methodological limit
Our sample size is limited to 40 cerebral hemispheres from 20 subjects due to lack of study funding. The realization of the MRI is not free, and it is expensive for our populations.
Given the great variability that exists in the cerebrovascular system, further study with a larger sample would improve this work.
Conclusion
The luminal diameter data obtained can contribute to advances in microcatheter and stent technology. Smaller stent diameters may help increase success rates for recanalization of distal segments of the MCA.
- Dubret G, Cousin F-R. Eléments d’Anatomie et de physiologie du système nerveux central. Paris: Flammarion. 1985 ; 376.
- Rouvière H, Delmas A. Système nerveux central, voies et centres nerveux. In : Anatomie humaine. Descriptive, topographique et fonctionnelle. Paris: Masson; 15e éd. révisée par Delmas V. 2002 ; 411.
- Vitte E, Chevallier J-M. Neuro-Anatomie. Paris: Flammarion; 4e éd. 1998 ; 234.
- Hima MA, Sakho Y, Ndiaye Ab, Dia A. Etude de la disposition topographique du cercle artériel de la base du cerveau et de ses branches collatérales : à propos de quinze cas. Université Cheikh Anta Diop de Dakar; 2009.
- Wade R, Plaisant O, Guédon A, Diop AD, Ndiaye A, Manyacka P, Gaye M, Ba S, Ndiaye A, Dia A. Morphology of the lateral fossa of the brain (sylvian valley): anatomo-radiological aspects and surgical application. Surg Radiol Anat. 2019 Jun;41(6):639-655. doi: 10.1007/s00276-019-02228-5. Epub 2019 Apr 6. PMID: 30955058.
- Elsharkawy A, Niemelä M, Lehečka M, Lehto H, Jahromi BR, Goehre F, Kivisaari R, Hernesniemi J. Focused opening of the sylvian fissure for microsurgical management of MCA aneurysms. Acta Neurochir (Wien). 2014 Jan;156(1):17-25. doi: 10.1007/s00701-013-1894-7. Epub 2013 Oct 8. PMID: 24101289.
- Gibo H, Carver CC, Rhoton AL Jr, Lenkey C, Mitchell RJ. Microsurgical anatomy of the middle cerebral artery. J Neurosurg. 1981 Feb;54(2):151-69. doi: 10.3171/jns.1981.54.2.0151. PMID: 7452329.
- Hacking C, Jones J. Middle cerebral artery [Internet]. Radiopaedia.org. Cité le 31 décembre 2017. Disponible sur: https://radiopaedia.org/articles/middle-cerebral-artery .
- Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke. 2000 Jul;31(7):1672-8. doi: 10.1161/01.str.31.7.1672. PMID: 10884472.
- Scholtens LH, de Reus MA, van den Heuvel MP. Linking contemporary high resolution magnetic resonance imaging to the von Economo legacy: A study on the comparison of MRI cortical thickness and histological measurements of cortical structure. Hum Brain Mapp. 2015 Aug;36(8):3038-46. doi: 10.1002/hbm.22826. Epub 2015 May 19. PMID: 25988402; PMCID: PMC6869041.
- Maslehaty H, Deuschl C, Kleist B, Göricke S, Sure U, Müller O. Computed Tomography- and Magnetic Resonance Image-based Analysis of the Anatomical Variations of the Sylvian Fissure and Characteristics of the Middle Cerebral Artery. Clin Pract. 2017 Feb 3;7(1):890. doi: 10.4081/cp.2017.890. PMID: 28243427; PMCID: PMC5304263.
- Piepgras A, Bise K, Schmiedek P. Morphometry of intraluminal side-to-side differences in human basal cerebral arteries. Ultrasound Med Biol. 1993;19(3):193-5. doi: 10.1016/0301-5629(93)90109-2. PMID: 8511825.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
