Journal of Neurology, Neurological Science and Disorders
Magnetic Resonance Imaging versus Proton Magnetic Resonance Spectroscopy in Neonatal Hypoxic Ischemic Encephalopathy in Egyptian Population: Pilot study
Ossama Y Mansour1*, Doaa Hanfy2, Sameh Fathy3 and Rania E Mohammed4
2Alexandria University, Neurology department, Egypt
3Zagazig University, radio diagnosis department, Egypt
4Zagazig University, radio diagnosis department, Egypt
Cite this as
Mansour OY, Hanfy D, Fathy S, Mohammed RE (2017) Magnetic Resonance Imaging versus Proton Magnetic Resonance Spectroscopy in Neonatal Hypoxic Ischemic Encephalopathy in Egyptian Population: Pilot study. J Neurol Neurol Sci Disord 3(1): 043-050. DOI: 10.17352/jnnsd.000020Background: Hypoxic-ischemic encephalopathy (HIE) is a serious condition that results of critical failure of the intrapartum gas exchange and may lead to a significant damage in the central nervous system.
Objective: to elucidate the role of brain magnetic resonance imaging (MRI) versus Proton magnetic resonance spectroscopy (1H-MRS) in the diagnosis and evaluating the severity of HIE in full-term Egyptian neonates.
Design: 49-months observational controlled study.
Setting: Pediatric neurology and Radiology Departments, Alexandria University Hospital
Patients and Methods: Thirty full-term neonates in the first two weeks with HIE (Sarnat staging) were included (18 males and 12 females), with exclusion of those with congenital brain malformation and/ or perinatal infection in addition to 15 age- and sex- matched healthy controls. All patients and controls were examined with brain MRI and 1H-MRS.
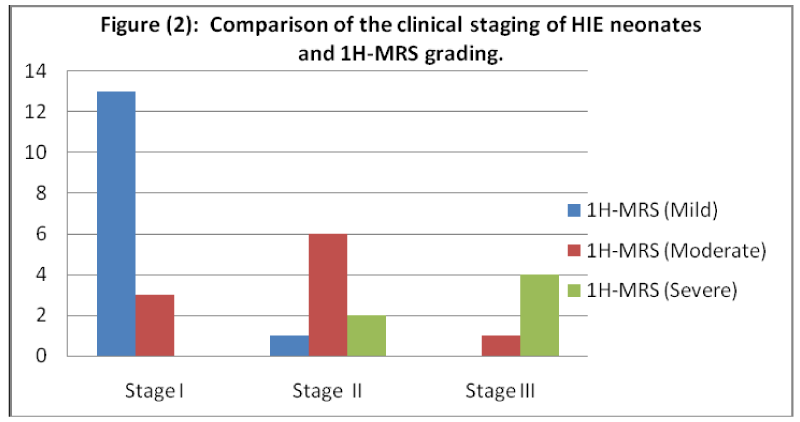
Results: In neonates with HIE, the percent of agreement between clinical staging, and MRI grading was 66.67 while it was 76.67 regarding 1H-MRS grading. The sensitivity for MRI was 66.67%, specificity was 75%, accuracy was 77.78%, PPV was 80% and NPV was 75%. Meanwhile, the sensitivity for 1H-MRS was 92%, specificity was 75%, accuracy was 84.44%, PPV was 82.14% and NPV was 88.24%.
Conclusion: 1H-MRS detected more percent of agreement, true positive and less false negative cases than MRI. Despite the equal specificity between them, still 1H-MRS got more sensitivity, accuracy, PPV and NPV than MRI i.e. when we have to choose early and single investigation it is recommended to choose 1H-MRS.
Abbrevations
1H-MRS: Proton Magnetic Resonance Spectroscopy; CHESS: Chemical Shift Selection; Cho: Choline; Cr: Creatine; FLAIR: Fluid-attenuated inversion recovery; Glx: Glutamate-glutamine; HIE: Hypoxic-Ischemic Encephalopathy; Lac: Lactate; MRI: Magnetic Resonance Imaging; NAA: N-Acetyl Aspartate; TE: Echo Time; TR/TE: Repetition Time/Echo Time; T2*GRE: T2* Gradient Echo
Introduction
Hypoxic-ischemic encephalopathy (HIE) is a significant cause of permanent damage to the central nervous system (CNS) cells. It remains a serious condition that may result in neonatal decease or be manifested later as cerebral palsy or mental deficiency [1,2]. It affects approximately 2 per 1000 births in the developed world. Prevention is vital because no specific therapy can reverse the CNS injury [3].
Acute cerebral hypoxia-ischemia (HI) occurs as a result of critical impairment of the intrapartum gas exchange [4]. However, the exact timing and underlying cause remains unknown [5]. Early and accurate prediction of outcome is difficult by clinical examination alone [6,7].
Abnormalities on conventional magnetic resonance imaging (MRI) may take several days to become obvious, during which the maximum benefit from therapeutic interventions can be achieved [8,9]. Because of this problem with conventional MRI, there has been a move towards defining quantitative cerebral biomarkers so as to increase prognostic objectivity [10,11]. Proton magnetic resonance spectroscopy (1HMRS) enables us to measure the various metabolite ratios in vivo both in normal and in pathologic conditions. The finding of a helpful method to detect abnormalities of metabolism even when normal structures are present on MRI studies will be appreciated [12].
The aim of this study is to elucidate the role of MRI versus 1H-MRS in the diagnosis and grading of severity of HIE in full-term neonates.
Patients and Methods
Between March 2009 and April 2011, a total of 30 consecutive full-term neonates in the first two weeks of their life were eligible and enrolled for admission to the study. They were diagnosed clinically as HIE complicating perinatal asphyxia and were admitted within 24 hours of presentation to the NICU, Pediatric Neurology Department, and the imaging procedures were done in the Radiology Department, Alexandria University Hospital. The criteria used for diagnosing HIE were described by Sarnat et al., (Table 1) [13,14]. The exclusion criteria included neonates with congenital brain malformation, inherited metabolic disorders, or perinatal infection.
Fifteen healthy age- and sex-matched neonates with no history of asphyxia, medical or neurological disorders were selected as control group. They were underwent the same imaging protocol for comparison. All Patients and controls were examined with brain MRI and 1H-MRS. After establishing the clinical diagnosis of HIE, a prescribed supportive treatment was started immediately in all cases. An official permission to carry out the study was obtained from the responsible authorities and the administrative staff. Parents’ consent to participate in the study was obtained.
Imaging procedures
The MRI and 1H-MRS procedures for all study neonates were done under either natural sleep or under sedation induced by 10% chloral hydrate syrup given orally or via a nasogastric tube. During radiological examination, patients were medically supervised, monitored by MR-compatible pulse oximetry and were maintained under 100% oxygen breathing. All MRI and 1H-MRS examinations were performed by using 1.5-Tesla unit system (Signa Horizon SR 120; General Electric Medical Systems, Milwaukee, WI, USA) with a quadrature head coil, Radiology department, Alexandria University Hospital.
MRI procedures
The MRI protocol included spin-echo and fast spin-echo MR pulse sequences. Spin-echo images were axial T1-weighted images (500/14/2 [TR/TE/NEX]). Fast spin-echo images were axial T2-weighted images (4000/126/2 [TR/TE/NEX]), and axial fluid-attenuated inversion recovery (FLAIR) (TR/TE/Inversion time (TI) = 6000/140/1200) sequences. Axial T2* gradient echo (T2*GRE) sequence (460, 15, 20 degrees [TR/TE/FA]) was done in selected cases for exclusion of micro-bleeds.
MRI grading of neonates with HIE
On conventional MRI the site of ischemic injury was identified and the severity of HIE was graded into three grades; (Table 2) [15].
1H-MRS Procedures
We used axial FLAIR images to locate the multivoxels for the 1H-MRS studies using a spin-echo (SE) mode sequence with long Echo Time (TE) [Repetition Time/Echo Time (TR/TE) = 1600/144] and short TE (TR/TE= 1600/35). Water suppression was achieved with chemical shift selection (CHESS) technique. Proton spectra were acquired in an area of 8 pixels that covered basal ganglia and thalami as regions of interest. The multi-voxel 1H-MRS data were processed by the spectroscopic analysis package on the workstation. The acquisition time for each sequence was 7 minutes 54 seconds. The resonances of main metabolites were quantified as follows: the glutamate-glutamine (Glx) peak at 3.75 ppm, the N-acetyl aspartate (NAA) peak at 2.02 ppm, the creatine (Cr) peak at 3.02 ppm, the choline (Cho) peak at 3.20 ppm, and the lactate (Lac) doublet at 1.33 ppm. Relative concentrations of metabolites were related to peak-area and expressed with reference to Cr. Ratios of NAA/Cr, NAA/Cho, Cho/ Cr, Lac/NAA and Lac/Cr were measured for the examined neonates with HIE and were compared to those of the control.
1H-MRS grading of neonates with HIE
Depending on Lac/Cr ratio, severity of HIE was graded into: mild (Lac/Cr ratio lower than 0.5), moderate (Lac/Cr ratio between 0.5 and 1.5) and severe (Lac/Cr ratio greater than 1.5) [12].
Statistical Analysis
Statistical analysis was performed with Statistical Package for Social Science (SPSS version 18). Statistical presentation and analysis of the present study was conducted using the mean, standard deviation, student t- test, Chi-square test (χ2) and Analysis of variance (ANOVA) tests. Significant value was when p≤0.05 [16,17].
Results
Regarding the personal data of the patients and control groups (Table 3), there were no significant differences between the two groups (p>0.05).
As shown in table 4, neonates with HIE showed significantly decreased mean peak-ratios of the NAA/Cr, and NAA/Cho when compared to normal control (p=0.019, and 0.044 respectively). On the other hand, they showed significantly increased mean peak-ratios of the Cho/ Cr, Lac/Cr, and Lac/NAA when compared to normal control (p<0.001 for each).
Additionally, Table 5 showed that the 1H-MRS mean peak-ratio of Lac/Cr was none significantly higher in patients with mild HIE compared to normal control (p= 0.577), while it was significantly higher in patients with moderate and severe HIE compared to normal control (p<0.001 for each). Also, it was significantly higher in moderate compared to mild (p<0.001), severe compared to moderate (p= 0.034), and in severe compared to mild HIE (p<0.001 for each).
Moreover, Table 6 and Figure 1 showed a significant percent of agreement (66.67%) between the clinical staging and MRI grading of the neonates with HIE (p< 0.001).
As well, Table 7 and Figure 2 showed a significant percent of agreement (76.67%) between the clinical staging and 1H-MRS grouping of the neonates with HIE (p< 0.001).
Moreover, Tables 8,9 showed a significant percent of agreement (63.3) between the 1H-MRS grouping and MRI grading of the neonates with HIE (p< 0.001).
Cases
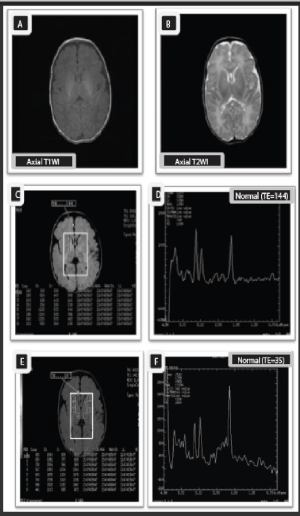
Case 1
Ten-days old normal full term male neonate (normal control).
Unenhanced axial T1WI (A) and axial T2WI (B),show normal progress of myelination with normally increased signal intensity of posterior limb of internal capsule by T1WI, and decreased its signal intensity by T2WI relative to basal ganglia and thalamus.
MRS multivoxel picture (C), and MRS spectrum (D) - (TE 144), as well as MRS multivoxel picture (E), and MRS spectrum (F)-(TE35) at the basal ganglia show normal NAA/Cr, NAA/Cho, and Cho/ Cr ratios with slight elevation of the Cho peaks which is accepted in neonates (NAA/Cr= 1.2, NAA/ Cho= o.7, Cho/Cr= 1.7, Lac/ Cr= 0.2).
Case 2
Two-days old female neonate with gestational age of 37 weeks presented clinically with Sarnat stage I normal signal intensity of both thalami. Axial T2WI (B) shows normally hypointense signal of posterior limb of internal capsule corresponds to normal progress of myelination. multivoxel picture (E), and MRS spectrum (F)-(TE35) at the basal ganglia show decreased peaks of NAA and slightly increased Cho, lactate, and Glx peaks which denoting hypoxic ischemic brain injury. Moreover, MRS revealed decreased NAA/Cr, and NAA/Cho ratios with increased Cho/ Cr, Lac/NAA and Lac/ Cr ratios in comparison to normal (NAA/Cr= 1.0, NAA/ Cho= o.5, Cho/Cr= 2.03, Lac/ Cr ratio= 0.4). Mild elevation of Lac/ Cr ratio denotes mild grade of HIE.
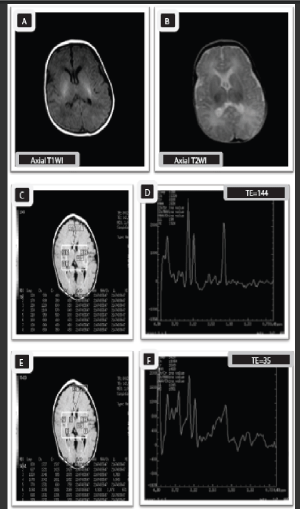
Case 3
Fifteen -days old male neonate with gestational age of 38 weeks presented clinically with Sarnat stage II hyperintensities with punctuate hyperintense foci in the basal ganglia. Prominent subarachnoid spaces. Moreover, early subacute subgalial hematoma in left parieto-occipital region is seen.
MRS multivoxel picture (C), and MRS spectrum (D) - (TE 144), as well as MRS, spectrum (E)-(TE35) at the basal ganglia show decreased peaks of NAA and elevated peaks of Cho, Lac, and Glx due to hypoxia –ischemia. Furthermore, MRS reveals decreased ratios of NAA/Cr and NAA/ Cho with increased Cho/ Cr and Lac/ Cr ratios in comparison to normal ratios (NAA/Cr= 0.7, NAA/ Cho= o.3, Cho/Cr= 2.2, Lac/ Cr ratio= 0.8 ( 0.5 and 1.5). The Lac/Cr ratio represents moderate grade of HIE.
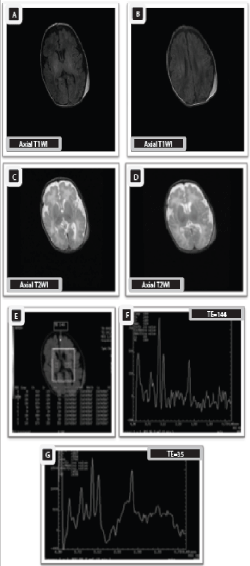
Case 4
Twelve -days old male neonate with gestational age of 38.5 weeks presented clinically with Sarnat stage III increased signal intensity in both basal ganglia and thalami. Decreased signal intensity of the posterior limb of internal capsule (absent posterior limb sign) is seen denoting poor thalami with hyperintense signal of posterior limb of internal capsule, indicative of poor myelination with hypoxic ischemic injury. Axial T2*GRE image (D) shows punctuate areas of abnormal low signal intensity in both thalami and putamen (Blooming artifact), indicating punctate areas of hemorrhage which exhibit hyperintense signal by T1WI and hypointense signal by T2WI denoting early subacute stage of accompanying hemorrhage. The MRI findings are consistent with severe neonatal hypoxic ischemic injury associated with hemorrhage.
multivoxel picture (G), and MRS spectrum (H)-(TE35) at the basal ganglia show decreased peaks of NAA and elevated peaks of Cho, Lac and Glx due to hypoxia –ischemia with marked increase of myo-inositol level in short TE sequences with increase in lipids due to necrosis. Furthermore, the NAA/Cr and NAA/Cho ratios are markedly decreased with significantly elevated Cho/Cr and Lac/Cr ratios in comparison to normal ratios (NAA/Cr= 0.6, NAA/Cho= o.2, Cho/Cr= 3.4, Lac/Cr ratio= 2.6). The Lac/Cr ratio represents severe grade of HIE.
Discussion
Hypoxic ischemic encephalopathy is a clinically defined syndrome of disturbed neurological function in the earliest days of life and usually affects the full term infant. It is one of the most common causes of severe neurologic deficits in children causing significant mortality and long-term morbidity.[18] Although the exact pathophysiology of HIE is not completely understood, the lack of sufficient blood flow along with decreased oxygen content in the blood produce a significant disturbance of normal cerebral autoregulation and a diffuse brain injury.[19,20] The predilection site and severity of HIE are correlated with the duration of HI and variation of cerebral metabolites levels. In full term neonates with HIE, accurate and early identification as well as characterization of the severity and extent of brain injury depend upon selecting the appropriate neuroimaging modalities [12,21].
The signal abnormalities of basal ganglia can be considered as an indicator of the severity of neonatal HIE, while the signal abnormality of thalamus is considered as a relatively specific and sensitive indicator of ischemic brain injury caused by hypoxia[21,22]. In the study of Thayyil, et al [6], the MRI of neonates with mild HIE showed normal basal ganglia with variable degrees of cortical signal abnormalities, while patients with most severe HIE showed mixed cortical and basal ganglia signal abnormalities. Similar findings were seen in the present study, except in one female neonate showed slight increase in the signal intensity of the basal ganglia by T1WI with normal thalamus. This might be attributed to her younger gestational age, younger age at time of examination and the history of prolonged labor.
The 1H-MRS, which is based on the phenomena of chemical shift, can reflect the main metabolite concentrations in the brain and play an important role in the determination of metabolic disturbance and impairment of neuronal functions which are caused by HIE. Moreover, 1H-MRS gives a good simultaneous imaging data from the cortex, basal ganglia and white matter [8].
In HIE, cerebral lactate (Lac) rises as a result of anaerobic metabolism, then it is rapidly cleared on resuscitation to be followed by a secondary increase after 12–24 hours because of renewed production of Lac in brain tissue rather than entry via the circulation [22,23]. The Cr was chosen as the metabolite of reference in 1H-MRS as it is not significantly changed after the onset of HIE. In the basal ganglia, an increased Lac/Cr peak-area ratio provides an early indication of the severity of brain injury in neonates following perinatal HIE, before changes are apparent on conventional MRI [24].
As such in other studies [2,12], we used Lac/Cr ratio as a marker for grading of the severity of neonatal HIE. So, the peak-area ratio of Lac/Cr was chosen as the marker of Lac levels in the brain and index of severity in cases of HIE. By using Lac/Cr ratio, neonatal HIE can be graded into mild (Lac/ Cr < 0.5), moderate (0.5
The present study showed a significant decrease of the mean peak-ratios of NAA/Cr and NAA/Cho as well as a significant increase of mean peak-ratios of the Cho/Cr, Lac/Cr and Lac/NAA, reflecting a poor neurodevelopmental outcome. This is also reported by other authors [2,12,24], who considered the lactate ratios as prognostic indices.
The NAA is a marker of neuro-metabolic fitness. Reduced NAA concentration is generally interpreted as neuronal/axonal dysfunction and/or loss. For normal cell function Cho is essential and it directly affects nerve cell signaling and cell membrane metabolism. It is a putative marker of cellularity and cell turnover. Reduction of Cho may be associated with decreased cellularity and delayed myelination [25].
In contrary to the present study and that of Cheong, et al.[2], which revealed a direct proportional increase of Cho peaks with the severity of HIE, Fan et al. [12], reported a decline in the peaks of Cho in HIE patients compared to their normal control. This difference might be due to the relatively smaller number of controls and younger gestational age of the patients in the study of Fan, et al.
Our results revealed reduction in peaks of Cr and NAA in most of patients with mild HIE, and in all patients with moderate and severe HIE compared to the control group. Moreover, peaks of lipid and Glx were obviously elevated in most of neonates with mild and moderate HIE and in all neonates with severe HIE. The elevated peaks of lactate showed typical double peaks appearance with a zigzag-like appearance of the elevated peaks of Glx. This is also reported by other studies [12,21,25].
Additionally, our results showed that the sensitivity for MRI was 66.67%, specificity was 75%, accuracy was 77.78%, PPV was 80% and NPV was 75%. Meanwhile, the sensitivity for 1H-MRS was 92%, specificity was 75%, accuracy was 84.44%, PPV was 82.14% and NPV was 88.24%. Also, the percent of agreement between the 1H-MRS and MRI grading of HIE neonates was 63.3, and the percent of agreement between clinical staging of the HIE neonates and MRI grading was 66.67, while it was 76.67 regarding 1H-MRS grading.
Hence, the evaluation of MRI and 1H-MRS in detecting HIE showed that 1HMRS got better percent of agreement, sensitivity, accuracy, PPV and NPV than MRI. In addition, there was more false negative results in MRI (5 cases) than with 1H-MRS (2 cases). Also, there was less true positive results in MRI (20 cases) than with 1HMRS (23 cases). This was well apparent in case (2) that shows nearly normal MRI but with mild affection in the 1H-MRS view. The low value of sensitivity and accuracy may be explained by the small number of cases.
Conclusion
1H-MRS detected more percent of agreement, true positive and less false negative cases than MRI. Despite the equal specificity between them, still 1H-MRS got more sensitivity, accuracy, PPV and NPV than MRI i.e. when we have to choose early and single investigation it is recommended to choose 1H-MRS which provide, even with unremarkable conventional MRI, further insight into the diagnostic protocol and the potential for possible therapeutic intervention in such serious condition especially in mild and moderate cases. It plays an important role in the grading of the severity in HIE. The limitation of our study included small number of cases due to the technical and medical difficulties during MRI and 1H-MRS examination especially with moderate and severe cases despite the long period of the study (49 months). So, multi-center large number studies are recommended for more accurate and specific statistics.
- Ferriero, Donna M (2004) Neonatal brain injury. N Engl J Med 351: 1985-95. Link: https://goo.gl/yv4jQj
- Cheong JL, Cady EB, Penrice J, Wyatt JS, Cox IJ et al. (2006) Proton MR spectroscopy in neonates with perinatal cerebral hypoxic-ischemic injury: Metabolite peak-area ratios, relaxation times and absolute concentrations. AJNR Am J Neuroradiol 27: 1546 -1554. Link: https://goo.gl/BQRy1D
- [Guideline] (1992) American Academy of Pediatrics. Relation between perinatal factors and neurological outcome. In: Guidelines for Perinatal Care. 3rd ed. Elk Grove Village, Ill: American Academy of Pediatrics 221-224. Link: https://goo.gl/jCAaCS
- Low JA (1997) Intrapartum fetal asphyxia: definition, diagnosis, and classification. Am J Obstet Gynecol 176: 957-959. Link: https://goo.gl/61WEgb
- [Guideline] (1996) Committee on fetus and newborn, American Academy of Pediatrics and Committee on obstetric practice, American College of Obstetrics and Gynecology. Use and abuse of the APGAR score. Pediatr 98: 141-142. Link: https://goo.gl/ezxjEm
- Thayyil S, Chandrasekaran M, Taylor A, Bainbridge A, Cady EB et al. (2010) Cerebral magnetic resonance biomarkers in neonatal encephalopathy: A meta-analysis. Pediatrics 125: e382-e395 Link: https://goo.gl/qFz7iL
- Cooke R (2005) Head cooling in neonatal hypoxic-ischemic encephalopathy. Lancet 365: 632-634. Link: https://goo.gl/QnyA6z
- Shanmugalingam S, Thornton JS, Iwata O, Bainbridge A, O'Brien FE et al. (2006) Comparative prognostic utilities of early quantitative magnetic resonance imaging spin-spin relaxometry and proton magnetic resonance spectroscopy in neonatal encephalopathy. Pediatrics 118:1467-77. Link: https://goo.gl/e1hDrX
- Ment LR, Bada HS, Barnes P, Grant PE, Hirtz D et al. (2002) Practice parameter: neuroimaging of the neonate- report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child. Neurology 58: 1726-1738 Link: https://goo.gl/66JkEs
- Chau V, Poskitt KJ, Miller SP (2009) Advanced neuroimaging techniques for the term newborn with encephalopathy. Pediatr Neurol 40:181-188. Link: https://goo.gl/1YRwX2
- Panigrahy A, Bluml S (2007) Advances in magnetic resonance neuroimaging techniques in the evaluation of neonatal encephalopathy. Top Magn Reson Imaging 18: 3-29. Link: https://goo.gl/Adjd25
- Fan G, Wu Z, Chen L, Guo Q, Ye B et al. (2003) Hypoxia-ischemic encephalopathy in full-term neonate: correlation proton MR spectroscopy with MR imaging. Eur J Radiol 45: 91-98. Link: https://goo.gl/sdfWN5
- Sarnat HB, Sarnat MS (1976) Neonatal encephalopathy following fetal distress: a clinical and electroencephalographic study. Arch Neurol 33: 696-705 Link: https://goo.gl/pGXcXS
- Rivkin MJ (1997) Hypoxic-ischemic brain injury in the term newborn: neuropathology, clinical aspects and neuroimaging. Clin Perinatol.; 24: 607-625. Link: https://goo.gl/CHxoAg
- Barkovich AJ, Westmark K, Partridge C, Sola A, Ferriero DM (1995) Perinatal asphyxia: MR findings in the first 10 days. AJNR Am J Neuroradiol 16: 427-438. Link: https://goo.gl/NzmYXd
- Dawson BD, Trapp RG (2001) Reading the medical literature: Basic & Clinical Biostatistics. Lange Medical Book/ McGraw - Hill. Medical Publication Division, New York. 3rd edition;.Chapter 7-9, p. 161-218 and Chapter 13, p. 305-14. Link: https://goo.gl/q4KJhd
- Williams B (1993) Biostatistics: Concepts and applications for biologists. Chapman & Hall, London 7-9: 94-177. Link: https://goo.gl/C5rrYJ
- Edwards AD, Nelson KB (1998) Neonatal encephalopathies. Time to reconsider the cause of encephalopathies. BMJ 317:1537-1538 Link: https://goo.gl/Y1C4As
- Huppi PS (2002) Advances in postnatal neuroimaging: relevance to pathogenesis and treatment of brain injury. Clin Perinatol 29: 827-856. Link: https://goo.gl/1VZicW
- Chao CP, Zaleski CG, Patton AC (2006) Neonatal hypoxic-ischemic encephalopathy: multimodality imaging findings, Radio Graphics. 26: S159-S172 Link: https://goo.gl/FqNSgL
- Heinz ER, Provenzale JM (2009) Imaging findings in neonatal hypoxia: A Practical Review. AJR Am J Roentgenol 192: 41-47. Link: https://goo.gl/Jv6E7S
- Penrice J, Cady EB, Lorek A, Wylezinska M, Amess PN et al. (1996) Proton magnetic resonance spectroscopy of the brain in normal preterm and term infants, and early changes after perinatal hypoxia-ischemia. Pediatr Res 40: 6-14. Link: https://goo.gl/MmDkjQ
- Penrice J, Lorek A, Cady EB, Amess PN, Wylezinska M et al. (1997) Proton magnetic resonance spectroscopy of the brain during acute hypoxia-ischemia and delayed cerebral energy failure in the newborn piglet. Pediatr Res 41: 795-802. Link: https://goo.gl/ca1zaf
- Soul JS, Robertson RL, Tzika AA, du Plessis AJ, Volpe JJ et al. (2001) Time course of changes in diffusion weighted magnetic resonance imaging in a case of neonatal encephalopathy with defined onset and duration of hypoxic-ischemic insult. Pediatrics 108: 1211-1214. Link: https://goo.gl/HQ9yKm
- Boichot Ch, Walker PM, Durand Ch, Grimaldi M, Chapuis S et al. (2006) Term neonate prognoses after perinatal asphyxia: contributions of MR Imaging, MR Spectroscopy, relaxation times, and apparent diffusion coefficients. Radiology 239: 839-848. Link: https://goo.gl/9g942L
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
