Journal of Neurology, Neurological Science and Disorders
Absence of CHRDL1 and FOXC1 sequence changes in two brothers with Megalocornea-Mental Retardation Syndrome
Gebril OH1*, Cheong SS2, Hardcastle AJ2, Abdelraouf ER1, Eid SR3 and Elsaied M1
2Institute of Opthalomology, University Collegue London, UK
3Pediatric department, Research Institute of Ophthalmology, Cairo, Egypt
Cite this as
Gebril OH, Cheong SS, Hardcastle AJ, Abdelraouf ER, Eid SR, et al. (2017) Absence of CHRDL1 and FOXC1sequence changes in two brothers with Megalocornea-Mental Retardation Syndrome. J Neurol Neurol Sci Disord 3(1): 028-032. DOI: 10.17352/jnnsd.000017Megalocornea is a defining feature of megalocornea-mental retardation (MMR) syndromealso calledNeuhäuser syndrome, a rare condition of unknown etiology.
Here we describe a family with two sons, who were diagnosed withmegalocornea, mild mental subnormality and microcephaly, in addition to limb anomalies in the form of clinodactyly in the younger brother, while extradigit and clinodactyly was seen in the older brother. Parents are second degree cousins with no obvious family history of similar problems.Mutations in CHRDL1 are known to cause X-linked megalocornea (MGC1) and FOXC1 mutations cause a wide range of syndromic or non-syndromic anterior segment dysgeneses (ASD) phenotypes. Sanger sequencing of CHRDL1 and FOXC1 did not identify any potential disease causing variants in this family.
Conclusions: Megalocornea-mental retardation (MMR) syndrome isa genetically and phenotypically heterogeneous condition. In this Egyptianfamily, CHRDL1 and FOXC1 have been excluded as the cause. Next generation sequencing is required to identify the genetic cause of the syndrome in this family
Background
X-linked megalocornea (MGC1) is a genetically homogeneous condition, characterized by congenital bilateralenlarged corneaswith a horizontal white-to-white diameter of ≥13 mm (after the age of 2 years), deep anterior chamber depth and reduced central corneal thickness (CCT) without increased intraocular pressure (IOP).Later onset clinical features includemosaic corneal degeneration, arcus juvenilis, lens dislocation, mild iris atrophy with pigment dispersion, and pre-senile cataracts. MGC1 is caused by mutations in the CHRDL1 (chordin –like 1) gene [1-3]. CHRDL1 encodes ventroptin, a secreted bone morphogenic protein (BMP) antagonist [4] and is expressed in the developing human cornea and anterior segment [1].
Megalocornea–mental retardation (MMR) syndrome is a rare and phenotypically heterogeneous condition, in which megalocornea is a defining feature. Extraocular features associated with MMR include intellectual disability (ID), hypotonia, seizures, and craniofacial abnormalities. Clinical features of previously reported MMR patients are summarized in Table 1. Due to the consistent presentation of megalocornea and ID in MMR patients, del Giudice suggested that these two features should be the minimal diagnostic criteria for MMR syndrome. Other features such as short stature, seizures, neurological symptoms, microcephaly or macrocephaly, and minor anomalies are considered asadditional clinical manifestations [5]. Transient hypothyroidism, epilepsy, cerebral palsy with choreoathetotic movements, and brain malformationshave also been described previously [6]. The genetic cause of MMR is still poorly understood. A previous study identified amissense CHRDL1 mutation in a patient with MMR syndrome. However, the lack of extraocular features in other MGC1 patients with CHRDL1 mutations suggests that the CHRDL1 mutation is only causative of the megalocornea phenotype but not the associated extraocular manifestations [3].
FOXC1 (Fork-head box C1) is a member of the forkhead family of transcription factors, which has a major role in epithelial‑mesenchymal transition, a process by which epithelial cells lose cell-to-cell adhesion and migrate at various stages of eye and nervous system development [7]. Mutations in the FOXC1 gene have been reported to cause a spectrum of autosomal dominant anterior segment dysgeneses (ASD), including Axenfeld-Rieger syndrome (ARS), Rieger anomaly, Peters anomaly, primary congenital glaucoma (PCG), iris hypoplasia, aniridia, with or without extraocular featuressuch as heart defects, craniofacial abnormalities and pituitary abnormalities [8-11]. Variable expressivity and incomplete penetrance for the associated phenotypes have also been observed [12-14].
Study Subjects and Clinical Description
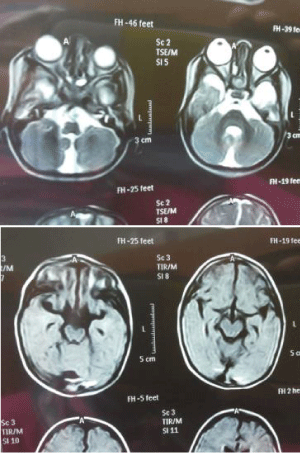
All studies were conducted in accordance with the principles of the Declaration of Helsinki. The study was approved by the local ethics committees. Informed written consent, including permission to publish photographs, was obtained from all participating individuals or parental guardians on behalf of the minors enrolled in this study. Blood samples were donated and genomic DNA was extracted from peripheral blood lymphocytes using conventional methodologies. Patients were clinically assessed by experienced ophthalmologists. Standard evaluation consisted of detailed ophthalmic examination, and the additional measurement of the axial length of the eye and imaging of the anterior segment of the eye were performed. The affected brothershad standard MRI with a 1.5 Tesla scanner.In the younger brother, MRI showed mild brain atrophy, and retro-cerebellar cyst (Figure 1). Metabolic screening as well as abdominal and pelvic ultrasound were normal. Thyroid profile and EEG showed no deviations from normal. In the older brother, brain scan, thyroid profile and EEG were normal.
The younger brother was born to unaffected parents who are first cousins. He presented at the age of 6 months with delayed developmental milestones (head support by 10 months) and decreased weight gain with no history of convulsions. Dysmorphic features, hypertelorism, prominent ears, and tower shaped head were noted in addition to clinodactyly. Following initial presentation, progressive delay in developmental milestones in relation to age was encountered.
He is the product of normal pregnancy with no complications, born by normal vaginal delivery, birth weight was 3.000 kg with no neonatal unit admission needed. The mother had taken folic acid supplement in the 1st trimester and inconsistent iron and calcium supplements. Foetal movements were felt by 16th week gestation with normal frequency and normal pregnancy scans. He is currently (2 years of age) suffering delayed walking (walking with support) and speech is developing more or less appropriately (about 10 words).
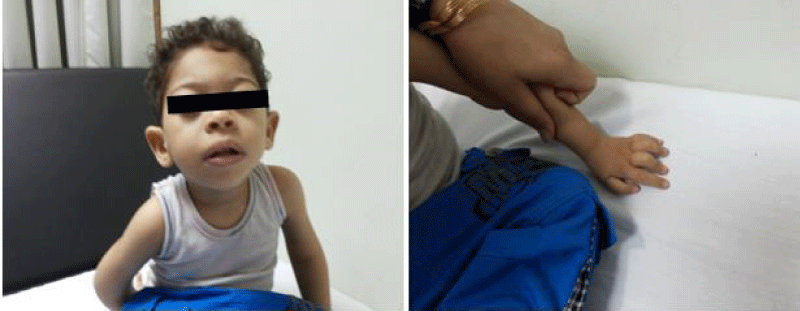
Hypertelorism, megalocornea, long philtrum, broad nasal root, recession of chin and forehead, fusiform fingers of hands, clinodactyly 2nd-3rd fingers (Lt hand) are evident (Figure 2). Complete simian crease in the left hand and low set prominent ears are present. Mouth examination showed normal palate, uvula and teeth. He has evidence of scoliosis with normal abdomen and chest.
Neurological examination revealed decreased motor power in both upper and lower limbs (grade II to grade IV) with moderate hypotonia, and normal deep reflexes. No obvious abnormalities in external genitalia were seen.
Ophthalmological examination revealed a corneal diameter of 14mm in Rt eye and 15 mm in left eye, moderate myopia, hypotrichosis, normal ocular reflexes with normal fundus and intraocular pressure. Head circumference was 43.5 cm (far < 5th), Height was 82 cm (0.4th) and weight was 8.5 kg (<0.4th).
The older brother has better developmental history with mild delay compared to the younger brother. He has 5 years, increased corneal diameter (13mm bilaterally), normal fundus, myopia with right convergent squint, hypertelorism, long philtrum, and mild hypotonia with normal reflexes (Figure 3).
Broad nasal root was evident, with history of an extra finger beside the little finger on the left hand, which was removed at the age of 6 months. There were variable toe insertion levels in both feet. Chest, abdomen, genitalia, skin, and back were normal. His head circumference was 49 cm (far < 5thcentile), height was 98 cm (about 25th centile) and his weight was 15 kg (25th- 50thcentile) at birth. He started walking at 1 2/12, had head support at the age of 7 months, and was sitting by 9 months of age. He had 4 words of speech at the age of 1 year.
He is the product of normal pregnancy with normal scans and foetal movements, and normal vaginal delivery. Birth weight was 3.100kg. He had mild neonatal jaundice and was incubated for3 days for phototherapy.
Molecular genetic analysis
All CHRDL1and FOXC1exons and intron-exon boundaries were directly Sanger sequenced from PCR amplimersas previously described [1,34]. No mutation was identified in CHRDL1or FOXC1in the two affected brothers.
Discussion and Conclusion
MMR syndromeis a phenotypically heterogeneous condition, with megalocornea and ID as pathognomonic features. Other clinical manifestations include neurological, craniofacial, digital anomalies and so forth. The genetic cause of MMR is yet to be fully elucidated. The similar ratio of reported male to female patients indicates no sex bias and therefore suggests an autosomal recessive or a de novo inheritance mode [16, 5, 21, 24, 25]. Interestingly, a recent study identified a missense mutation, p.(Cys155Tyr) in the CHRDL1 gene in a male patient with a diagnosis of MMR syndrome [3]. However, extraocular phenotypes have not been observed in any previously reported MGC1 patients with confirmed CHRDL1 mutations. This finding suggests that in this patient, MMR may be a digeniccondition, where CHRDL1 mutation accounts for the ocular phenotype, and the genetic cause of the extraocular phenotypes remains unexplained. FOXC1 is a ubiquitously expressed transcription factor, which regulates cell viability and resistance to oxidative stress in the eye [35,36]. FOXC1 mutations have been associated with a wide range of ASD, such as ARS, a genetically diverse group of developmental disorders, including peters anomaly, aniridia, and PCG, which affect several anterior segment structures of the eye, with a high risk of blindness due to glaucoma [37]. In addition to ocular findings, systemic anomalies such as ID, hypotonia, facial dysmorphism, and bilateral sensorineural deafness have been described in patients with 6p25microdeletion, which encompassed the FOXC1 gene [38]. These mutations were encountered to be associated with carcinogenesis mainly breast cancer and melanoma by activating MST1R/PI3K/AKT [39-40]. Follow up of affected brothers is done regularly by systemic clinical examination and some laboratory tests including full blood count and occult blood in stools.
In this study, two brothers diagnosed with MMR syndrome were recruited suggesting X-linked or recessive inheritance of the condition. No mutation was identified in the CHRDL1 or FOXC1 genes by Sanger sequencing of the coding regions, howeverthe genes cannot be fully excluded as copy number variations or mutations in non-coding regionswere not tested using this approach. Due to the rarity of this condition, next generation sequencing (NGS) in a cohort of MMR patients is essential to identify the underlying genetic cause(s) of this poorly understood syndrome.
Funding
This work was funded by UCL Opthalmology institute, London and National Research centre, Egypt.
- Webb TR, Matarin M, Gardner JC, Kelberman D, Hassan H, et al. (2012) X linked megalocornea caused by mutations in CHRDL1 identifies an essential role for ventroptin in anterior segment development. American journal of human genetics 90: 247–259. Link: https://goo.gl/7KKT9i
- Han J, Young JW, Frausto RF, Isenberg SJ, Aldave AJ (2015) X linked Megalocornea Associated with the Novel CHRDL1 Gene Mutation p.(Pro56Leu*8). Ophthalmic Genet 36:145-148. Link: https://goo.gl/ojz7Cv
- Davidson AE, Cheong SS, Hysi PG, Venturini C, Plagnol V, et al. (2014) Association of CHRDL1 mutations and variants with X-linked megalocornea, Neuhäuser syndrome and central corneal thickness. PLoS One. 9: e104163. Link: https://goo.gl/QN3LHq
- Gao WL, Zhang SQ, Zhang H, Wan B, Yin ZS (2013) Chordin-like protein 1. Mol Med Rep 7:1143-1148. doi: 10.3892/mmr.2013.1310. Link: https://goo.gl/G4afjc
- Del Giudice E, Sartorio R, Romano A, Carrozzo R, Andria G (1987) Megalocornea and mental retardation syndrome: two new cases. Am J Med Genet 26: 417-420. Link: https://goo.gl/xHZchs
- Margari L, Presicci A, Ventura P, Buttiglione M, Dicuonzo F, et al. (2006) Megalocornea and mental retardation syndrome: clinical and instrumental follow-up of a case. J Child Neurol 21: 893-896. Link: https://goo.gl/i65uVM
- Ou-Yang L, Xiao SJ, Liu P, Yi SJ, Zhang XL, et al. (2015) Forkhead box C1 induces epithelial‑mesenchymal transition and is a potential therapeutic target in nasopharyngeal carcinoma. Mol Med Rep doi 12: 8003-8009 Link: https://goo.gl/JmNkxh
- Nishimura DY, Swiderski RE, Alward WL, Searby CC, Patil SR, et al. (1998) The forkhead transcription factor gene FKHL7 is responsible for glaucoma phenotypes which map to 6p25. Nature Genetics, 19:140–147. Link: https://goo.gl/ukgKvk
- Lehmann OJ, Ebenezer ND, Jordan T, Fox M, Ocaka L, et al. (2000) Chromosomal duplication involving the forkhead transcription factor gene FOXC1 causes iris hypoplasia and glaucoma. American Journal of Human Genetics; 67:1129–1135. Link: https://goo.gl/TbXoWz
- Nishimura DY, Searby CC, Alward WL, Walton D, Craig JE, et al. (2001) A spectrum of FOXC1 mutations suggests gene dosage as a mechanism for developmental defects of the anterior chamber of the eye. American Journal of Human Genetics 68: 364–372. Link: https://goo.gl/4Z62tB
- Khan AO, Aldahmesh MA, Al-Amri A (2008) Heterozygous FOXC1 mutation (M161K) associated with congenital glaucoma and aniridia in an infant and a milder phenotype in her mother. Ophthalmic Genetics 29: 67–71. Link: https://goo.gl/EXP9DQ
- Fitch N, Kaback M (1978) The Axenfeld syndrome and the Rieger syndrome. Journal of Medical Genetics 15: 30–34. Link: https://goo.gl/kfYPYM
- Alward WL (2000) Axenfeld-Rieger syndrome in the age of molecular genetics. American Journal of Ophthalmology 130: 107–115. Link: https://goo.gl/3dT7aG
- Lines MA, Kozlowski K, Walter MA (2002) Molecular genetics of Axenfeld-Rieger malformations. Human Molecular Genetics 11: 1177–1184. Link: https://goo.gl/88wAKG
- Frank Y, Ziprkowski M, Romano A, Stein R, Katznelson MB, et al. (1973) Megalocornea associated with multiple skeletal anomalies: a new genetic syndrome?. J Genet Hum 21: 67-72. Link: https://goo.gl/3DoLU9
- Neuhauser G, Kaveggia EG, France TD, Opitz JM (1975) Syndrome of mental retardation, seizures, hypotonic cerebral palsy and megalocorneae, recessively. Z Kinderheilkd 120: 1-18. Link: https://goo.gl/gPtKf8
- Ohno K, Suzuki Y (1978) A case of the syndrome of megalocornea, mentalretardation and seizures (Neuhauser). Shounika Rinshou 31:1977–1979.
- Schmidt R, Rapin L (1981) The syndrome of mental retardation and megalocornea. Am J Hum Genet 30:90A. Link: https://goo.gl/Etcp1v
- Raas-Rothshild A, Berkenstadt M, Goodman RM (1988) Megalocornea and mental retardation syndrome. Am J Med Genet 29: 221– 223. Link: https://goo.gl/hbKq6v
- Grønbech-Jensen M (1989) Megalocornea and mental retardation syndrome: A new case. Am J Med Genet 32:468–469. Link: https://goo.gl/4haUgD
- Frydman M, Berkenstadt M, Raas-Rothschild A, Goodman RM (1990) Megalocornea, macrocephaly, mental and motor retardation (MMM). Clin Genet 38:149–154. Link: https://goo.gl/AuhUUr
- Kimura M, Kato M, Yoshino K, Ohtani K, Takeshita K (1991) Megolocornea: mental retardation syndrome with delayed myelination. Am J Med Genet. 38: 132-133. Link: https://goo.gl/EshVW6
- Temtamy S, Abdel-Hamid J, Hussein F (1991) Megalocornea mental retardation syndrome (MMR): Delineation of a new entity (MMR-2). Am J Hum Genet 49: 125A. Link: https://goo.gl/1k9LAq
- Santolaya JM, Griyalbo A, Delgado A, Erdozain G (1992) Additional case of Neuhauser megalocornea and mental retardation syndrome with retardation syndrome: An additional case. Am J Med Genet 52: 56 Link:
- Verloes A, Journel H, Elmer C, Misson JP, Le Merrer M, et al. (1993) Heterogeneity versus variability in megalocornea-mental retardation (MMR) syndromes: Report of new cases and delineation of 4 probable types. Am J Med Genet 46: 132–137. Link: https://goo.gl/wJE8SX
- Antinolo G, Rufo M, Borrego S, Morales C (1994) Megalocornea-mental Assignment of a locus (GLC3A) for primary congenital glaucoma (Buphthalmos) to 2p21 and evidence for genetic heterogeneity. Genomics 30: 171–7. Link:
- Gibbs ML, Wilkie AOM, Winter RM ( 1994 ) Megalocornea, developmental retardation and dysmorphic features: Two further patients. Clin Dysmorphol; 3: 132–138. Link: https://goo.gl/FJC6tu
- Barisic I, Ligutic I, Zergollern L (1996 ) Megalocornea-mental retardation syndrome: Report of a new case. J Med Genet 33: 882–883. Link: https://goo.gl/zc6Ptb
- Naritomi K, Chinen Y, Tohma T (1997) Megalocornea-mental retardation syndrome: An additional case report. Jpn J Hum Genet 42: 461–465. Link: https://goo.gl/p9jBAK
- Tominaga N, Kondoh T, Kamimura N (1999) A case of megalocorneamental retardation syndrome complicated with bilateral sensorineural hearing impairment. Pediatr Int 41:392–394. Link: https://goo.gl/HuJyTR
- Sarkozy A, Mingarelli R, Brancati F, Dallapiccola B (2002) The syndrome of mental retardation and megalocornea. Am J Hum Genet 30:90 A. Link:
- Balci S, Teksam O, Gedik S (2002) Megalocornea, macrocephaly, mental and motor retardation: MMMM syndrome (Neuhauser syndrome) in two sisters with hypoplastic corpus callosum. Turk J Pediatr 44: 274-277. Link: https://goo.gl/b9yrxW
- Derbent BM, Oto S, Alehan F (2004) Megalocornea-mental retardation (MMR or Neuhauser) syndrome: Another case associated with cerebral cortical atrophy and bifid uvula. Genet Couns 15: 477– 480. Link: https://goo.gl/16G7QT
- Mears AJ, Jordan T, Mirzayans F, Dubois S, Kume T, et al. (1998) Mutations of the forkhead/winged-helix gene, FKHL7, in patients with Axenfeld-Rieger anomaly. American Journal of Human Genetics 63: 1316–1328. Link: https://goo.gl/STTsUx
- Pierrou S, Hellqvist M, Samuelsson L, Enerbäck S, Carlsson P (1994) Cloning and characterization of seven human forkhead proteins: binding site specificity and DNA bending. The EMBO Journal 13: 5002–5012. Link: https://goo.gl/yop3rj
- Berry FB, Skarie JM, Mirzayans F, Fortin Y, Hudson TJ, et al. (2008) FOXC1 is required for cell viability and resistance to oxidative stress in the eye through the transcriptional regulation of FOXO1A. Human Molecular Genetics 17: 490–505. Link: https://goo.gl/aQJXYS
- Idrees F, Vaideanu D, Fraser SG, Sowden JC, Khaw PT (2006) A review of anterior segment dysgeneses. Surv Ophthalmol. 51: 213-231. Link: https://goo.gl/pWGSyT
- Kapoor S, Mukherjee SB, Shroff D, Arora R (2011) Dysmyelination of the cerebral white matter with microdeletion at 6p25. Indian Pediatr. 48: 727-729. Link: https://goo.gl/BV9qY9
- Xu YL, Yao R, Li J, Zhou YD, Mao F, et al. (2017 ) FOXC1 overexpression is a marker of poor response to anthracycline-based adjuvant chemotherapy in sporadic triple-negative breast cancer. Cancer Chemother Pharmacol. 79: 1205-1213. Link: https://goo.gl/bCiPsP
- Han B, Qu Y, Jin Y, Yu Y, Deng N, et al. (2015) FOXC1 Activates Smoothened-Independent Hedgehog Signaling in Basal-like Breast Cancer. Cui XCell Rep. 13: 1046-1058. Link: https://goo.gl/mHNqri
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
