Journal of Neurology, Neurological Science and Disorders
Cerebral Microbleeds in a Small Cohort of Patients with First Ever Lacunar Stroke. A 3Tesla MRI Longitudinal Case Series
Giosue Gulli1*, Francesca B Pizzini2, Giuseppe Moretto2, Alberto Beltramello2 and Nicola Micheletti3
2Depatment of Neuroscience, Unit of Neurology, Verona, Italy
3Department of Pathology and Diagnostic, Unit of Neuroradiology, Verona, Italy
Cite this as
Gulli G, Pizzini FB, Moretto G, Beltramello A, Micheletti N (2016) Cerebral Microbleeds in a Small Cohort of Patients with First Ever Lacunar Stroke. A 3Tesla MRI Longitudinal Case Series. J Neurol Neurol Sci Disord 2(1): 001-003. DOI: 10.17352/jnnsd.000006Background: High resolution imaging may help detect early development of cerebral microbleeds (CMB) and clarify mechanisms of small vessel disease (SVD).
Methods: 19 patients with lacunar stroke were recruited and 3T MRI scan was performed after the acute event and at four months. All patients were started on anti-platelet treatment after the first event. SVD severity was assessed by the age-related white matter changes (ARWMC) scale.
Results: First MRI - 13 patients had No or Mild SVD (ARWMC 1-2), 6 patients had Moderate to Severe SVD (ARWMC 3-6). 3/13 of NO-MILD patients and 5/6 of MOD-SEV patients have at least 1 CMB in the first scan.
Only 2 patients in the MOD-SEV group showed either enlarged or additional CMB at follow up. 3 patients in the MOD-SEV group and no patients in the NO-MILD group had at least one new asymptomatic subcortical ischemic lesion in the follow-up scan.
Conclusions: Rapid development of new CMB after starting antiplatelet therapy seems to be confined in patients with severe SVD only. Subclinical vascular events in patients with moderate to severe SVD occur even when antiplatelet treatment is started. Long term effects of antiplatelet treatment in MOD-SEV patients with GRE lesions must be tested.
Introduction and Methods
Cerebral microbleeds (CMB) are small haemosiderin deposits in the brain detected by blood sensitive MRI sequences. CMB are associated with cerebrovascular disease, in particular cerebral small vessel disease (SVD), and cerebral amyloid angiopathy [1]. On Gradient Echo MRI techniques CMB appear as black signal voids and the size may vary according to acquisition parameteVrs including field strength [2]. Presence of CMB often coincides with other radiological features of SVD (lacunar infarction, white matter hyperintensity and asymptomatic microinfarction) and may be regarded as a marker of underlying vasculopathy severity [3,4]. CMB may be deemed as an important prognostic factor since their presence correlates with higher risk of recurrent stroke, both ischemic and hemorrhagic [5]. Up to few months ago there was no prospective evidence about presence of CMB and risk of recurrent ischemic or haemorrhagic stroke, in patients with newly diagnosed lacunar stroke [5]. However there is still lack of neuroimaging correlates.
Herein we present a small prospective series of patients with newly diagnosed lacunar stroke started on secondary prophylaxis studied by early and delayed 3Tesla brain MRI.
Nineteen patients (mean age 59.8y, range 41-83y, 16males) presenting with a clinical lacunar stroke syndrome and with a corresponding lacunar stroke [1].
Patients had the 3Tesla MRI scan after 4.5 days from the index event. The structural MRI protocol, including standard MR sequences (DWI axial image, 3D T1 MP RAGE, 3D FLAIR, GRE) was applied to identify MR visible abnormalities. Axial diffusion-weighted images were acquired by using a spin-echo echo-planar imaging sequence (repetition time (TR)/echo time (TE), 2800/77 ms; field of view (FOV), 230 mm; matrix size, 128/128; slice thickness, 4 mm; averages, 2, maximum b value, 1000 s/mm2). The parameters of the other imaging sequences were as follows:
1. TOF: TR/TE 46/6.15, FOV, 215, matrix size, 208/512, slice thickness, 1.
2. 3D FLAIR: TR/TE of 5000/353, FOV, 270, matrix size of 220/256, slice thickness, 1.
3. GRE: TR/TE, 453/18, FOV, 230, matrix size 256/256, slice thickness, 5.
4. T1 MPRAGE: TR/TE 2300/3.93, FOV, 256, matrix 256/256, slice thickness, 1.
White matter disease grading was performed on the basis of visual grading scales (ARWMC) scale [1,6], by two stroke Physicians (NM and GG) independently. In few cases only there was not agreement between the two evaluations and the final grading was made after common evaluation. Microbleeds were defined as focal round or ovoid areas of signal loss devoid of signal hyperintensity on T1- or T2-weighted spin-echo images that show a blooming effect on Gradient Echo images [1]. With the same above cited principles the identification of microbleeds was made by the same Physicians. Ischemias were defined as acute hyperintense lesions on diffusion-weighted imaging with matched reduced signal on apparent diffusion coefficient map. Patients were prospectively followed up for 4-5 months (range 118 – 142 days) to identify recurrent TIA or stroke and reviewed at follow up by a consultant neurologist/stroke physician. At follow up patients underwent the same MRI protocol.
All patients were treated with a standard regimen of aspirin 100 mg. In patients with aspirin intolerance or contraindications clopidogrel 75mg od was used. Hypertension was treated at medical discretion aiming an average blood pressure below 140/90 mmHg. All patients were started on Atorvastatin 80 mg or Atorvastatin 40 mg when total cholesterol level was >5.2 mmol/L or normal respectively.
Results and Discussion
No patients recruited had history or signs of ischemic heart disease, structural cardiac diseases or carotid artery stenosis. 15 patients had been found to be hypertensive.
At follow up all patients were still on secondary prophylaxis regimen started after discharge and none of them showed blood pressure values above 140/90 mmHg. None of them had clinical recurrent events.
Five patients recruited in our study underwent systemic thrombolysis with rTPA. All MRI scans were performed after thrombolysis.
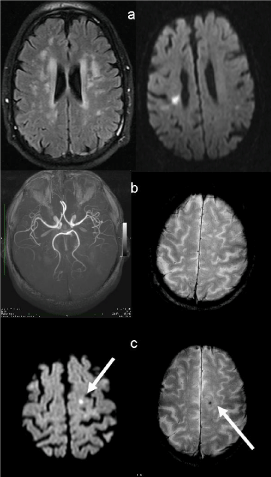
Figure 1 reports imaging of a representative case.
Table 1 summarizes the neuroradiology findings at time 1 and time 2. Of the 19 patients enrolled, two patients missed the follow up MRI. The first 3T MRI was performed 4 or 5 days from the index event. Thirteen patients with ARWMC<3 had been classified as having no to mild (No-Mild) white matter disease while six patients with a ARWMC scale >2 had moderate to severe (Mod-Sev) white matter disease.
None of the patients in the No-Mild group showed new CMB. Two patients in the Mod-Sev group showed, either an enlarged preexistent CMB, or a new CMB.
No patients in the No-Mild group showed incidental subcortical ischemias in the follow up scan, while 3 patients in the Moderate-Severe group showed acute incidental subcortical ischemias.
All patients who underwent thrombolysis did not showed signs of bleedings or microbleeds both in the first and in the follow up scan.
We found that in some patients with lacunar stroke and Moderate-Severe leukoaraiosis CMB developed in a relatively short period of time. On the other hand patients with No-Mild leukoaraiosis did not develop new CMBs over the 4 months follow up. To our knowledge this is the first longitudinal observational case series based on neuroimaging employing high field MRI techniques specifically designed for patients with small vessel disease stroke followed up in a short period of time. Other longer term longitudinal studies [7], showed the development of CMB in stroke patients in general.
It is well accepted that hypertension is a strong predictor of cerebral microbleeds and a number of studies suggest that hypertensive vasculopathy is the predominant underlying pathology of CMB [3,4]. In our patients CMB developed despite good blood pressure control. One possible explanation accounting for our observation may be regarded as accidental because leukoencephalopathic brain tissue would be susceptible to develop CMB over time regardless of antiplatelet therapy. This is indirectly confirmed by the observation that CMB are common findings in elderly patients with no history of cerebrovascular disease [8]. However, we rather support a second possible explanation which is that antiplatelet therapy may have facilitated micro hemorrhages in a vasculopathic vulnerable tissue.
Given the implications of increased risk of intracerebral hemorrhage in the presence of CMB [5] and some evidence suggesting that CMB are related to antithrombotic-related hemorrhage [9], starting antiplatelet therapy in patients with moderate to severe leukoaraiosis and CMB might not be the ideal therapeutic approach. Under this respect, it has to be emphasized that in our cohort of patients three subjects with Moderate-Severe leukoaraiosis showed incidental subcortical ischemias at follow up. One of these patients showed also a new developed CMB (Figure 1). As for our three patients with Moderate-Severe SVD, incidental subcortical ischemias may occur despite antiplatelet therapy suggesting that at least part of the small subcortical infarcts seen in patients with SVD is not related to thrombotic processes.
As a secondary observation we showed that thrombolysis was a safe medical procedure in our cohort of patients with lacunar stroke. Intravenous thrombolysis was not associated with early risk of intracerebral bleed which is in contrast with the recent meta-analysis of Charidimou et al. [10]. However Charidimou’s meta-analysis combines patients with all stroke subtypes and risk of hemorrhage in these patients may vary. We also showed that none of the patients who underwent thrombolysis developed CMB over the follow up period suggesting that thrombolysis did also not increase the risk of delayed CMB.
Our study has the limitations of being a small study and also prevalence of male patients may be related to the small sample size. However, we think that our observations highlight the importance of conducting large cohort as well as long term prospective studies to clarify the prognostic significance of CMB particularly in patients with lacunar stroke and Moderate-Severe SVD.
Well characterized patients with Moderate-Severe SVD and CMB may not benefit from anti-thrombotic therapy. New trials are underway for clarifying the role of cerebral microbleeds in haemorrhage risk stratification in patients started on anticoagulant therapy (Cromis UKCRN ID 4152; Cromis 2 UKCRN ID 10697). Similarly studies should be designed for patients started on antithrombotic therapy.
- Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, et al. (2013) Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 12: 822-838.
- Stehling C, Wersching H, Kloska SP, Kirchhof P, Ring J, et al. (2008) Detection of asymptomatic cerebral microbleeds: a comparative study at 1.5 and 3.0 T. Acad Radiol 15: 895-900.
- Vernooij MW, van der Lugt A, Ikram MA, Wielopolski PA, Niessen WJ, et al. (2008) Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology 70: 1208-1214.
- Shoamanesh A, Kwok CS, Benavente O (2011) Cerebral microbleeds: histopathological correlation of neuroimaging. Cerebrovasc Dis 32: 528–534.
- Imaizumi T, Inamura S, Nomura T, Kanno A, Kim SN (2015) The Severity of White Matter Lesions Possibly Influences Stroke Recurrence in Patients with Histories of Lacunar Infarctions. J Stroke Cerebrovasc Dis 24: 2154-2160.
- Wahlund LO, Barkhof F, Fazekas F, Bronge L, Augustin M, et al. (2001) A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke 32: 1318-1322.
- Lee SH, Lee ST, Kim BJ, Park HK, Kim CK, et al. (2011) Dynamic Temporal Change of Cerebral Microbleeds: Long-Term Follow-Up MRI Study. PLoS ONE 6: e25930.
- de Leeuw FE, de Groot JC, Achten E, Oudkerk M, Ramos LM, et al. (2001) Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry 70: 9–14.
- Gregoire SM, Jäger HR, Yousry TA, Kallis C, Brown MM, et al. (2010) Brain microbleeds as a potential risk factor for antiplatelet-related intracerebral haemorrhage: hospital-based, case-control study. J Neurol Neurosurg Psychiatry 81: 679–684.
- Charidimou A, Shoamanesh A, Wilson D, Gang Q, Fox Z, et al. (2015) Cerebral microbleeds and postthrombolysis intracerebral hemorrhage risk Updated meta-analysis. Neurology 85: 927-924.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
