Journal of HIV for Clinical and Scientific Research
Feasibility of HIV-1 RNA Extraction and Viral Load Testing of Bone Marrow Plasma Using the Abbott m2000 Platform and RealTime Quantitative HIV-1 Assay
Jason Rippe1, William Kabat1, Kristian Schafernak2 and Ram Yogev1*
2Department of Pathology and Laboratory Medicine, Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL, and Division of Pathology and Laboratory Medicine, Phoenix Children’s Hospital, Phoenix, AZ, USA
Cite this as
Rippe J, Kabat W, Schafernak K, Yogev R (2017) Feasibility of HIV-1 RNA Extraction and Viral Load Testing of Bone Marrow Plasma Using the Abbott m2000 Platform and RealTime Quantitative HIV-1 Assay. J HIV Clin Sci Res 4(1): 003-005. DOI: 10.17352/2455-3786.000020Current efforts to find a cure for HIV disease require that viral load assays be available to accurately detect and measure viral load in compartments other than peripheral blood. Over the years, HIV viral load assays have been adapted to measure viral presence in cerebral spinal fluid (CSF), genital secretions and various other compartments. In this study we describe our efforts to quantitatively recover HIV-1 from pooled pediatric bone marrow plasma spiked with known concentrations of HIV-1 virus using the Abbott RealTime HIV-1 assay and the m2000 platform. We focused on nucleic acid extraction, sample dilution, viral copy recovery, linearity and limit of detection.
We found that HIV-1 viral load can be reliably detected down to 250 copies/mL in bone marrow plasma diluted with BM53-processed EDTA plasma and extracted with the Abbott mSystem. The assay was linear from 250 to 100,000 copies/mL. Bone marrow composition may contribute to some loss of HIV-1 recovery, and a high quality serum diluent is essential for optimal viral recovery and detection.
Our results suggest that the Abbott RealTime HIV assay with the m2000 platform is a useful method to quantify HIV-1 viral load in human bone marrow.
Introduction
Viral load measurements are important for assessing antiretroviral drug effectiveness as part of HIV disease management. Viral loads are normally derived from peripheral blood plasma but only serve as a surrogate for viral activity in other body compartments. Robust assays are required to demonstrate clearance of HIV from all compartments if a true cure of HIV-1 is to be proven. Determination of viral activity in lymph nodes, oral and genital secretions and various other tissues have previously been described [1-3]. A limited number of studies have described evidence of cellular HIV-1 DNA reservoirs of virus in bone marrow [4], but quantification of HIV-1 in bone marrow plasma has not been described. In this study we examined the feasibility of using the Abbott m2000sp and m2000rt platforms with the Abbott RealTime quantitative HIV-1 assay to detect viral loads in spiked human bone marrow. Pooled pediatric human bone marrow plasma spiked with HIV-1 was used, as primary bone marrow samples from HIV-1 infected subjects were not available to us. The Abbott RealTime assay described below is the same as the FDA-approved assay for peripheral blood plasma assay with the pre-analytical modifications noted below specific to bone-marrow sample type.
Methods and Materials
Bone marrow collection and processing
Seventy deidentified residual bone marrow aspirate samples obtained from pediatric patients (age 1 to 15 years of age) as part of their work-up for suspected cancer were submitted to our laboratory after all required clinical testing had been completed. Bone marrow was centrifuged at 800 x g for 10 minutes and the plasma was separated and pooled. The pooled plasma was spun a second time at 800 x g for 10 minutes and aliquots of 1 to 2 mL were frozen at -70 oC or lower until used for testing. Because pediatric bone marrow is typically, collected in small volumes, we pooled several bone marrows samples in order to achieve the necessary volume for our study. The use of these specimens was approved by the Institutional Review Board of the Ann & Robert H. Lurie Children’s Hospital of Chicago.
Reagents
High concentration HIV-1 standard at 1.5 x 106 copies/mL was kindly provided by the Virology Quality Assurance (VQA) Laboratory, Rush University, Chicago, IL (provided under the NIAID Virology Quality Assurance Contract [J. Bremer, HHSN272201200023C, HHSN266200500044C]). The following Abbott RealTime reagents were obtained from the manufacturer (Abbott Molecular, Des Plaines, IL): RealTime HIV-1 Controls (Part # 6L-18-80), RealTime Calibrators (Part #6L-18-70), mSystem RNA general purpose reagent (Part # 04J70-24), mSystem DNA GPR modified for total nucleic acid extraction (Part # 06K12-24), Bulk Lysis Buffer (Part # 02N77-01) and RealTime HIV-1 amplification kit (Part # 6L-18-90). Sera Care Base Matrix BM53 serum (Sera Care Life Sciences, Milford, MA) was used for bone marrow plasma dilutions. This reagent has been validated for plasma dilution assays for viral load testing by the VQA, the AIDS Clinical Trails Group (ACTG) and the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) Laboratory Committees.
Viral load testing
Pooled bone marrow plasma were spiked with known HIV-1 concentration (500,000 copies/ml) used for standard quality assurance. Dilutions from this concentration were made to achieve the range of HIV concentrations used in the study. The prepared bone marrow plasma concentrations were further diluted 5-fold at the time of assay in either Abbott lysis buffer or Sera Care base matrix to final HIV-1 nominal concentration. All assays were performed on the Abbott m2000sp and m2000rt platforms following the same algorithms used for peripheral blood testing. Assays were performed on the Abbott platforms using 0.6 mL input volumes (0.8 mL with void volume). RNA extraction reagents were used for sample extraction according to manufacturer’s procedures [5]. Extractions were performed on the m2000sp automated extraction platform with PCR reactions completed on the m2000rt real time PCR platform.
Results
Bone marrow preparation for extraction
Initially, Abbott Lysis Buffer was used to dilute the spiked bone marrow, but recovery of HIV-1 was noted to be less than expected particularly with lower nominal viral concentrations. As a result, the assays were repeated using SeraCare BM53 as the diluent. HIV-1 recovery with the Abbott lysis buffer as the diluent was about 10 fold less efficient than with Sera Care BM53. Many centrifuged bone marrow samples had a lipid layer on top and with an indistinct interface with the plasma that could not be removed without significant loss of harvested marrow plasma. We suspect that the lipid layer does not adequately disperse in the Abbott Lysis Buffer leading to extraction interference but does solubilize in BM53 resulting in better viral recovery. A similar assay was performed without bone marrow plasma by diluting HIV-1 VQA Standard directly in either Abbott Lysis buffer or BM53 to determine if the discrepancy in recovery was influenced by the diluent. While there were small differences in viral recovery, the differences did not explain the bone marrow/diluent differences (data not shown).
Linearity
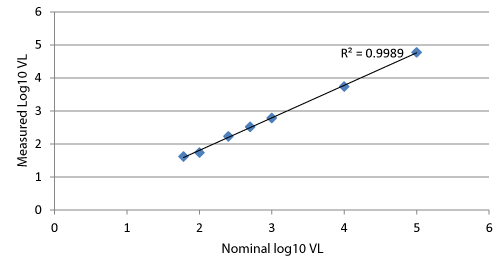
Pooled bone marrow plasma were spiked with HIV-1 VQA standard and diluted with BM53 to desired nominal concentrations of 100,000, 10,000, 1,000, 100, 50 and 20 copies/mL were tested in duplicate on the Abbott m2000 platform. Figure 1 shows the assay’s excellent linearity over the tested range.
Lower Limit of Detection (LLoD)
To better define the lowest level of HIV-1 recovery, the following concentrations were prepared using BM53 as the diluent: 1,000, 500, 250, 100, 60, 40 and 20 copies/mL. Two independent assays with replicate testing of nominal concentrations varied with 2 or 3 replicates of (1,000, 500 and 250 copies/mL), 2 or 7 replicates of 100 copies/mL, 15 total replicates of 60 copies/mL and 20 total replicates of 40 and 20 copies/mL. Table 1 depicts the results of the LLoD assays. The data suggest that the Abbott assay can reliably detect HIV-1 viral load in bone marrow plasma down to 250 copies/mL and sometimes as few as 40 copies/mL.
Discussion
Our study shows that the Abbott RealTime HIV-1 assay has potential utility in quantifying HIV-1 viral load in bone marrow plasma despite some limitations related in part to differences in bone marrow composition relative to peripheral blood plasma. The delay in processing the samples in our study resulted in some bone marrow plasma with hemolysis which we attempted to minimize by eliminating heavily hemolyzed samples from the pools we used. The limited availability of pediatric bone marrow plasma will likely require routine dilutions of bone marrow plasma in the Abbott assay clinically and if so the commercial Abbott lysis buffer may not be well suited for bone marrow plasma viral load testing. Instead SeraCare Basematrix BM53 should be used as it was better suited for bone marrow plasma viral load determinations in our study.
Our quantitative bone marrow plasma viral load determinations with the Abbott RealTime HIV-1 assay had a higher than expected lower limit of detection. The normal lower limit of quantitation of this assay in peripheral blood plasma is 40 copies/mL as compared to 250 copies/mL for bone marrow plasma found in this study. In addition we could not determine linear ranges beyond 100, 000 copies/mL. Higher viral load samples would need additional dilutions to fall within the assay linear range that we found. Further studies comparing the viral load ratio between the blood and bone marrow plasma are needed to verify the utility of our method. Additionally, bone marrow viral load may have applications in other viral infections such as hepatitis C virus.
In conclusion, our data suggest that the Abbott RealTime HIV-1 assay can be adapted by using BM53 serum diluent to accurately detect HIV-1 viral activity in human bone marrow plasma over a range of 250 to 100,000 copies/mL.
- Canestri A, Lescure FX, Jaureguiberry S, Moulignier A, Amiel CA, et al. (2010) Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis 50: 773-778. Link: https://goo.gl/8k9koN
- Susan A Fiscus, Susan Cu-Uvin, Abel Tilahun Eshete, Michael D Hughes, Yajing Bao, et al. (2013) Changes in HIV-1 subtypes B and C in genital tract RNA in women and men after initiation of antiretroviral therapy. Clin Infect Dis 57: 290-297. Link: https://goo.gl/Vl28cp
- Celum C et al. (2002) Recovery of infectious human immunodeficiency virus type-1 from the oropharynx: implications for oral transmission of HIV-1. Ninth Conference on Retroviruses abstract 379-M, Seattle, 24-28 February.
- Christine M Durand, Gabriel Ghiaur, Janet D Siliciano, S Alireza Rabi, Evelyn E Eisele, et al. (2012) HIV-1 DNA PCR Is Detected in Bone Marrow Populations Containing CD4+ T-Cells but not Found in Purified CD34+ Hematopoietic Progenitor Cells in Most Patients on Antiretroviral Therapy. J Infect Dis 205: 1014-1018. Link: https://goo.gl/9sDQEY
- Abbott RealTime HIV-1 RNA PCR Kit Product Insert.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
