Journal of Gynecological Research and Obstetrics
Novel therapy for COVID-19 does intravenous ozonated-saline affect blood and tissue oxygenation?
Thorp JA*, Hollonbeck SA, Viglione DD, Green PC, Hodge JR, Tamburro JA, Tran TN and Glassman DS
Cite this as
Thorp JA, Hollonbeck SA, Viglione DD, Green PC, Hodge JR, et al. (2020) Novel therapy for COVID-19 does intravenous ozonated-saline affect blood and tissue oxygenation? J Gynecol Res Obstet 6(2): 046-050. DOI: 10.17352/jgro.000085Introduction: Adjunctive ozone therapy for COVID-19 is being used successfully in China, Spain, Italy, and South America. One proposed mechanism is by improving blood / tissue oxygenation thus averting multiorgan system failure due to hypoxia. The purpose of this study was to determine if ozonated-saline administered intravenously affects the oxygenation and duration of time spent in a hypoxia chamber.
Materials and methods: This was a prospective pilot study that used one volunteer who underwent seven experiments. Each included two runs in a hypoxia chamber that resulted in symptomatic oxygen desaturation. One subject was used as his own control in the hypoxia chamber before and after infusion of intravenous ozonated-saline in four paired experiments.Another 3 experiments were performed identically except ozone was not administered. The primary outcome was to test the null hypothesis that ozonated-saline infusion does not affect oxygenation.
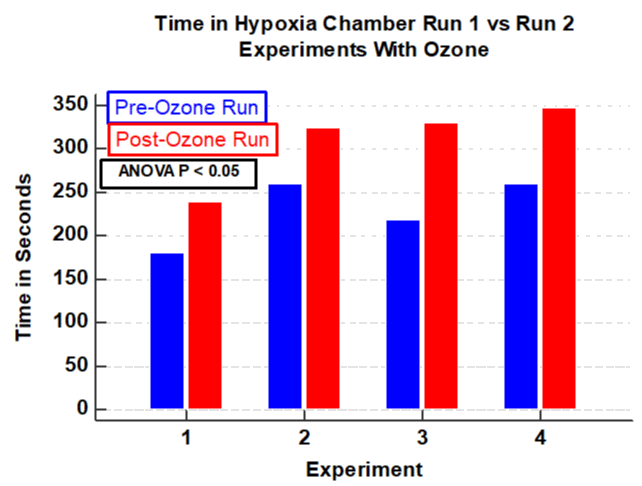
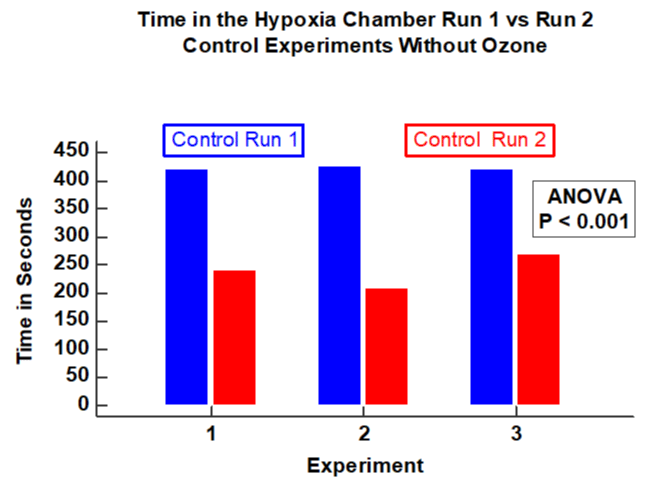
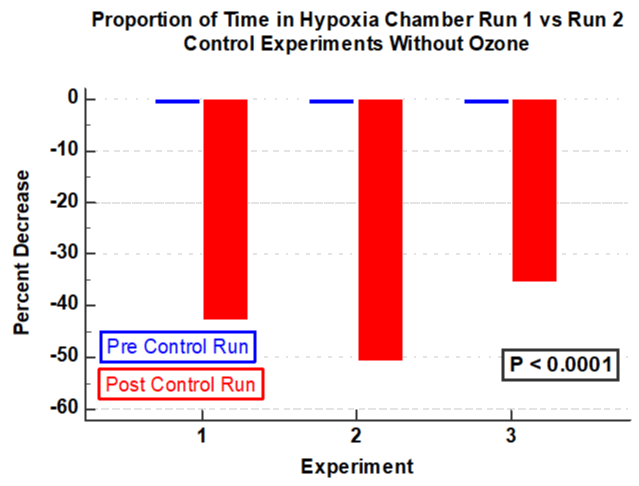
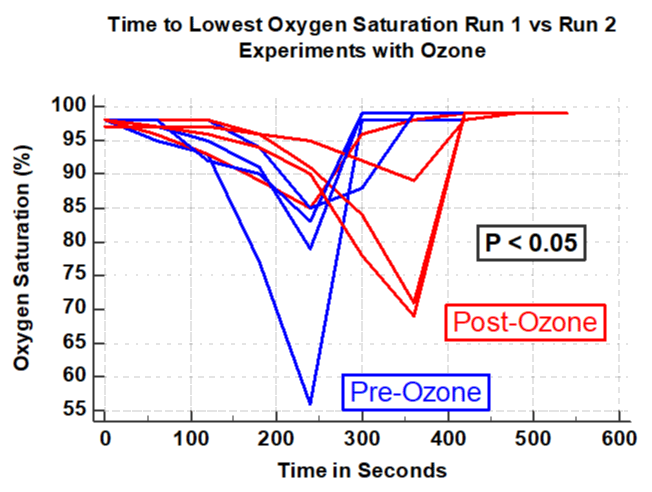
Results: In four experiments, ozone was associated with a significant increase in time the subject could remain in the hypoxia chamber (P< 0.05). In three control experiments without ozone, there was a significant decrease in time in the hypoxia chamber in the second run compared to the first (P <0.001). Compared to the first run there was a 32.4% increase in the proportion of time in the second run (after ozone) compared to the first run (P <0.0001). In contrast, in the three control experiments without ozone, there was significant decrease in proportion of time the subject could remain in the hypoxia chamber with an average decrease of -43.1% (P < 0.0001). Ozone therapy was associated with a significant delay in lowest oxygen desaturation (P <0.05). In contrast, in the three experimental runs without ozone there was a significant reduction in time to reach the nadir of the desaturation curve in the second run compared to that of the first (P < 0.05).
Conclusions: Infusion of intravenous ozonated-saline significantly increases the duration of time that a subject can remain in hypoxia and delays the nadir of the oxygen de-saturation curve.
Introduction
Ozone therapy has been used in medicine for over a century after it was discovered in the mid-1800’s. It is extensively used in the USA, especially in the pre-antibiotic era for infectious diseases including combat injuries. Most recently reports from China, Italy, Spain and South America report the beneficial use of ozone therapy in extremely ill patients with COVID-19 associated acute respiratory insufficiency [1-8]. Despite the extensive use of ozone among Integrative / Wellness providers in the US and world wide there are very few prospective studies [9-11]. There are multiple proposed mechanisms by which adjuvant ozone therapy for COVID-19 could possibly improve outcomes: 1) Ozone is virucidal; 2) Ozone increases oxygenation of blood and tissues; 3) Ozone stimulates immune function via nuclear factor erythroid 2–related factor 2 (NRF2); 4) Ozone inhibits inflammatory mediators (interleukins, cytokines, and tumor necrosis factors; 5) Ozone activates endogenous antioxidant immune defenses; 6) Ozone inhibits viral replication; 7) Ozone inhibits micro thrombus formation; 8) Ozone up-regulates HO-1 in endothelial cells and; 9) Ozone stimulates the 2-3 diphosphoglycerate that shifts the oxygen saturation curve to the right, thus delivering more oxygen to the tissues [12]. The purpose of this investigation is to determine if ozone-gas infused saline administered intravenously affects oxygen desaturation and recovery in a hypoxia chamber.
Materials and methods
One volunteer (author JAT) consented to undergo serial testing in a reproducible hypoxia model. The subject was continuously monitored with oxygen saturations and vital signs. All experiments were supervised by physicians and health care providers. The subject himself is extensively trained in aggressive aerobic exercise and thus was well acquainted with exercise induced hypoxia. The hypoxia model was accomplished by a closed, hermetically sealed hypoxia chamber that was instilled with approximately 8 liters of air (at sea level) thus constituting about 1.6 liters of oxygen. The air inserted into the hermetically sealed hypoxia chamber was measured by a standard air pump. The subject has an oxygen consumption of approximately 375 ml per minute thus it was estimated that the oxygen would be consumed in about 4.27 minutes. Ozone was generated by a high quality medical cold plasma technology generator.
The subject was placed in the hypoxia model for the first run of each of the seven experiments. In three of the experiments after the first run in the hypoxia chamber, the subject waited for the exact same time period of 30 minutes and then underwent the second run in the hypoxia chamber. In four of the experiments after the first run in the hypoxia chamber, the subject was infused intravenously with 500 ml of ozonated normal saline. The normal saline was ozonated by infusion of 600 ml of ozone gas infused into a liter bag of only 500 ml of normal saline at a concentration of 100 microgram/ml (gamma). It was shaken vigorously then immediately administered intravenously to the subject. Upon completion of the 30 minutes of ozonated-saline infusion, the subject was again placed in an identical hypoxia model. In each of the seven experiments the amount of time (seconds) before and after the intravenous ozonated saline infusion (or control) was compared using one-way ANOVA. In a similar fashion the percent of time gained or lost in the second hypoxia run was calculated using the first run as a reference in seven experiments before and after ozone (or control). The percent change was tested by comparison of proportions assuming the null hypothesis that ozone / control did not affect these proportions.
Continuous hemoglobin saturations were obtained using a standard pulse oximeter. The oxygen saturation curves were analyzed using standard statistical measurements to compare the time to the lowest value (nadir in seconds). Based upon a 10% improvement in the primary outcomes and a power of 80% , we calculated that seven experiments would be required (alpha <0.05, beta <0.2). All observers were trained and aware of the signs and behavioral symptoms of hypoxia, specifically anxiety, agitation, excessive movement of limbs, changing position, fidgeting, tremors, tachypnea with increased inspirational tidal volumes. Standard statistical methods were used MedCalc.org statistical package using the serial measurements time to reach minimum oxygen saturation (nadir) and comparison of one proportion compared to the null hypothesis.
Results
Vital signs remained stable before, during and after the two sequential hypoxia runs. There was a significant increase in duration of time the subject could stay in the hypoxia model after receiving ozone compared with that prior to the ozone therapy (ANOVA; P < 0.05, F = 7.8, Figure 1). In contrast, three experiments using control demonstrate a significant reduction in time that the subject could remain in the hypoxia chamber in the second run compared to the first and (ANOVA; P = 0.001, F=109, Figure 2). The percent change of time in the hypoxia chamber after ozone was calculated using the pre-ozone time in the hypoxia chamber as a reference. The null hypothesis was rejected as there was as substantial increase in the percent of time the subject could stay in the hypoxia chamber. (increase by 31%, P < 0.0001, Figure 3). Likewise, in the three control experiments there was a substantial reduction in the proportion of time in the hypoxia chamber in the second run compared with the first run (decrease by 43%, P < 0.0001, Figure 4).
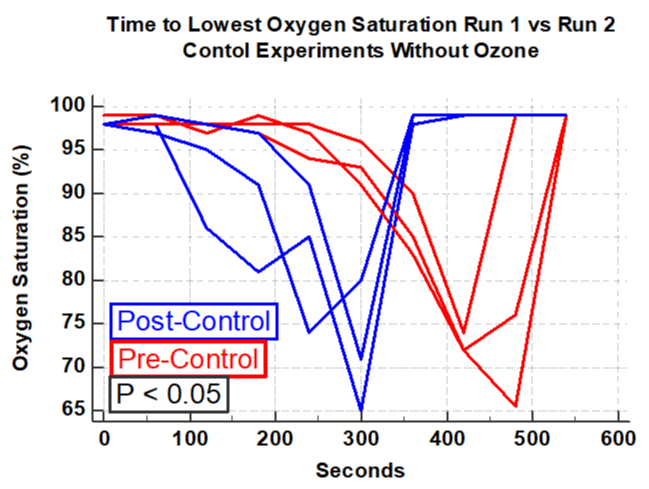
The hemoglobin oxygen saturation curve was also analyzed in the four experiments using ozone and the three control experiments without ozone. In the four experiments using ozonated-saline there was a substantially longer time to the lowest point of oxygen desaturation compared with the run immediately prior to the ozone therapy (Figure 5 P < 0.05). In contrast, in the three control experiments there was a significantly shorter time to reach the nadir of oxygen desaturation compared to the first runs (Figure 6, P < 0.05). All observers noted a substantially more rapid appearance of hypoxia signs / symptoms in the four pre-ozone experiments compared to the post ozone hypoxia run. In contrast, in the three identical experiments without ozone, there was a substantially shorter time in onset of hypoxia signs/symptoms of tachypnea, agitation, motor restlessness, deeper breathing and discomfort of air hunger in the hypoxia run compared to that of the first run of the experiment.
Discussions
Intravenous ozonated-saline was associated with clinically and statistically significant improvements in oxygenation. This was evidenced by the duration of time in the hypoxia chamber and by the delay of the lowest oxygen saturation (Figures 1-4). There was a substantial increase in the duration of time in seconds that the subject could remain in the hypoxia chamber after receiving ozone compared with that of the pre-ozone run (ANOVA; P < 0.05, F = 7.8, Figure 1). Remarkably the exact opposite occurred in the three experiments that were performed without ozone, that being a significant decrease in the duration of time in seconds that the subject could remain in the hypoxia chamber in the post-control run compared with that of the pre-control run (ANOVA; P = 0.001, F=109, Figure 2). Similarly, in the 4 experiments using ozone, the proportion of time in the hypoxia model in the post-ozone runs were compared using the pre-ozone runs as the reference and there was an average 31% increase(P < 0.0001 Figure 3). In striking contrast, in the three experiments using the exact same technique without ozone, there was an average 43% reduction in the proportion of time that the subject could stay in the hypoxia chamber in the second compared to the first run (P < 0.0001, Figure 4). Of great interest to corroborate the findings outlined above there was dramatic improvement in oxygenation measured by the continuous oxygen saturation monitor associated with the four experiments with the runs before and after ozone. There was a striking and consistent increase in time to achieve the lowest oxygen saturation level (nadir) measured by pulse oximetry in the post ozone run compared to that of the pre ozone run (Figure 5, P < 0.05). Consistent with the patterns observed above, there was a complete opposite effect in the three control experiments in which ozone was not used. Instead of having a longer time to reach the oxygen desaturation nadir in the post ozone run, there was a striking reversal of that pattern in the post control run (without ozone). In the post-control run there was a significant reduction in time to achieve the lowest level of oxygen saturation as measured by pulse oximetry compared to that of the pre control run(Figure 6, P < 0.05).
Independent observers unanimously observed a lessening and delay in hypoxia symptoms observed in the post-ozone run compared with that of the pre-ozone run. To our knowledge this is the first documentation of intravenous ozonated-saline improving the ability to remain in hypoxia with the same endpoint, that is, severely symptomatic hemoglobin desaturation documented by pulse oximetry. This study did NOT address the efficacy of adjuvant ozone therapy on COVID-19. However, it does support one of the mechanisms postulated to improve outcomes, that is, the improvement in oxygenation of blood and tissues.
Like oxygen and water, high concentrations of ozone can be hazardous to the lungs if directly inhaled for extended periods of time. There are a variety of modalities of ozone administration that have been extensively utilized [12]. Although controversial, direct ozone gas infusion into the venous blood is the most simple and efficient ozonation method and is widely used as the route of choice by some experts. In contrast to air, ozone gas infusion intravenously does not pose the same risk since it is not composed of 80% nitrogen and ozone is highly soluble in blood & plasma. Ozone is about 10 to 13-fold more soluble in water than is oxygen [12]. Depending upon the temperature and pressure, about 50 ml of ozone gas dissolves in 100 ml of water compared to only about 4 ml of oxygen. [12]. Many experts prefer the use of extracorporeally ozonated autologous blood transfusion of 100 ml to 200 ml, widely known as “major autohemotherapy” (also referred to as “MAH”) [12]. Various protocols for MAH typically use volumes of only 100-200 ml of blood. The drawbacks are that it requires bloodletting, heparinization, and ozonation of the blood followed by autologous transfusion. This process is further complicated by the necessity of heparin with its attendant significant side effects (allergic reactions and heparin induced thrombocytopenia) and also by care providers requiring personal protective equipment for COVID-19. Additionally, MAH could not be “blinded” in future studies.
Infusion of intravenous ozonated-saline has been utilized by many clinicians as avoids the concerns and inconveniences of extracorporeal blood ozonation and heparin. There are multiple other safe routes of administration of ozone gas including insufflation into the external ear canal, vagina, rectum, and bladder as well as direct ozone gas injection into muscle, joint spaces, facets and discs for back pain relief [12]. Drinking ozonated water is safe and allows absorption through the GI tract [12]. The World Federation of Ozone Therapy Scientific Advisory Committee in 2015 published an extensive 117-pagereview on evidence-based ozone therapy [12]. In a sample of over a million treatments they quoted a complication rate of only 0.0007% which the authors claim is one of the lowest in all of medicine [12]. They discuss four associated deaths attributed to the uses by non-qualified personnel injecting large volumes of intravenous ozone gas [12]. In contrast Rowen states that intravenous ozone gas therapy is exceptionally safe and has extensive personal use with a reported complication rate of only 0.7 per 100,000 treatments, most all of which he attributed to improper administration [13].
Singh, et al. provided an excellent detailed review of the three different coronaviruses in the past two decades delineating their differences in viral dynamics and clinical features [14]. As these authors point out the treatment is primarily supportive therapy and they conclude that there is no anti-viral therapy proven to be effective against COVID-19 [14]. A few small-scale studies have claimed benefit with chloroquine, hydroxychloroquine and other drugs including lopinavir/ritonavir, remdesivir, favipiravir, oseltamivir, ribavirin, interferon beta, tocilizumab and abidol [14]. In a separate publication, Singh et al review innovative technologies that may provide more effective screening and treatments for COVID-19 in the outpatient setting [15].
The limitations of this study are that the experiments were performed in just one subject, using himself as the control. Another limitation is that the subject was not blinded to the intervention. A rather unique consideration is whether the impressive oxygen desaturations that this subject achieved in the hypoxia chamber were physiologic or pathophysiologic. The subject has underlying chronic lung disease from a 17-segment spinal fusion for severe scoliosis earlier in life. Whether or not this contributed to the impressive oxygen desaturations that were achieved is unknown but regardless, this study convincingly demonstrated that ozone therapy was associated with dramatic improvements in oxygenation. Whether or not young healthy subjects will desaturate as easily will be determined by the follow up phase two study. Our future planned study will evaluate the use of ozone with a more sophisticated hypoxia “altitude simulator” device in 30 young healthy adults with ozonated-saline administered in a double blinded fashion.
The study supports that intravenous ozonated-saline improves oxygenation of blood and tissues and prolongs the ability of an individual to remain in a hypoxic environment. This study supports the potential benefit of adjuvant ozone therapy for COVID-19 as observed in China, Spain, Italy, and South America [1-8]. Based upon the findings of this study a large randomized controlled prospective trial should be conducted in the United States. Ultimately a multicenter randomized controlled clinical trial will be necessary to prove whether adjuvant ozone therapy can improve outcomes in COVID-19 patients as purported by observational studies from China, South America, Spain and Italy [18]. Based upon a reduction in the progression of disease to require mechanical ventilation by 50% (from 10% to 5%), a sample size of approximately 1000 patients would achieve a power of greater than 80% to demonstrate this difference (alpha < 0.05, beta < 0.20). We are attempting to organize and fund this trial.
- Guangjian N, Hongzhi Y (2020) Clinical study for ozonated autohemotherapy in the treatment in the treatment of Novel Coronavirus Pneumonia (COVID-19). ChiCTR2000030165. Academy of Medical Engineering and Translation Medicine. Link: https://bit.ly/2MFH9Ji
- Linlin, H, Xiangdong C (2020) A randomized controlled trial for the fficacy of ozonatedautohemotherapy in the treatment of Novel Coronavirus Pneumonia (COVID-19). Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. Link: https://bit.ly/2XIJaLi
- Huiling H, Tong X (2020) A multicenter randomized controlled trial for ozone autohemotherapy in the treatment of novel coronavirus pneumonia (COVID-19). Tianjin Huanhu Hospital. Link: https://bit.ly/2AeNSar
- Mariano F (2020) Scientific Society Oxygen Ozone Therapy (SIOOT) First Report on the Use of SIOOT Ozone Oxygen in Patients Hospitalized with COVID-19., Scientific Society of Oxygen-Ozone Therapy, Bergamo, Italy. Link: https://bit.ly/3h1UeL0
- Mariano F (2020) Scientific Society Oxygen Ozone Therapy (SIOOT) First Report on the Use of SIOOT Ozone Oxygen in Patients Hospitalized with COVID-19., Scientific Society of Oxygen-Ozone Therapy, Bergamo, Italy. Link: https://bit.ly/3h1UeL0
- José B (2020) Scientific Society Oxygen Ozone Therapy (SIOOT), World Federation of Ozone Therapy,Success of the first Spanish clinical trial with ozone therapy for COVID-19 patients in Polyclinic Group, Ibiza, Spain. Link: https://bit.ly/2UjGVMo
- Hernández A, Papadakos PJ, Torres A, González DA, Vives M, et al. (2020) Two Known therapies could be useful as adjuvant therapy in critical patients infected by COVID-19. Rev Esp Anestesiol Reanim 67: 245-252. Link: https://bit.ly/30i0eJG
- De Monte A (2020) To stop Covid-19, more and more hospitals are using Oxygen-Ozone, Santa Maria dellaMisericordia Udine, Italy. Link: https://bit.ly/3h9KA9o
- Martinex-Sanchez G, Schwartz A, Di Donna V (2020) Potential cytoprotective activity of ozone therapy in SARS-CoV-2/COVID-19. Antioxidants 9: 389. Link: https://bit.ly/2UkuD6e
- Elvis AM, Ekta JS (2011) Ozone therapy: A clnical review. J Nat Sci Biol Med 2: 66-70. Link: https://bit.ly/3gYWsL4
- Andrade RR, Oliveira-Neto OB, Barbosa LT, Santos IO, Sousa-Rodrigues CF, et al. (2019) Effectiveness of ozone therapy compared to other therapies for low back pain: a systematic review with meta-analysis of randomized clinical trials. Rev Bras Anestesiol 69: 493-501. Link: https://bit.ly/37cnfiL
- World Federation of Ozone Therapy Review on Evidence Based Ozone Therapy (WFOT). WFOT Scientific Advisory Committee 2015. Link: https://bit.ly/2XGbeij
- Rowen RJ, Robins H (2020) A Plausible “Penn’ Costing Effective Treatment for Corona Virus – Ozone Therapy. J Infect Dis Epidemiol 6: 113. Link: https://bit.ly/3eWNCvq
- Singh RP, Javaid M, Kataria R, Tyagi M, HaleemA, et al. (2020) Significant applications of virtual reality for COVID-19 pandemic. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 14: 661-664. Link: https://bit.ly/37iKYOg
- Singh RP, Javaid M, Kataria R, Tyagi M, HaleemA, et al. (2020) Significant applications of virtual reality for COVID-19 pandemic. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 14: 661-664. Link: https://bit.ly/37iKYOg
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
