Journal of Gynecological Research and Obstetrics
Rise & Fall of Fetal Heart Rate, the Principle of Fetal Monitoring: Hypoxia Index prevents Cerebral Palsy
Kazuo Maeda1* and Masaji Utsu2
2Department of Obstetrics and Gynecology, Seirei Mikatahara Hospital, Hamamatsu, Japan
Cite this as
Maeda K, Utsu M (2018) Rise & Fall of Fetal Heart Rate, the Principle of Fetal Monitoring: Hypoxia Index prevents Cerebral Palsy. J Gynecol Res Obstet 4(3): 036-038. DOI: 10.17352/jgro.000056As FHR rises by the fetal brain excitation with fetal movement, and FHR falls by the excitation of fetal vagus nerve center with hypoxic stimulation, hypoxic grade is numerically known by the hypoxia index, which is the sum of FHR deceleration duration (min) divided by the lowest FHR (bpm), and multiplied by 100, where all of 6 cerebral palsy cases’ hypoxia index were 25 or more, while the hypoxia index of all 16 no cerebral cases were 24 or less, thus, cerebral palsy is prevented, if the hypoxia index is 24 or less in full course of fetal monitoring. It is the first numeric threshold setting to prevent cerebral palsy in 50 years’ history of fetal monitoring.
Introduction
Fetal heart rate (FHR) transient bradycardia is reverse triangle, of which nadir is the lowest FHR in FHR deceleration in fetal monitoring. The deceleration is classified into early, late, mild and severe variable decelerations, where late and severe variable ones were hypoxia predicting patterns, particularly late deceleration was ominous even in very shallow one in original pattern classification. Normal FHR baseline is 110 to 140 bpm, where the baseline variability was 5 to 24, which develops by the stimulation of minor fetal movements, while the loss of FHR variability is severe fetal brain damage similar to anencephaly. Sinusoidal FHR develops in severe fetal anemia, and it is separated from favorable physiological sinusoidal pattern by authors actocardiogram, analysis of fetal movement and heart rate. The FHR pattern classification was common into early, late and variable decelerations in visual FHR record diagnosis, which was vague several times and controversy in CTG.
Continuous FHR
There is the baseline FHR found in resting fetal state, continuous FHR rise is tachycardia, transient FHR rise is triangular acceleration synchronized with fetal movement bursts. Continuous FHR fall is bradycardia. Stable fetal heart rate is the sign of stable fetal oxygenation.
FHR rise
Although you may detect abnormal FHR changes and fetal heart rate patterns in early, late, mild & severe variable decelerations, discussing fetal outcomes [1], however, FHR diagnosis with FHR pattern classifications were controversy in several cases. Thus, the author firstly calculated objective FHR score in 1969 [2], by which predicted Apgar score and UApH [3], then fetal outcome diagnosis with artificial neural network analysis [4]. The author created actocardiogram (ACG), which analyses fetal heart rate with fetal movement, and clarified the relation between fetal heart rate and movement [5]. Triangular FHR acceleration is originated in fetal midbrain [6], which is caused by square shaped fetal movement burst to triangular acceleration by the integral function of brain with 7 sec time constant in the electric simulation. Physiologic simulation of adult also developed triangular heart rate from square shape leg movements [7], thus, it was clear that FHR increases by excited fetal brain to fetal movements. The logic clarified the developing mechanism of physiologic sinusoidal fetal heart rate, where the physiologic sinusoidal is the reaction of fetal brain to fetal cyclic mouthing or respiratory fetal movements, separating physiologic sinusoidal FHR from pathologic sinusoidal one [8].
As fetal brain function to rise heart rate is suppressed by hypoxia, where firstly FHR acceleration is lost in “non-reactive heart rate” in biophysical profile in maternal perception of fetal kicking, while FHR baseline variability is preserved in mild hypoxia, and the baseline variability was the final fetal brain reaction to minor fetal movements in hypoxia, while the variability is lost in severe hypoxic fetal brain damage in severe irreversible hypoxic fetal brain damage. Thus, early caesarean delivery with the purpose to prevent fetal damage should be performed before the loss of FHR baseline variability, namely, the delivery after the loss of variability cures fetal life but unable to prevent cerebral palsy due to irreversible fetal brain damage in severe fetal hypoxia, where the variability is lost [9], while the numeric threshold to develop the loss of variability was unknown in the past, and firstly introduced in fetal monitoring of the present study.
FHR fall
FHR falls in fetal hypoxia, where the vagus nerve center located in medulla oblongata is excited by the stimulation of hypoxia to reduce FHR developing fetal bradycardia, where FHR deceleration appears in transient hypoxia, and continuous bradycardia in prolonged hypoxia, where hypoxic fetal bradycardia is a vagus nerve excitation, but not immediate fetal brain damage, namely frequently repeated decelerations or prolonged hypoxia will develop fetal brain damage, which is predicted by hypoxia index as follows in the present report.
Threshold value of hypoxia index to develop fetal brain damage
It was mandatory to verify numeric threshold of hypoxia index to develop fetal brain damage represented by the loss of baseline variability, while there had been no threshold in the past fetal monitoring, thus, the author tried to analyze late FHR deceleration to detect its hypoxic effect, however, a case of 3 connected typical late decelerations with 45 sec lag time resulted fully normal neonate after caesarean delivery, where the Apgar score was 9 points, while a case of late decelerations repeated for 50 minutes due to refusal of caesarean delivery developed severe neonatal asphyxia of which Apgar score was 3 points, associated severe brain damage, which ended by infantile brain hemorrhage and death, namely, typically characteristic late deceleration repeated 3 times was ineffective to develop fetal damage, while frequently repeated decelerations were effective to develop fetal brain damage. In addition, there was another definition of late deceleration, that a late deceleration was diagnosed after 15 or more minutes’ repetition, namely, it was confirmed that frequent repetition of decelerations are effective to develop hypoxic damage of fetal brain, but not by the characteristic late deceleration pattern, thus, the author decided to set the threshold level, to develop fetal brain damage by the repetition of decelerations.
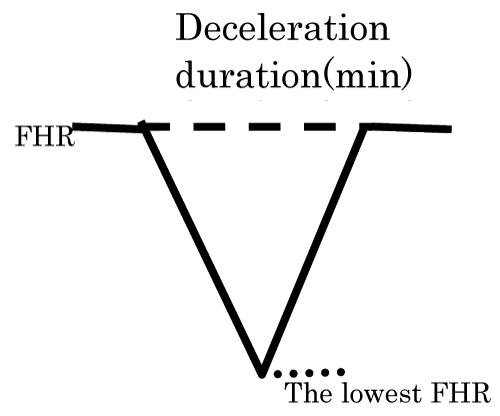
Thus, the novel hypoxia index was the sum of all deceleration durations (min) in full course of the fetal monitoring, divided by the lowest FHR (bpm), which indicates the intensity of hypoxia, then multiplied by 100 to keep integer of index (Figure 1). It was sure that fetal brain damaging threshold exists between 3 times decelerations and 50 minutes’ repetition of decelerations.
We collected retrospectively 22 cases of infants who were diagnosed by pediatric clinic, and their intrapartum FHR records were kept in obstetric clinic, where 6 cases were cerebral palsy and 16 cases were no cerebral palsy. Hypoxia index of two groups were compared (Table 1). Comparison of several kinds of cerebral palsy was unable due to small number of cerebral palsy cases.
Results and discussion
The hypoxia index of all 6 cerebral palsy cases was 25 or more, and no case of no cerebral palsy, while the index of all 16 cases of no cerebral palsy was 24 or less, and there was no case of cerebral palsy. The possibility to be the same probability was almost zero (p=0.000008<0.05). The digital numeric threshold existed between 24 and 25 of hypoxia index (Table 1).
As there was no cerebral palsy in 16 cases, whose hypoxia index was 24 or less, cerebral palsy is prevented, if the index is kept at the level of 24 or less in fetal monitoring.
The cases whose hypoxia index is 25 or more can receive early treatment of cerebral palsy even in neonatal period, as there is possibility to develop infantile cerebral palsy, if the index is 25 or more. Early treatment may improve the therapeutic effect.
As late deceleration develops by the stop of maternal placental blood flow by the compression of pelvic artery by contracted pregnant uterus [10] and late deceleration disappeared by changing maternal posture to lateral one from supine [11], the author recommends to take maternal lateral posture when any deceleration appears during labor, to prevent the increase of hypoxia index.
Conclusion
FHR changes are complicated due to the mixture of FHR acceleration of which loss is related the decrease of fetal brain function, and FHR fall, which is related fetal hypoxia, thus, it is mandatory to study the roles of FHR rise and fall in fetal monitoring, not only to rely on the FHR pattern classification, of which use is changing by the digital numeric analysis of FHR, where the rise and fall of FHR should be correctly analysed according to scientific facts. The author’s hypoxia index and Cahill’s deceleration area [12], are good example of update tendency of modern FHR diagnosis.
- Hon EH (1968) An Atlas of Fetal Heart Rate Patterns. Harty Press, New Haven. Link: https://goo.gl/9SwGfJ
- Maeda K, Kimura S, Nakano H (1969) Pathophysiology of Fetus, Fukuoka Printing, Fukuoka.
- Maeda K, Noguchi Y, Matsumoto F, Nagasawa T (2006) Quantitative fetal heart rate evaluation without pattern classification: FHR score and artificial neural network analysis. In Kurjak, Chervenak Eds. Textboook of Perinatal Medicine, 2nd ed. London, Informa. 1487-1495. Link: https://goo.gl/o2dKHf
- Maeda K, Utsu M, Makio A, Srizawa M, Noguchi Y, et al. (1998) Neural network computer analysis of fetal heart rate. J Matern Fetal Investig 8: 163-171. Link: https://goo.gl/r1qxMp
- Maeda K (2016) Actocardiogram, Analysis of Fetal Motion and Heart Rate. Jaypee, New Delhi. Link: https://goo.gl/6CXYSu
- Terao T, Kawashima Y, Noto H, Inamoto Y, Lin TY, et al. (1984) Neurological control of fetal heart rate in 20 cases of anencephalic fetuses. Am J Obstet Gynecol 140: 201-208. Link: https://goo.gl/QLnfZA
- Maeda K (2013) Actocardiographic analysis of fetal hypoxia detected by the bradycardia, loss of fetal heart rate acceleration and long term variability. Health & Med Inform 4: 118-122. Link: https://goo.gl/RvWKv8
- Ito T, Maeda K (1994) Differentiatio between physiologic and pathologic sinusoidal FHR pattern by fetal actocardiogram. J Perinat Med 22: 39-48. Link: https://goo.gl/fXnpXg
- Maeda K (2014) Modalities of fetal evaluation to detect fetal compromise prior to the development of significant neurological damage. JOGR 40: 2089-2094. Link: https://goo.gl/z268MJ
- Caldeyro-Barcia R, Poseiro JJ, Mendez-Bauer C, Gulin LO (1967) Effects of abnormal uterine contraction on fetal heat rate during labor. Proc. 5th World Cong Gynecol Obst. Sydney. Link: https://goo.gl/c8AT9i
- Poseiro JJ, Mendex-Bauer SV, Caldeyro-Barcia R (1969) Effect of uterine contractions on maternal blood flow through the placenta. 8 PAHO Advisary Committee on Medical Research 161-171.
- Cahill AG, Tuuli MD, Stout MJ (2018) A prospective cohort study of fetal heart rate monitoring: deceleration area is predictive of fetal acidemia. AmerJ Obstet Gynecol 5: 523-e1-523. Link: https://goo.gl/JvrNhP
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
