Journal of Gynecological Research and Obstetrics
Can the response to three months Ibuprofen for controlling Heavy Menstrual Bleeding with IUD be predicted at baseline visit?
Mohammed k Ali, Ahmed M Abbas*, Ali H Yosef, Osama S Abdalmageed and Mustafa Bahloul
Cite this as
Ali Mk, Abbas AM, Yosef AH, Abdalmageed OS, Bahloul M (2017) Can the response to three months Ibuprofen for controlling Heavy Menstrual Bleeding with IUD be predicted at baseline visit? J Gynecol Res Obstet 3(4): 093-097. DOI: 10.17352/jgro.000046Objective: The present study examines the hypothesis that the clinical and ultrasonography data reported at baseline visit can predict the responsiveness of ibuprofen in controlling heavy menstrual bleeding with Cu-IUDs at 3 months follow-up visit.
Materials and methods: We used data revealed from a single center open label prospective cohort study conducted at Woman’s Health Hospital, Assiut University, Egypt in the period between the 1st of October 2015 and the 30th of September 2016. This study included 128 women complaining of heavy/prolonged menstrual bleeding with Cu-IUD; they received ibuprofen for three consecutive cycles. Seventy patients (54.68%) respond to ibuprofen and while 58 patients (45.31%) did not respond. Logistic regression was done to show the significant predictors of ibuprofen response and a receiver operating characteristic (ROC) curves was used to evaluate the sensitivity and specificity of the potential predictors revealed by logistic regression.
Results: The responders had fewer number of bleeding days per month (5.1 ± 1.7 vs. 7.41 ± 1.7 days in the non-responders, p<0.001). Additionally, the uterine volume was significantly smaller in the responders than the non-responders with statistically significant difference (47.01 ± 6.5 mL vs. 60.9 ± 25.5 mL, p=0.001; respectively). Women reporting higher number of bleeding days per month (OR 3.26, 95% CI 1.06-10.03, p<0.001), larger uterine volume (OR 1.26, 95% CI 1.00-1.59, p=0.03) and lower uterine PI (OR 0.15, 95% CI 0.23-0.95, p=0.005 were likely to not succeed to be controlled by ibuprofen. Our predictive model can predict the failure of ibuprofen when utilize the 3 prior predictors with 93.5% sensitivity, 57.5%specificity, 67.4% PPV, 90.5% NPV and 75.0%accuracy.
Conclusion: Women reporting a lot of bleeding days per month, high uterine volume and low uterine artery PI during baseline visit may not respond to ibuprofen in controlling of heavy menstrual bleeding with Cu-IUD.
Introduction
Copper intrauterine device (Cu-IUD) is a common method of reversible contraception worldwide [1]. Abnormal uterine bleeding and pain are the most common medical indications for the discontinuation of its use [2,3].
Many factors may exert influences on the frequency of bleeding with Cu-IUDs such as the type of device used, the duration of usage, the previous menstrual pattern and the woman’s parity [4]. Moreover; the uterine volumetric measurement and uterine blood flow pattern may be also having a role in development of such bleeding [5].
Excessive prostaglandin release appears to play an important role in provoking both bleeding and pain related to IUDs [6]. There are many types of prostaglandin metabolites that present in the endometrium; one of them is prostacyclin which causes vasodilatation and inhibits platelet aggregation. Another one is thromboxane which induces vasoconstriction and blood clotting [7].
The Cochrane Review states that non-steroidal anti-inflammatory drugs (NSAIDs) are the most effective treatment to reduce the bleeding with IUD use [8]. They are prostaglandin synthetase inhibitors acting by decreasing production of endometrial prostaglandins; thus can improve both uterine bleeding and pain [9].
Since its discovery; several drugs in NSAIDs class have been used to treat heavy uterine bleeding and pain associated with IUD use such as mefenamic acid, ibuprofen and naproxen [10]. In addition, there is also a recent systematic review reported that NSAIDs are the first line used for reduction of the menstrual blood and pain associated with Cu-IUD [11].
However, some women respond well to NSAIDs and show high acceptability and satisfaction, on the other hand; a group of women may not respond to this line of treatment and opt to shift to other treatment line or have IUD removal.
For this reason; the prediction of the responsiveness to these drugs in controlling prolonged/heavy bleeding associated with Cu-IUDs is an interesting issue which should be addressed. The present study examines the hypothesis that patient’s data reported at baseline visit before starting ibuprofen can help in prediction of the responsiveness for this treatment at 3 months. Up to our knowledge; no clinical trial had been registered or conducted to study the potential predictors of responsiveness for ibuprofen in controlling the uterine bleeding with Cu-IUDs.
Materials and Methods
We reanalyzed data from a single-center open label prospective cohort study, conducted at Woman’s Health Hospital, Assiut University, Egypt between the 1st of October 2015 and the 30th of September 2016. This study had the approval of our Institutional Review Board and registered at www.clinicaltrials.gov (NCT02580344).
In this study, we included women who were complaining from prolonged/ heavy bleeding with Cu-IUDs. Other inclusion criteria required that participants were 20–45 years old, had a regular cycle, planning for birth spacing for at least 6-12 month, with no history of any medical problem or drug intake, no contraindications for ibuprofen, had a normal pelvic examination and living in the nearby vicinity to make the follow-up feasible. All eligible participants included in the study signed a written informed consent before participation after explaining the nature of the study.
We excluded women with evidence of defective coagulation, adnexal abnormality on ultrasound, history of hyperplasia in a previous endometrial biopsy, history of endometritis and history of allergy to NSAIDs. Additionally, we excluded women with adenomyosis, those receiving any haemostatic medications, and those declined to utilize ibuprofen or to maintain the menstrual diary.
Demographic characteristics, menstrual bleeding pattern as well as uterine volumetric measures and uterine Doppler blood flow indices related details were recorded before intervention. Then the eligible women were asked to receive ibuprofen 1200 mg per day for 5 days starting from first day of the cycle for the next three menses. The women were followed up monthly by menstrual diary, uterine volumetric measures by 2D TV US and uterine Doppler blood flow assessment.
Altogether, 140 women were recruited in the study period. We excluded five women who preferred to remove the Cu-IUD as a final option. The remaining women accepted to receive ibuprofen. Finally; seven cases were lost follow up and excluded from final analysis. This left 128 women at the end of the three months. Out of them, 70 patients (54.68%) respond to treatment and showed significant decreased in number of bleeding days per month, number of sanitary pads as well as significant increase in number of bleeding free days by menstrual diary after three consecutive months of use. While 58 patients (45.31%), at three months follow up, were still suffering from prolonged/heavy bleeding and choose to withdrawal their consent and leave the study.
Data analysis was performed using SSPS Statistics 21.0. The dichotomous dependent variable in the model was responsiveness to ibuprofen in controlling prolonged/heavy bleeding associated Cu-IUD after 3 months of use. The independent variables consisted of patient’s clinical baseline criteria, selected on the basis of previous published studies, uterine volumetric measure and uterine blood flow follow-up results, which might be affect the effectiveness of ibuprofen in controlling Cu-IUD associated prolonged/heavy bleeding.
To examine the relationship between individual patient, clinical and ultrasonographic, characteristics (at admission) and ibuprofen responsiveness in controlling the uterine bleeding with Cu-IUD at 3 months, while controlling for all other characteristics, we estimated a maximum likelihood model using multivariate linear regression. The model included all the variables reported, on theoretical grounds, might affect the ibuprofen response. Then, we represented the model’s b-coefficients and cleared them in the form of odds ratios with 95% confidence intervals (CI).
Finally; we constructed a receiver operating characteristic (ROC) curves to evaluate the sensitivity, specificity, positive and negative predictive values (PPV, NPV) and accuracy of the potential predictors revealed by logistic regression. We considered p- value < .05 as significant.
Results
Table 1 shows the clinical baseline characteristics of the study participants. Both groups, responders and non-responders, were homogenous regard most of the clinical baseline criteria with no significant differences, however; the responders had fewer number of bleeding days per month (5.1 ± 1.7 vs. 7.41 ± 1.7, p<0.001; respectively), fewer number of sanitary pads per day (3.8 ± 1.0 vs. 4.5 ± 1.2, p=0.001; respectively) and higher number of bleeding free days (23.6 ± 1.3 vs. 20.6 ± 1.2, p<0.001; respectively ) than non-responders at time of recruitment with statistically significant differences (Table 1).
As regard the uterine volumetric measurement and uterine blood flow at baseline, the uterine volume was significantly smaller in responders than non-responders with statistically significant difference (47.01 ± 6.5 mL vs. 60.9 ± 25.5 mL, p=0.001; respectively). Moreover; the uteri of non-responders had relatively higher blood flow than the responders with statically significant difference (p<0.001) (Table 2).
Table 3 showed the variables from baseline visit which might be significant predictors of increased likelihood of failure of ibuprofen after 3 months of use (Table 3). Women reporting higher number of bleeding days per month (OR [odds ratio] 3.26, 95% CI [confidence interval] 1.06-10.03, p<0.001), larger uterine volume (OR 1.26, 95% CI 1.00-1.59, p=0.03) and lower uterine PI (OR 0.15, 95% CI 0.23-0.95, p=0.005 were likely to fail to be controlled by ibuprofen (Table 3).
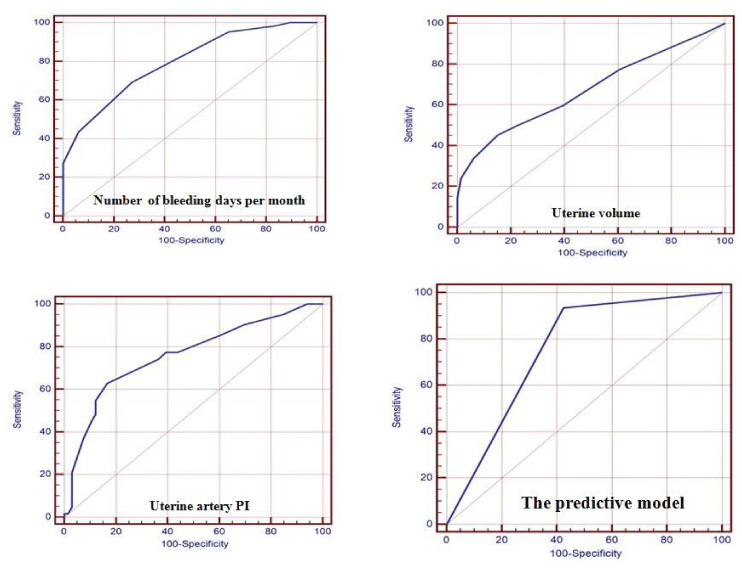
A ROC curve analysis including the number of bleeding days, uterine volume and uterine pulsatility index in a predictive model produced an area under the curve of (.795, .674, and .759; respectively). The analysis demonstrated that more than 6 days of menstrual bleeds clearly predicts the failure of ibuprofen therapy with a sensitivity (69.3%), specificity (72.7%), PPV (70.5%), NPV (71.6%) and accuracy (71.1%). While for the uterine size, a volume more than 49 ml could predict the non-response with a sensitivity (45.1%), specificity (84.8%), PPV (73.7%), NPV (62.2%) and accuracy (65.6%). A uterine PI < 2.02 yielded 62.9% sensitivity, 83.3% specificity, 78.0% PPV, 70.5% NPV and 73.4% accuracy.
A predictive model was constructed to predict the responsiveness to ibuprofen, and utilized the 3 prior predictors will have a 93.5% sensitivity, specificity (57.5%), PPV (67.4%), NPV (90.5%) and accuracy (75.0%). The ROC curves and corresponding sensitivities, specificities are presented in figure 1.
Discussion
We found in this study that more of bleeding days per months, higher uterine volume and lower uterine artery PI are independently associated with poor response to ibuprofen. The hypothesis behind this study was that none of the researchers studied or found an accepted explanation to those who did not respond to that important non hormonal line of treatment for prolonged/heavy uterine bleeding associated with Cu-IUDs.
For this reason; we examined the value of baseline clinical and ultrasonographic criteria of the women using Cu-IUDs and complaining of prolonged/heavy uterine bleeding in predicting the responsiveness to ibuprofen at 3 months follow up visit.
First of all; we reported comparable effectiveness for ibuprofen in controlling prolonged/heavy uterine bleeding with Cu-IUD (54.68%) as reported by Yarkoni [12], and Mercorio [13], who reported that NSAIDS controlled the uterine bleeding in about 57% and 46%, respectively. In spite, they used different type of NSAIDs (Mefenamic acid and indomethacin, respectively); we are all in the same track due to prostaglandin synthetase inhibiting activity of all mentioned drugs.
Although the Cochrane Review which included 15 randomized controlled trials with more than 2700 women found 15 trials addressed the role of NSAIDs in controlling pain and bleeding associated with Cu-IUD; none of them studied the potential predictors associated with successful effect of various NSAIDs in controlling pain and bleeding associated with IUD [8].
On the other hand, they are many studies in literature addressed the predictors associated with the discontinuation of Cu-IUDs due to pain and bleeding. One of these studies was reported by Stanback who concluded that women reporting intermenstrual bleeding or excessive menstrual flow at their 1-month follow-up are at increased risk for early discontinuation of the IUD [14].
More recent study done by Modesto and his colleagues and reported that the counseling strategy which expects and respects the abnormal bleeding patterns associated with long acting reversible contraception including IUD will followed by low rates of premature discontinuations and high rates of continuation and satisfaction [15]. Maguire et al in their study found that the preexisting dysmenorrhea may predict IUD removal within 1 year [16].
Also Kaislasuo et al investigated the relation between uterine cavity size and bleeding/pain as reasons for discontinuation of IUD and found that smaller uterine dimensions in nulligravid women do not predict difficulties or complications during IUD use [17]. Also Kaislasuo et al in their study found that baseline menstrual characteristics prior to IUD insertion were also strongly associated with both bleeding profile and pain experience during the use of this IUD.
Lee et al in 2016 used the uterine volume as a predictor for the effectiveness of levonorgestrel releasing IUD in controlling bleeding and pain with adenomyosis [18]. Our results were in agreement with this study which proved that failure of the LNG-IUS in controlling pain and bleeding with adenomyosis was associated significantly with a large volume uterus (> 150 mL). However; we reported much less uterine size (>49 ml), this is because the nature of his adenomyotic patients. Also Shaaban et al and Cho et al found that smaller uterine volume may lead to obvious improvement of uterine bleeding as well as the pain associated with adenomyosis when using LNG-IUS [19,20].
Many studies investigated the uterine blood flow in women using Cu-IUD and showed that those who developed heavy bleeding might be due to decrease vascular resistance in the uterine artery and subsequently, they reported decreased Doppler indices in those women [21-24].
We found that lower uterine PI (< 2.02), more uterine blood flow, was significantly associated with ibuprofen failure. Additionally; Hurskainen et al investigated the relation between uterine PI and menorrhagia and found that women with lower uterine flow resistance bleed more [25]. However, contrary to our finding; a study demonstrated that diclofenac, surprisingly, reduced uterine PI in absence of IUD and did not show any effect on the PI when IUDs were used [26]. The study population of this trial did not include any women with excessive blood loss related to the IUD insertion.
This study has both strengths and weaknesses. Strengths of our study include firstly that we are the first who addressed not only the potential clinical predictors but also the ultrasonographic predictors associated with ibuprofen failure in treating the uterine bleeding associated with Cu-IUD. Secondly, the data of this part of study was given from a prospective cohort study with calculated sample size for achieving sufficient power to detect a clinically significant difference in our primary outcome. However; the present work had some limitations. First, the subjectivity in reporting menstrual blood loss through the menstrual diary and objective parameters as hemoglobin or hematocrit levels were not included. Second, although our predictive model reported high sensitivity of 93.5%, but the specificity is still low (57.5%).
Conclusion
Women have excessive bleeding days, small uterine volume, low uterine artery PI are at high risk for unsatisfactory effect of ibuprofen on controlling heavy menstrual bleeding with Cu-IUDs. These data can further modulate the promotion of gynecologists for using NSAIDS for controlling heavy menstrual bleeding with Cu-IUDs.
- Evans T, Ramaswamy M, Satterwhite CL (2017) Availability of Long-Acting Reversible Contraception in Kansas Health Departments. J Rural Health 14: 186-189. Link: https://goo.gl/xiZR8P
- Phillips SJ, Hofler LG, Modest AM, Harvey LF, Wu LH, et al. (2017) Continuation of copper and levonorgestrel intrauterine devices: a retrospective cohort study. Am J Obstet Gynecol 217: 57. Link: https://goo.gl/QZ1h88
- Hubacher D, Reyes V, Lillo S, Pierre-Louis B, Zepeda A, et al. (2006) Preventing copper intrauterine device removals due to side effects among first-time users: randomized trial to study the effect of prophylactic ibuprofen. Hum Reprod 21: 1467-1472. Link: https://goo.gl/1Bqb9H
- Jatlaoui TC, Riley HE, Curtis KM (2016) The safety of intrauterine devices among young women: a systematic review. Contraception 95: 17-39. Link: https://goo.gl/jPv3xr
- Liang H, Li L, Yuan W, Zou Y, Gao ES, et al. (2014) Dimensions of the endometrial cavity and intrauterine device expulsion or removal for displacement: a nested case-control study 121: 997-1004. Link: https://goo.gl/dkBpYw
- Friedlander E, Kaneshiro B (2015) Therapeutic Options for Unscheduled Bleeding Associated with Long-Acting Reversible Contraception. Obstet Gynecol Clin North Am 42: 593-603. Link: https://goo.gl/9gvHVA
- Bradley LD, Gueye NA (2016) The medical management of abnormal uterine bleeding in reproductive-aged women. Am J Obstet Gynecol 214: 31-44. Link: https://goo.gl/4pCUog
- Grimes DA, Hubacher D, Lopez LM, Schulz KF (2006) Non-steroidal anti-inflammatory drugs for heavy bleeding or pain associated with intrauterine-device use. Cochrane Database Syst Rev. Link: https://goo.gl/UnUWqq
- (2014) Royal College of Obstetricians and Gynecologists. Advice for Heavy Menstrual Bleeding (HMB) Services and Commissioners.
- Forthofer KV (2009) A Clinical Review of the Intrauterine Device as an Effective Method of Contraception. J Obst Gynecol Neonat Nursing 38: 693-698. Link: https://goo.gl/wYPGLi
- Godfrey EM, Folger SG, Jeng G, Jamieson DJ, Curtis KM (2013) Treatment of bleeding irregularities in women with copper-containing IUDs: a systematic review. Contraception 87: 549-566. Link: https://goo.gl/h8Ufmk
- Yarkoni S, Anteby SO (1984) Treatment of IUD related menorrhagia by indomethacin. Clin Exper Obstet Gynecol 11: 120–122. Link: https://goo.gl/SoTVky
- Mercorio F, De Simone R, Di Carlo C, Bifulco G, Tessitore G, et al. Effectiveness and mechanism of action of desmopressin in the treatment of copper 18: 2319–2322. Link: https://goo.gl/zHeB6J
- Stanback J, Grimes D (1998) Can intrauterine device removals for bleeding or pain be predicted at a one-month follow-up visit? A multivariate analysis. Contraception. 58: 357-360. Link: https://goo.gl/rTQVw7
- Maguire K, Joslin-Roher S, Westhoff CL, Davis AR (2015) IUDs at One Year: Predictors of Early Discontinuation. Contraception 92: 575-577. Link: https://goo.gl/LEbRDk
- Modesto W, Bahamondes MV, Bahamondes L (2014) A randomized clinical trial of the effect of intensive versus non-intensive counselling on discontinuation rates due to bleeding disturbances of three long-acting reversible contraceptives. Hum Reprod 29: 1393-1399. Link: https://goo.gl/siaRDA
- Kaislasuo J, Heikinheimo O, Lähteenmäki P, Suhonen S (2014) Predicting painful or difficult intrauterine device insertion in nulligravid women. Obstet Gynecol 124: 345-353. Link: https://goo.gl/DWF2PX
- Lee KH, Kim JK, Lee MA, Ko YB, Yang JB, et al. (2016) Relationship between uterine volume and discontinuation of treatment with levonorgestrel-releasing intrauterine devices in patients with adenomyosis. Arch Gynecol Obstet 294: 561-566. Link: https://goo.gl/LVs2a8
- Shaaban OM, Ali MK, Sabra AM, Abd El Aal DE (2015) Levonorgestrel-releasing intrauterine system versus a lowdose combined oral contraceptive for treatment of adenomyotic uteri: a randomized clinical trial. Contraception 92: 301-307 . Link: https://goo.gl/x3Qevz
- Cho S, Nam A, Kim H, Chay D, Park K, et al. (2008) Clinical effects of the levonorgestrel-releasing intrauterine device in patients with adenomyosis. Am J Obstet Gynecol 198: 1-7. Link: https://goo.gl/eVkfvF
- Momtaz M, Zayed M, Rashid K, Idriss O (1994) Doppler study of the uterine artery in patients using an intrauterine contraceptive device. Ultrasound Obstet Gynecol 4: 231-234. Link: https://goo.gl/ULf1Gk
- Frajndlich R, von Eye Corleta H, Frantz N (2000) Color Doppler sonographic study of the uterine artery in patients using intrauterine contraceptive devices. J Ultrasound Med 19: 577-579. Link: https://goo.gl/bAHPPa
- de Souza MAM, Geber S (2006) Doppler Color Flow Analysis of the Uterine Arteries Before and After Intrauterine Device Insertion A Prospective Study. J Ultrasound Med 25: 153-157. Link: https://goo.gl/uZiXev
- Yigit N, Kacar M, Yigit H, Kosar P, Kosar U (2009) The effects of copper contraceptive intrauterine device on the uterine blood flow: A prospective transvaginal Doppler study. J Clin Ultrasound 37: 380-384. Link: https://goo.gl/x1YtM6
- Hurskainen R, Teperi J, Paavonen J (1999) Cacciatore B Menorrhagia and uterine artery blood flow. Hum Reprod 14: 186-189. Link: https://goo.gl/rE7rFT
- Jarvela I, Tekay A, Jouppila P (1998) The effect of diclofenac on uterine artery blood flow resistance during menstruation in patients with and without a copper intrauterine device. Human Reprod 13: 2480-2483. Link: https://goo.gl/2rh9de
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
