Journal of Gynecological Research and Obstetrics
Pseudomemberanous Colitis following Cesarean Delivery with Adjunctive Intracolonic Vancomycin
Farahnaz Sadat Ahmadi*, Zahra Rezaee, Fatemeh Davari Tanha, Sepideh Nekuie, Soleiman Abbasi and Sara Abbasi2
Cite this as
Ahmadi FS, Rezaee Z, Tanha FD, Nekuie S, Abbasi S, et al. (2016) Pseudomemberanous Colitis following Cesarean Delivery with Adjunctive Intracolonic Vancomycin. J Gynecol Res Obstet 2(1): 055-060. DOI: 10.17352/jgro.000020Background: Pseudomembranous colitis is a rarely reported condition in obstetrics. The clinical presentation varies from a self-limiting diarrhea to a severe pseudomembranous colitis with toxic megacolon and subsequent death. The process of this disease is mainly associated with prior antibiotic intake.
Case presentation: A 32 year-old, G4L4RIV woman was admitted 3 days after her delivery with severe diffuse abdominal pain and distention. The patient had intravenously cefazolin after cord clamping. The colonoscopy showed pseudomembranous colitis. She was managed conservatively and after 12 days of medical treatment the patients was discharged in a good condition.
Conclusion: In the patients with low grade fever, abdominal distention and diarrhea after delivery, pseudomembranous colitis should be considered a possible diagnosis in order initiate the treatment as soon as possible.
Introduction
Pseudomembranous colitis (PMC) is a type of colitis (and sometimes enteritis), with special pathologic and oftentimes radiologic features, which is usually associated with the overgrowth of a bacterium called Clostridium difficile. Although this condition normally occurs following antibiotic consumption, it may rarely happen in other settings such as bowel ischemia, intestinal obstruction, and intestinal surgery or chemotherapy. Moreover, gastrointestinal infections (e.g. Staphylococcus aureus) can induce a similar condition [1].
A recent increase in the incidence rate and severity of clostridium difficile infection has been reported, which is thought to be due to the great use of broad spectrum antibiotics worldwide and the development of a hypervirulant strain of this organism [2-4]. The presentation of PMC can vary from a self-limiting diarrhea to toxic megacolon and death [5]. Yet, many cases have confusing clinical features and their condition may not be diagnosed immediately [1]. History of previous antibiotic intake especially cephalosporins and flouroquinolones is the most important risk factor for PMC [6-8]. This risk is at its peak within the first month after antibiotic use [9].
Immediate diagnosis and treatment of this condition can decrease the adverse features to a great deal. In this article we are going to present a patient with previous history of PMC who had been saved from definite death through emergent diagnosis and treatment.
Case Report
The patient is a 32-year-old gravida 4 , para 4 , repeat 4 woman who presented to hospital Shohada in Gonbadekavus due to abdominal pain and distention three days after cesarean section. She reported a history of same symptoms after the past cesarean section for which she had been hospitalized and treated for 7 days.
According to the operation report numerous adhesions were detected during surgery, many of which especially those in the uterine incision site had been released. The patient received one dose of prophylactic cefazolin after clamping of the umbilical cord. Before discharge (discharged with personal consent) she had gas passage and defecation.
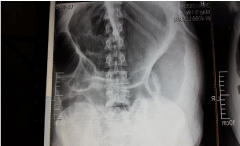
Three days after C/S the patient was hospitalized with a presumed ileus. At that point she seemed well and had no fever and tachycardia; the abdomen was distended; bowel sounds were normal. On palpation, tenderness in left lower quadrant without rebound was present. In chest auscultation no abnormal finding except dextrocardia was detected (Figures 1,2).
Initially the patient was managed conservatively, laboratory tests and surgery consult were ordered.
The patient was not allowed to take anything by mouth and it was well emphasised to her to stay mobile. Despite gas passage her abdominal distention fluctuated during the first two days of hospitalization. During this period the laboratory findings were normal, no leucocytosis was present and hemoglobin and electrolytes were normal. Just on abdominal x ray( upright and flat) hyperinflation was to be seen.
After the second day, diarrhea and a 38 C fever were added to the patients’ signs. The diarrhea contained greenish liquid with white cheese -like parts. The patient was not ill, the abdominal palpation revealed no change and the laboratory findings were still normal.
On the third day, abdominal distention was noticeably increased and the abdomen became tenser. Emergent surgery and gastrointestinal consult were ordered and the patient was admitted to the intensive care unit. At that point potassium was as low as 2.4 and coagulation profile was disturbed, too. Hematology consult was ordered. Still, no leucocytosis was present. Abdominal ultrasound reported moderate ascites, compared to the first day of hospitalisation.
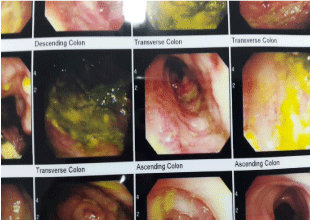
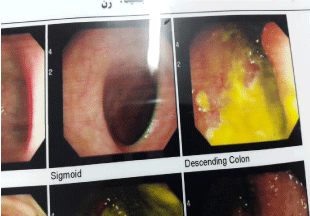
On colonscopy, typical appearance of PMC was detected and vancomycin was poured onto the lesions and decompression was performed. Treatment was continued with parenteral metronidazol and oral vancomycine (Figures 3,4). Pathological report was consistent with PMC.
In the ICU, the patient received TPN for 7 days, after which oral nutrition was initiated. After 10 days she was released from the ICU and being hospitalized in the surgery ward for 2 days; the patient was discharged in good health.
Discussion
The first pseudomembranous lesion of the intestines was reported in 1893, on the bulletin of Johns Hopkines hospital [10]. The case was a woman of 22 years old who had developed progressive diarrhea, which eventually led to her death, after a gastric tumor resection. Dr. Osler who was her physician used the description of “a miserable emaciated creature in a wretched physical condition” for her. Her autopsy revealed a diphteritic membrane in the small bowel [10].
Pseudomembranous enterocolitis (PMEC) is a condition described by presence of pseudomembranes on the colonic or small bowel mucosa. This disease usually involves the colonic mucosa (PMC), yet it may sometimes appear in the small bowel (PMEC). These two conditions have a specific similar characteristic and that is the presence of pseudomembranes. Although clinical features, severity, and potential fatal outcome are other similar features [10].
Clostridium difficile is a spore forming gram positive anaerobic bacillus which damages the mucosa through two toxins: A and B. Toxin A loosens junctions between epithelial cells and is enterotoxic as well as cytotoxic. Toxin B induces breakdown of cytoskeleton as it is a powerful cytotoxin. Besides their cytotoxic effects, these two productions induce production of cytokines resulting in a noticeable inflammatory response [11].
Clinical presentation of this disease can be very confusing; therefore, diagnosis may be delayed. These patients usually undergo radiologic surveys for abdominal pain, fever and presence of leukocytosis [10].
Over the past two decades there has been a dramatic increase in the prevalence of C.difficil due to its widespread nature and the increasing number of immune compromised patients [12-16]. This organisms finds its way through the GI tract early in life [16]. With a colonization rate of more than 50%, neonates frequently acquire this organism. This colonization rate is reduced to 5-10% at 6 months, reaching the 3% of adult carriage rate [17].
Durung periods in which C.difficile is not epidemic , the incidence rate of C.difficile colitis is reported to be 0.1–30 in 1000 patients in nosocomial settings and 8–12 in 100,000 persons in year in community settings [18-26].
96%–100% of pseudomembranous colitis cases, 60%–75% of antibiotic-associated colitis cases, and 11%–33% of cases with diarrhea due to antibiotic intake are associated with C. difficile [27-42].
According to available evidence, this organism can readily be transmitted among hospitalized patients, as its spores are not only resistant to heat but they also are resistant to drying and therefore are persistent in the hospital environment [43].
PMC, due to previous antibiotic intake is strongly associated with C difficile. Although all antibiotics can presumably result in C difficile-associated PMC, clindamycin and lincomycin have been shown to be the most responsible ones [16].
Cephalosporins either first, second or third generation increase the risk of C.difficile; yet, 3rd generation cephalosporins are proved to have the highest influence on bacterial flora of the intestines due to an active metabolite which is secreted into small intestines from gallbladder [44,45-47].
The clinical presentation of PMC may vary from a mild diarrhea to severe septic shock due to colitis (1–3%); but it should be taken into consideration that rare cases of PMC without diarrhea have been reported and that in patients with mild or moderate disease abdominal pain, fever, and leukocytosis may be absent. Noteworthy is that even in some severe cases with ileus and toxic megacolon, diarrhea could be absent and sepsis, haemodynamic instability and perforation may occur [48].
Although colectomy is required very rarely, it is of necessity in patients with toxic megacolon peritonitis, perforation or massive bloody diarrhea [49]. Using stool samples, small numbers of C.difficile can be cultured (in up to 35% of cases), nevertheless in 90%-lOO% of patients with PMC related to antibiotics its toxin can be detected in stool [17,50,51].
This disorder, the bowel is often dilated and on pathological survey mucosal patterns are lost. Besides that, the mucosal surface is raised, slightly, with yellow-green foci of different size and appearance (from bran-like parts to large yellow friable membrane); due to the clearly defined demarcation and fibrinous surface, these foci, may be interpreted as ulceration by inexperienced eyes. Ulcers may occur and perforate resulting in peritonitis. But according to one study in most cases death preceded before ulceration occurred.the exudate has a specific appearance microscopically. It consists of fibrin, mucus, and variable number of leucocytes [52].
By fibrinoid necrosis of the mucosa small foci are formed and it is from this parts that fibrinous material breaks open and propagate laterally. Concomitantly, excessive amounts of mucus is secreted from adjacent glandular tubules. This mucus is then mixed with the transuded fibrin. Exudates, together with the necrotic superficial parts of mucosa blend together and form a broad membrane. Capillaries of the mucosa may be a part of the fibrinoid necrosis but vasculitis is not a characteristic of this lesion [52].
The change from normal mucosa to dilated, actively secreting glandular tubules covered by a layer of exudate is characteristic for pseudo-membranous colitis [52].
Current treatment for C. difficile diarrhea is supportive care and withdrawing the offending antibiotic, in addition to using Metronidazole or Vancomycin to eradicate the bacteria [53]. In most patients this approach produces a quick response; however, some cases may have a more refractory course.
Newer treatments are more focused on binding to the toxin (Anion resins, Immunoglobulins), improving host immune response (vaccination), using alternative antibiotics (Rifaxamin, Nitazoxanide, OPT-80, Tinidazole), and changing the intestinal flora (probiotics, faecal bacteriotherapy) [54].
Although these treatments help reduce inflammation, they are not targeted at the offending process directly. PMC patients have noticeable inflammatory response in the mucosa of the colon with histological similarities (epithelial necrosis and neutrophil infiltration) to inflammatory bowel disease (IBD), for which steroids are greatly used.
Before identification of C. difficile as the responsible agent, steroids were widely used in the treatment of pseudomembranous colitis. In a case series from 1976, the use of steroids was reported to be of good efficacy in up to 47% of patients [55,56].
In some cases, severity of the disease can lead to toxic megacolon, ileus, or toxic enterocolitis. Treating with oral or intravenous antimicrobial regimens has been shown to be unsuccessful mainly due to inadequate concentration of the drug in the colon [57-59]. There is some evidence available that adding intra colonic vancomycin, as an alternative route, can be promising as in 57%–75% of patients [60,61-70]. Intravenous metronidazole, in some circumstances, produces fecal concentrations greater than the MIC needed for C. difficile; therefore, it may be an effective therapy [71]. However, the efficacy of intravenous metronidazole therapy still remains debatable, since its excretion occurs mostly in the upper parts of gastrointestinal tract and as low as 14% of each intravenously administered dose is excreted in the feces [68].
Despite some data suggesting effectiveness of intravenous metronidazole in antibiotic-associated [71,72], there are some reports of treatment failure in the presence of [73].
Moreover, the delivery of metronidazole was not predictable in patients with ileus [30], and as clinical resolution took place the amount of the medication and its detected metabolite decreased [71].
Consequently, addition of ICV, administered by rectal enema or long catheters, is an alternative regimen especially in severely ill patients who have not responded to first-line therapy or are not eligible for it [60,61-70].
George and colleagues [74], were the first group who recommended the use of adjunctive ICV in patients with ileus or toxic megacolon.
Griebie and Adams [66], reported effectiveness of adjunctive ICV therapy for C. difficile colitis in a patient who had had head and neck surgery and had severe ileus and colonic obstruction. There are also several case reports available, which have maintained the effectiveness of adjunctive ICV therapy in treatment of patients with severe ileus or impaired oral intake [60,61-70].
Olson and colleagues have reported the success of a standard initial dose of adjunctive ICV therapy in 6 of 8 (75%) patients who had C. difficile–associated colitis and subsequent ileus [60].
Similarly, Shetler and colleagues have claimed that colonoscopic decompression along with adjunctive ICV can be used in treatment of severe pseudomembranous colitis [68].
Taking all these into account, it can be concluded that adequate amounts of clindamycine can be delivered to the toxin production site and conquer the process. Several recommendations are given regarding adjunctive ICV treatment of C. difficile colitis [59,74-79]. Orally administered vancomycin can result in significant.
Sserum levels in the presence of severe mucosal inflammation or decreased drug excretion due to renal failure [49,80].
In order to avoid neuro- and nephrotoxicity, the adjunctive ICV therapy dose is preferred to be reduced to keep the serum concentration between 30 and 40 mg/L in patients with renal insufficiency [49,80]. Therefore, after administering ICV in such patients, monitoring the serum level of vancomycin should be considered, in order to increase the safety of this regimen [79,81].
There are several concerns regarding the delivery of ICV to the transverse and ascending colon [60,61-70]. Inadequate delivery of ICV to the proximal colon is a contributory factor in treatment failure of some cases.
In our study it was first decided to manage the patient conservatively, but after deterioration of her condition obstruction was considered a good diagnosis, especially with the reported adhesion bands during surgery. Yet, laboratory studies, physical examination and having defecation were against this hypothesis.
In the performed colonoscopy an extensive yellow membrane, green feces and great hyperinflation were detected. Descending colon and about one half of transverse colon were involved, while ascending colon was involved in patchy pattern. Considering the colonoscopic appearance, vancomycine was poured on all parts of the colon, but whether to continue the course with oral or intravenous metronidazol was disputed. In the end, 500 mg metronidazol was administered intravenously every 6 hours and oral vancomycin was prescribed every 12 hours. We did not use corticosteroids in our treatment course. It should be mentioned that infectious disease specialist had added tinidazol for 5 days to the regimen and this has helped the patient to a great deal, from our view. After 7 days of great challenges, abdominal distention and ascites started to subside.
Conclusion
PMS should be considered as a possibility in all patients with ileus after operation, especially when symptoms are accompanied by diarrhea and fever.
We would like to thank our general surgeons Dr. soleiman Alaghi and Dr. shokrollah yadegari; our GI specialist Dr. Abdolsamad Gharavy, our pathologist Dr. Hendi, our infectious disease specialist Dr. Bahram Alaghi, our internal medicine specialists Dr. Esmaili and Dr. Ajami, all the ICU staff of Shahid Motahari hospital of Gonbadekavus, Mrs. Beski the matron of the Beski hospital.
- Ros PR, Buetow PC, Brown LP, Forsmark CE, Sobin LH (1996) Pseudomembranous colitis. Radiology 198: 1-9.
- Rouphael NG, O’Donnell JA, Bhatnagar J, Lewis F, Polgreen PM, et al. (2008) Clostridium difficile-associated diarrhea: an emerging threat to pregnant women. Am J Obstet Gynecol 198: 635. e1-6.
- Ghai S, Ghai V, Sunderji S (2007) Fulminant postcesarean Clostridium difficile pseudomembranous colitis. Obstet Gynecol 109: 541-543.
- Pépin J, Valiquette L, Alary ME, Villemure P, Pelletier A, et al. (2004) Clostridium difficile-associated diarrhea in a region of Quebec from to 2003: a changing pattern of disease severity. Can Med Assoc J 171: 466-472.
- Price MF, Dao-Tran T, Garey KW, Graham G, Gentry LO, et al. (2007) Epidemiology and incidence of Clostridium difficileassociated diarrhoea diagnosed upon admission to a university hospital. J Hosp Infect 65: 42-46.
- Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, et al. (2010) Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol 31: 431–455.
- Nelson DE, Auerbach SB, Baltch AL, Desjardin E, Beck-Sague C, et al. (1994) Epidemic Clostridium difficile-associated diarrhea: role of second and third-generation cephalosporins. Infect Control Hosp Epidemiol 15: 88–94.
- Pépin J, Saheb N, Coulombe MA, Alary ME, Corriveau MP, et al. (2005) Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis 41: 1254–1260.
- Hensgens MP, Goorhuis A, Dekkers OM, Kuijper EJ (2012) Time interval of increased risk for Clostridium difficile infection after exposure to antibiotics. J Antimicrob Chemother 67: 742–748.
- Kleckner MS, Burgen JA, Baggenstoss AH (1952) Pseudomembranous enterocolitis: cinicopathologic study of fourteen cases in which the disease was not preceded by an operation. Gastroenterology 21: 212-217.
- Hookman P, Barkin JS (2009) Clostridium difficile associated infection, diarrhea and colitis. World J Gastr oenterol 15: 1554-1580.
- Klingler PJ1, Metzger PP, Seelig MH, Pettit PD, Knudsen JM, et al. (2000) Clostridium difficile infection: risk factors, medical and surgical management. Dig Dis 18: 147–160.
- Anand A, Glatt AE (1993) Clostridium difficile infection associated with antineoplastic chemotherapy: a review. Clin Infect Dis 17: 109–113.
- Aronsson B, Mollby R, Nord CE (1985) Antimicrobial agents and Clostridium difficile in acute enteric disease: epidemiological data from Sweden, 1980–1982. J Infect Dis 151: 476-481.
- Trnka YM, LaMont JT (1984) Clostridium difficile colitis. Adv Intern Med 29: 85-107.
- Fenoglio-Preiser CM, Lantz PE, Listrom MB, Davis M, Rilke FO (1989) Gastrointestinal pathology: an atlas and text. New York, NY: Raven 661-663: 1033-1037.
- Bartlett JG (1990) Clostridium difficile: clinical considerations. Rev Infect Dis 12: S243-S251.
- Kyne L, Farrell RJ, Kelly CP (2001) Clostridium difficile. Gastroenterol Clin North Am 30: 753–777.
- Alfa MJ, Du T, Beda G (1998) Survey of incidence of Clostridium difficile infection in Canadian hospitals and diagnostic approaches. J Clin Microbiol 36: 2076–2080.
- Lai KK, Melvin ZS, Menard MJ, Kotilainen HR, Baker S (1997) Clostridium difficile–associated diarrhea: epidemiology, risk factors, and infection control. Infect Control Hosp Epidemiol 18: 628–632.
- Olson MM, Shanholtzer CJ, Lee JT Jr, Gerding DN (1994) Ten years of prospective Clostridium difficile–associated disease surveillance and treatment at the Minneapolis VA Medical Center, 1982–1991. Infect Control Hosp Epidemiol 15: 371–381.
- Samore MH (1999) Epidemiology of nosocomial Clostridium difficile diarrhea. J Hosp Infect 43: S183–190.
- Samore MH, DeGirolami PC, Tlucko A, Lichtenberg DA, Melvin ZA, et al. (1994) Clostridium difficile colonization and diarrhea at a tertiary care hospital. Clin Infect Dis 18: 181–187.
- Struelens MJ, Maas A, Nonhoff C, Deplano A, Rost F, et al. (1991) Control of nosocomial transmission of Clostridium difficile based on sporadic case surveillance. Am J Med 91: 138S–144S.
- Hirschhorn LR, Trnka Y, Onderdonk A, Lee ML, Platt R (1994) Epidemiology of community-acquired Clostridium difficile–associated diarrhea. J Infect Dis 169: 127–133.
- Levy DG, Stergachis A, McFarland LV, Van Vorst K, Graham DJ, et al. (2000) Antibiotics and Clostridium difficile diarrhea in the ambulatory care setting. Clin Ther 22: 91–102.
- Jobe BA, Grasley A, Deveney KE, Deveney CW, Sheppard BC (1995) Clostridium difficile colitis: an increasing hospital-acquired illness. Am JSurg 169: 480–483.
- Tedesco FJ (1982) Pseudomembranous colitis: pathogenesis and therapy. Med Clin North Am 66: 655–664.
- McFarland LV, Mulligan ME, Kwok RY, Stamm WE (1989) Nosocomial acquisition of Clostridium difficile infection. N Engl J Med 320: 204–210.
- McFarland LV, Stamm WE (1996) Review of Clostridium difficile–associated diseases. Am J Infect Control 14: 99–109.
- Bartlett JG (1984) Antibiotic-associated colitis. Dis Mon 30: 1–54.
- Rosen L (1991) Review of Clostridium difficile associated diseases. In: Schrock TR, ed. Perspectives in colon and rectal surgery. St Louis, MO: Quality Medical Publishing 205–214.
- Kofsky P, Rosen L, Reed J, Tolmie M, Ufberg D (1991) Clostridium difficile--a common and costly colitis. Dis Colon Rectum 34: 244–248.
- George WL, Rolfe RD, Finegold SM (1980) Treatment and prevention of antimicrobial agent-induced colitis and diarrhea. Gastroenterology 79: 366–372.
- Gerding DN, Olson MM, Johnson S, Peterson LR, Lee JT Jr (1990) Clostridium difficile diarrhea and colonization after treatment with abdominal infection regimens containing clindamycin or metronidazole. Am J Surg 159: 212–217.
- Tedesco FJ, Napier J, Gamble W, Chang TW, Bartlett JG (1979) Therapy of antibiotic-associated pseudomembranous colitis. J Clin Gastroenterol 1: 51–54.
- Bartlett JG (1985) Treatment of Clostridium difficile colitis. Gastroenterology 89: 1192–1195.
- Bartlett JG, Laughon BE (1985) The microbiology and pathogenesis of pseudomembranous colitis. Surv Synth Pathol Res 4: 152–162.
- Fekety R, Kim KH, Batts DH, Browne RA, Cudmore MA, et al. (1980) Studies on the epidemiology of antibiotic-associated Clostridium difficile colitis. Am J Clin Nutr 33: 2527–2532.
- Foulke GE, Silva J Jr (1989) Clostridium difficile in the intensive care unit: management problems and prevention issues. Crit Care Med 17: 822–826.
- Finney JMT (1893) Gastroenterostomy for cicatrizing ulcer of the pylorus. Bull Johns Hopkins Hosp 4: 53–55.
- Morris JB, Zollinger RM Jr, Stellato TA (1990) Role of surgery in antibioticinduced pseudomembranous enterocolitis. Am J Surg 160: 535–539.
- Gerding DN, Olson MM, Peterson LR, et al. (1986) Clostridium difficile-associated diarrhea and colitis in adults: a prospective case controlled epidemiologic study. Arch Intern Med 146: 95-100.
- Hensgens MP, Goorhuis A, Dekkers OM, Kuijper EJ (2012) Time interval of increased risk for Clostridium difficile infection after exposure to antibiotics. J Antimicrob Chemother 67: 742–748.
- Baxter R, Ray GT, Fireman BH (2008) Case–control study of antibiotic use and subsequent Clostridium difficile-associated diarrhea in hospitalized patients. Infect Control Hosp Epidemiol 29: 44–50.
- Al-Obaydi W, Smith CD, Foguet P (2010) Changing prophylactic antibiotic protocol for reducing Clostridium difficile-associated diarrhoeal infections. J Orthop Surg (Hong Kong) 18: 320–323.
- Al-Eidan FA, McElnay JC, Scott MG, Kearney MP (2000) Clostridium difficile-associated diarrhoea in hospitalised patients. J Clin Pharm Ther 25: 101–109.
- Mylonakis E, Ryan ET, Calderwood SB (2001) Clostridium difficile-associated diarrhea: a review. Arch Intern Med 161: 525–533.
- Kelly CP, Pothoulakis C, LaMont JT (1994) Clostridium difficile colitis. N Engl J Med 330: 257–262.
- Boland GW, Lee MJ, Cats A, Mueller PR (1994) Pseudomembranous colitis: diagnostic sensitivity of the abdominal pain radiograph. Clin Radiol 49: 473-475.
- Taylor NS, Thorne GM, Bartlett JG (1981) Comparison of two toxins produced by Cbstridium difficile. Infect Immun 34: 1036-1043.
- Goulston SJM, McGovern VJ (1965) Pseudo-membranous colitis Pablo. Gut 6: 207.
- (2008) Department of Health. Clostridium difficile infection: How to deal with the problem 2008:
- Kelly CP, LaMont JT (2008) Clostridium difficile--more difficult than ever. N Engl J Med 359: 1932-1940.
- Goodman MJ, Truelove SC (1976) Intensive intravenous regimen for membranous colitis. Br Med J 2: 354.
- Keeffe EB, Katon RM, Chan TT, Melnyk CS, Benson JA, Jr (1974) Pseudomembranous enterocolitis. Resurgence related to newer antibiotic therapy. West J Med 121: 462-472.
- Bartlett JG (2002) Clinical practice. Antibiotic-associated diarrhea. N Engl J Med 346: 334–339.
- Counihan TC, Roberts PL (1993) Pseudomembranous colitis. Surg Clin North Am 73: 1063–1074.
- Maggiolo F, Bianchi W, Ohnmeiss H (1989) A new approach to the treatment of pseudomembranous colitis? J Infect Dis 160: 170–171.
- Olson MM, Shanholtzer CJ, Lee JT Jr, Gerding DN (1994) Ten years of prospective Clostridium difficile-associated disease surveillance and treatment at the Minneapolis VA Medical Center, 1982–1991. Infect Control Hosp Epidemiol 15: 371–381.
- Griebie M, Adams GL (1985) Clostridium difficile colitis following head and neck surgery: report of cases. Arch Otolaryngol 111: 550–553.
- Goodpasture HC, Dolan PJ, Jacobs ER, Meredith WT (1986) Pseudomembranous colitis and antibiotics. Kans Med 87: 133: 146.
- Osler T, Lott D, Bordley J 4th, Lynch F, Ellsworth C, et al. (1986) Cefazolin-induced pseudomembranous colitis resulting in perforation of the sigmoid colon. Dis Colon Rectum 29: 140–143.
- Johnson S, Adelmann A, Clabots CR, Peterson LR, Gerding DN (1989) Recurrences of Clostridium difficile diarrhea not caused by the original infecting organism. J Infect Dis 159: 340–343.
- Bagwell CE, Langham MR Jr, Mahaffey SM, Talbert JL, Shandling B (1992) Pseudomembranous colitis following resection for Hirschsprung’s disease. J Pediatr Surg 27: 1261–1264.
- Pasic M, Jost R, Carrel T, Von Segesser L, Turina M (1993) Intracolonic vancomycin for pseudomembranous colitis. N Engl J Med 329: 583.
- Bublin JG, Barton TL (1994) Rectal use of vancomycin. Ann Pharmacother 28: 1357–1358.
- Shetler K, Nieuwenhuis R, Wren SM, Triadafilopoulos G (2001) Decompressive colonoscopy with intracolonic vancomycin administration for the treatment of severe pseudomembranous colitis. Surg Endosc 15: 653–659.
- Nathanson DR, Sheahan M, Chao L, Wallack MK (2001) Intracolonic use of vancomycin for treatment of Clostridium difficile colitis in a patient with a diverted colon: report of a case. Dis Colon Rectum 44: 1871–1872.
- Apisarnthanarak A, Khoury H, Reinus WR, Crippin JS, Mundy LM (2002) Severe Clostridium difficile colitis: the role of intracolonic vancomycin? Am J Med 112: 328–329.
- Bolton RP, Culshaw MA (1986) Faecal metronidazole concentrations during oral and intravenous therapy for antibiotic associated colitis due to Clostridium difficile. Gut 27: 1169–1172.
- Kleinfeld DI, Sharpe RJ, Donta ST (1998) Parenteral therapy for antibioticassociated pseudomembranous colitis. J Infect Dis 157: 389.
- Guzman R, Kirkpatrick J, Forward K, Lim F (1988) Failure of parenteral metronidazole in the treatment of pseudomembranous colitis. J Infect Dis 158: 1146–1147.
- George WL, Rolfe RD, Finegold SM (1980) Treatment and prevention of antimicrobial agent-induced colitis and diarrhea. Gastroenterology 79: 366–372.
- Fekety R, Shah AB (1993) Diagnosis and treatment of Clostridium difficile colitis. JAMA 269: 71–75.
- Silva J Jr (1989) Update on pseudomembranous colitis. West J Med 151: 644–648.
- Thielman NM (2000) Antibiotic-associated colitis. In: Mandell GL, Douglas RG, Bennett JE, eds. Principles and practice of infectious diseases. 5th ed. New York: Churchill Livingstone 1111–1126.
- Guerrant RL, Van Gilder T, Steiner TS, Thielman NM, Slutsker L, et al. (2001) Practice guidelines for the management of infectious diarrhea. Clin Infect Dis 32: 331–351.
- Gerding DN, Johnson S, Peterson LR, Mulligan ME, Silva J Jr (1995) Clostridium difficile–associated diarrhea and colitis. Infect Control Hosp Epidemiol 16: 459-477.
- Spitzer PG, Eliopoulos GM (1984) Systemic absorption of enteral vancomycin in a patient with pseudomembranous colitis. Ann Intern Med 100: 533–534.
- Dzink J, Bartlett JG (1980) In vitro susceptibility of Clostridium difficile isolates from patients with antibiotic-associated diarrhea or colitis. Antimicrob Agents Chemother 17: 695–698.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
