Journal of Gynecological Research and Obstetrics
Lack of Association between the 4234G/C X-Ray Repair Cross-Complementing 2 (XRCC2) Gene Polymorphism and the Risk of Endometrial Cancer among Polish Population
Hanna Romanowicz1*, Magdalena Bryś2, Ewa Forma2 and Beata Smolarz1
2Department of Cytobiochemistry, Faculty of Biology and Environmental Protection, University of Lodz, Pomorska, Lodz, Poland
Cite this as
Romanowicz H, Brys M, Forma E, Smolarz B (2016) Lack of Association between the 4234G/C X-Ray Repair Cross-Complementing 2 (XRCC2) Gene Polymorphism and the Risk of Endometrial Cancer among Polish Population. J Gynecol Res Obstet 2(1): 047-050. DOI: 10.17352/jgro.000018Objective: One of the major causes of carcinogenesis is loss of genome stability. The double-strand break DNA repair pathway, including X-ray repair cross complementing group 2 (XRCC2) gene, is implicated in maintenance integrity of genome and therefore could affect endometrial cancer (EC) risk. The purpose of this study was to evaluate the clinical significance of the XRCC2 4234G/C (rs3218384) gene single nucleotide polymorphism (SNP) in endometrial cancer patients.
Material and Methods: The study included 1632 patients: 808 with endometrial cancer and 824 healthy controls. XRCC2 4234G/C (rs3218384) polymorphism was genotyped by the PCR-RFLP (restriction fragment-length polymorphism) method. The associations of the analysed genotypes and clinical data at diagnosis have been evaluated.
Results: The frequencies of genotype of the 4234G/C XRCC2 polymorphism did not differ significantly between patients and controls. The current study failed to show the correlation between XRCC2 genotypes and histological grading. Analyzed polymorphism was also unrelated to the patient age, body mass index, number of pregnancies, uterine bleeding, endometrial ultrasound transvaginal, diabetes and hypertension.
Conclusion: This is the first article of 4234G/C polymorphism in XRCC2 gene and EC risk. The current study failed to show the association between the 4234G/C XRCC2 polymorphism and clinical data of patients with endometrial cancer.
Introduction
There are several biochemical pathways that can lead to cancerogenesis, one of which involves DNA damage induced by exogenous carcinogens or by endogenous metabolic processes. The double-strand break DNA repair pathway, including XRCC2 gene, is implicated in maintaining genomic stability and therefore could affect cancer risk. Common genetic polymorphisms in DNA repair genes might affect protein function and thus the capacity of repair DNA damage, which in turn could lead to genetic instability [1,2].
Single nucleotide polymorphisms (SNPs) were found in nearly all human DNA repair genes that have been investigated so far, and some of them were shown to modulate levels of DNA damage, individual DNA repair capacity and cancer risk. Among them, polymorphisms of X-ray repair cross complementing group 2 (XRCC2) have been studied extensively [3-5].
The XRCC2 gene, located at 7q36.1, is an essential part of the homologous recombination repair pathway and a functional candidate for involvement in cancer progression. Common variants within XRCC2, including Arg188His polymorphism, have been identified as potential cancer susceptibility loci in recent studies, although association results are controversial. The Arg188His polymorphism has been proposed to be a genetic modifier for pancreatic cancer and was associated with an increased risk of breast, laryngeal and oral cancers [6-9].
Recently, a large number of studies have attempted to identify the association between this polymorphism and other human cancers such as ovarian cancer, thyroid cancer, and colorectal cancer. However, results of these studies still remain inconsistent rather than conclusive [4,10].
XRCC2 -41657C/T polymorphism was associated with the risks of many cancers, such as esophageal squamous cell carcinoma (ESCC), gastric cardia adenocarcinoma (GCA), smoking- drinking-related laryngeal cancer or ovarian cancer [8,11,12].
Some reports provide the proof that the XRCC2 41657C/4234C and 41657T/4234G haplotypes were related to increased risk of GCA [11]. To find reports that directly link SNP 4234G/C in DNA repair gene XRCC2 with endometrial cancer (EC) incidence poses a serious difficulty. Yet, to our knowledge, there are no reports that assess the effect of XRCC2 4234G/C polymorphism on the risk of EC.
The purpose of this study was to evaluate the clinical significance of the 4234G/C (rs3218384) XRCC2 gene polymorphism in patients with EC.
Materials and Methods
Patients
The study included 808 patients with endometrial cancer. Analysed patients were hospitalized in Department of Surgical Gynaecology and Gynaecologic Oncology, Institute of Polish Mothers Memorial Hospital, between 2000-2013. All patients involved into the study were Caucasians. The age of patients ranged in from 50 to 85 years (the mean age 59.1 ± 10.12). 824 individuals treated in the parallel period for uterine fibroids constituted the control group (age range 49–83, mean age 54.24±11.16).
Only patients with confirmed pathology diagnosis of endometrial carcinoma were included into the study. The associations of the analysed genotypes and clinical data at diagnosis have been evaluated. The following demographic and clinical data have been analysed: age, histological grade, body mass index, number of pregnancies, uterine bleeding, endometrial ultrasound transvaginal, diabetes and hypertension.
All the diagnosed tumours were graded by criteria of the International Federation of Gynaecology and Obstetrics (FIGO). Histological grade was based on the degree of glandular differentiation, and tumors were graded as: G1 (percentage of solid growth in the tumor mass up to 5%); G2 (percentage of solid growth between 6 and 50%); G3 (percentage of solid growth above 50%). Histological typing and grading were done according to the WHO classification. The Local Ethic Committee approved the study and each patient gave a written consent (No 4/2011).
DNA isolation
Formalin-fixed paraffin-embedded (FFPE) endometrial tissue samples were obtained from all analysed patients. Genomic DNA was prepared using QIAamp DNA FFPE Tissue Kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer instruction.
Determination of XRCC genotype
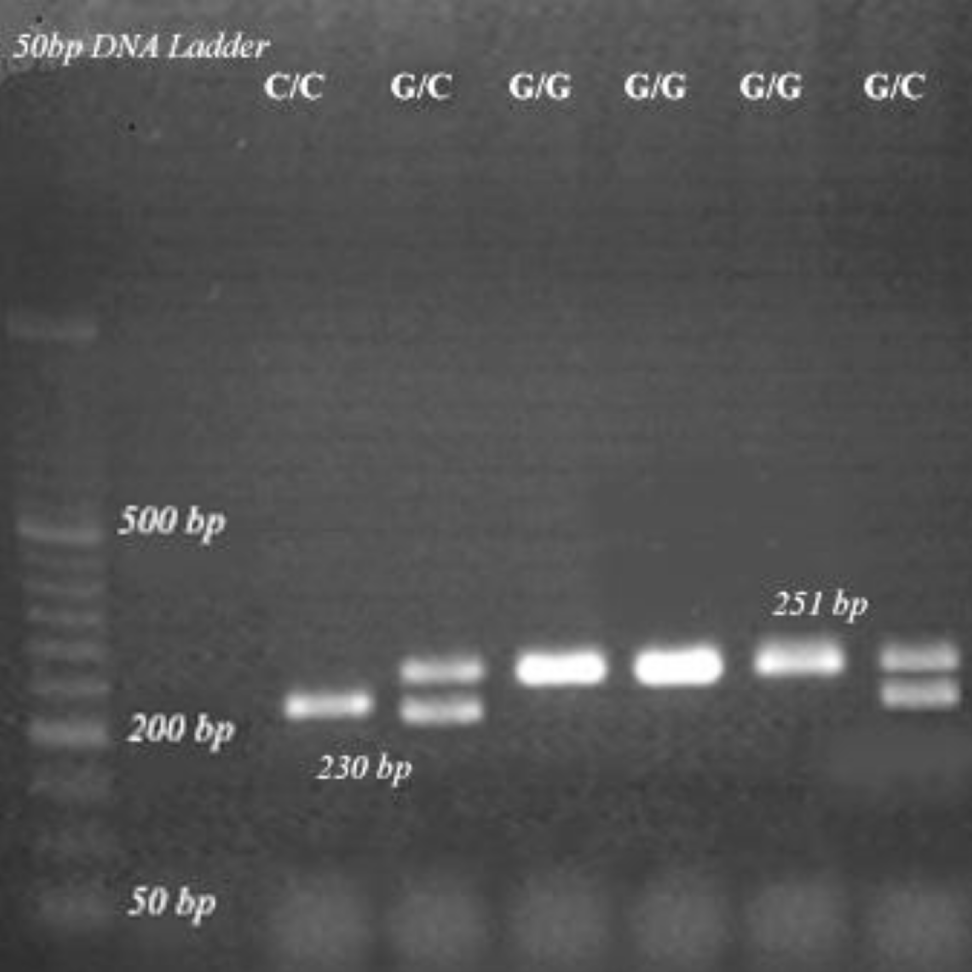
Polymorphism 4234G/C of the XRCC2 gene was determined by PCR-RFLP (restriction fragment-length polymorphism) [11]. The primers: forward 5’- GTGCGCACGCGCGCGGGTGGAC-3’ and reverse 5’- GCGCCGCCCCAAGCCTCCCAATC-3’ were used to amplify the region containing the 4234G/C XRCC2 variant. PCR amplification was performed in a final volume of 25 μl containing 100 ng of DNA, 1.5 mM of MgCl2, 10mM Tris-HCl (pH 8.3), 50 mM KCl, 0.2 mM of dNTP, each primer at 1.0 μM and 1.0 unit of Taq polymerase (Takara, Japan) in PTC-100 TM (MJ Research, INC, Waltham, MA, USA) Thermocycler. PCR cycle conditions were the following: 95°C for 45s, 70°C for 45s and 72°C for 60s, repeated in 35 cycles. PCR products were electrophoresed in a 2% agarose gel and visualized by ethidium bromide staining. The cleavage with MluI (New England BioLabs, Frankfurt am Main, Germany) produced fragments of 251, 251/230/21 and 230/21 bp corresponding to the G/G, G/C and C/C genotypes of the XRCC2 gene, respectively (21 bp have been out of the gel) (Figure 1).
Statistics
To determine differences between groups, standard Chi square test (χ2) or Fisher’s exact tests were used. Clinical significance of analyzed polymorphism was determined using logistic regression analysis and presented in tables as odds ratios (OR) with their 95% confidence intervals. The deviations from Hardy-Weinberg equilibrium were analyzed using the χ2 test. Differences with a p value less than 0.05 were considered significantly.
Results
The genotype distributions of analyzed the 4234G/C XRCC2 gene polymorphism are summarized in Table 1. All allele distributions were consistent with Hardy–Weinberg equilibrium. The distribution of gene variants was similar between all cases and controls, the maximal difference of 3,5% was found for the XRCC2 polymorphism with the homozygous C/C genotype being less frequent in cases (19.8%) than in controls (23.3%). This difference was, however, not significant (p> 0.05).
The potential relationship between XRCC2 genotype distribution and clinical data of EC patients was investigated. However, the current study failed to show the correlation between analysed gene polymorphism and histological grading. XRCC2 gene polymorphism was also unrelated to the patient’s age, body mass index, number of pregnancies, uterine bleeding, endometrial ultrasound transvaginal, diabetes and hypertension (Table 2).
Discussion
Several studies have investigated the possible role of XRCC2 gene polymorphisms in neoplastic diseases. The 188His allele and 188His/His homozygous variant of XRCC2 Arg188His polymorphism were associated with increased risk of breast cancer in Polish population [7].
Other authors observed that XRCC2 polymorphisms might play important role in colorectal cancer tumorigenesis, conferring susceptibility to rectal tumors [13].
This polymorphism may be also associated with increased risk of gastric and pharyngeal cancers [14,15].
However, in the recently published metaanalysis, statistical significant association between XRCC2 Arg188His polymorphisms and neoplastic diseases was found in ovarian cancer but not in other studied cancers [16].
According to the recently published analysis, the XRCC2 -41657C/T polymorphism might be also a risk factor for ESCC, GCA, laryngeal and ovarian cancer [8,11,12].
The 41657C/4234C and 41657T/4234G haplotypes of this gene may be responsible for significantly increased risk of gastric cardia adenocarcinoma.
Until this moment, there have been no studies that analyse the association between 4234G/C polymorphism of the XRCC2 gene and EC.
Because a proper functioning of the XRCC2 gene is important for the genomic stability, its alternations may be associated with higher cancer susceptibility.
In the current study, the XRCC2 4234G/C genotype distribution was similar in patients with endometrial carcinoma and control group. According to our data, the analysed polymorphisms were also unrelated to the histologic grade, age, number of pregnancies, body mass index, uterine bleeding, endometrial ultrasound transvaginal, diabetes and hypertension.
To our best knowledge, only a few authors investigated the genetic variants of XRCC2 gene and the risk of developing endometrial cancer [17,18].
Earlier, Han et al., demonstrated that the heterozygous and homozygous variant alleles of XRCC2 Arg188His are not associated with risk of endometrial cancer [17].
No significant associations were observed between the Arg188His genotype and endometrial cancer in Polish population [18].
This is the first article of XRCC2 4234G/C polymorphism and endometrial cancer risk. A limitation of the study was the relatively small population of participants. The sample for the present study comprised of 808 EC patients. This sample is only a very small proportion of the entire population of endometrial cancer women in the country. Therefore the obtained results cannot be considered as definitive and require further, more extensive evaluations, performed on bigger groups of patients.
In conclusion, current evidence did not suggest that analysed XRCC2 polymorphism was directly associated with endometrial cancer risk. Our study failed to show any correlation between 4234G/C polymorphism and clinical data of endometrial cancer patients. Our results should be explained with some caution and be re-evaluated in the future when more studies with larger sample size are conducted.
- Tambini CE, Spink KG, Ross CJ, Hill MA, Thacker J (2010) The importance of XRCC2 in RAD51-related DNA damage repair. DNA Repair 9: 517–525 .
- Yuan C, Liu X, Yan S, Wang C, Kong B (2014) Analyzing association of the XRCC3 gene polymorphism with ovarian cancer risk. Biomed Res Int 2014: 648137 .
- Popanda O, Schattenberg T, Phong CT, Butkiewicz D, Risch A, et al. (2004) Specific combinations of DNA repair gene variants and increased risk for non-small cell lung cancer. Carcinogenesis 25: 2433-4241 .
- Banescu C, Trifa AP, Demian S, Benedek Lazar E, Dima D, et al. (2010) Polymorphism of XRCC1, XRCC3, and XPD genes and risk of chronic myeloid leukemia. Biomed Res Int 2014: 213790 .
- Park DJ, Lesueur F, Nguyen-Dumont T, Pertesi M, Odefrey F, et al. (2012) Rare Mutations in XRCC2 Increase the Risk of Breast Cancer. The American Journal of Human Genetics 90: 734-739 .
- He Y, Zhang Y, Jin C, Deng X, Wei M, et al. (2014) Impact of XRCC2 Arg188His Polymorphism on Cancer Susceptibility: A Meta-Analysis. PLOS ONE 9: e91202 .
- Smolarz B, Makowska M, Samulak D, Michalska MM, Mojs E, et al. (2015) Association between single nucleotide polymorphism (SNPs) of XRCC2 and XRCC3 homologous recombination repair genes and triple-negative breast cancer in Polish women. Clin Exp Med 15: 151-157 .
- Romanowicz-Makowska H, Smolarz B, Gaj?cka B, Kiwerska K, Rydzanicz M, et al. (2012) Polymorphism of the DNA repair genes RAD51 and XRCC2 in smoking- and drinking-related laryngeal cancer in a Polish population. Arch Med Sci 8: 1065-1075 .
- Jiao L, Hassan M, Bondy ML, Wolff RA, Evans DB, et al. (2008) XRCC2 and XRCC3 gene polymorphism and risk of pancreatic cancer. Am J Gastroenterol 103: 360-367 .
- Brooks J, Shore RE, Zeleniuch-Jacquotte A, Currie D, Afanasyeva Y, et al. (2008) Polymorphisms in RAD51, XRCC2, and XRCC3 are not related to breast cancer risk. Cancer Epidemiol Biomarkers Prev 17: 1016-1019 .
- Wang N, Xiu-juan Dong, Rong-miao Zhou, Wei Guo, Xiao-juan Zhang, et al. (2009) An investigation on the polymorphisms of two DNA repair genes and susceptibility to ESCC and GCA of high-incidence region in northern China. Mol Biol Rep 36: 357-364 .
- Michalska MM, Samulak D, Smolarz B (2014) An association between the -41657 C/T polymorphism of X-ray repair cross-complementing 2 (XRCC2) gene and ovarian cancer. Med Oncol 31: 300 .
- Curtin K, Lin WY, George R, Katory M, Shorto J, et al. (2009) Genetic variants in XRCC2: new insights into colorectal cancer tumorigenesis. Cancer Epidemiol Biomarkers Prev 18: 2476-2484 .
- Gok I, Baday M, Cetinkunar S, Kilic K, Bilgin BC (2014) Polymorphisms in DNA repair genes XRCC2 and XRCC3 risk of gastric cancer in Turkey. Bosn J Basic Med Sci 13: 214-218 .
- Benhamou S, Tuimala J, Bouchardy C, Dayer P, Sarasin A, et al. (2014) DNA repair gene XRCC2 and XRCC3 polymorphisms and susceptibility to cancers of the upper aerodigestive tract. Int J Cancer 112: 901-904 .
- Zhand Y, Wang H, Peng Y, Liu Y, Xiong T, et al. (2014) The Arg188His polymorphism in the XRCC2 gene and the risk of cancer. Tumour Biol 35: 3541-3549 .
- Han J, Hankinson SE, Hunter DJ, De Vivo I (2004) Genetic variations in XRCC2 and XRCC3 are not associated with endometrial cancer risk. Cancer Epidemiol Biomarkers Prev 13: 330-331 .
- Romanowicz-Makowska H, Smolarz B, Polac I, Sporny S (2012) Single nucleotide polymorphisms of RAD51 G135C, XRCC2 Arg188His and XRCC3 Thr241Met homologous recombination repair genes and the risk of sporadic endometrial cancer in Polish women. J Obstet Gynaecol Res 38: 918-924 .
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
