J Food Sci Nutr The
Evaluation the Viability of the Saccharomyces Boulardii Bld-3 and its Influence on the Colonic Microbiota Composition by SHIME
Hai-Bo Zhang, Ning Peng, Qian Cheng, Zhi-Xian Chen and Yan Zhang*
Cite this as
Zhang HB, Peng N, Cheng Q, Chen ZX, Zhang Y (2018) Evaluation the Viability of the Saccharomyces Boulardii Bld-3 and its Influence on the Colonic Microbiota Composition by SHIME. J Food Sci Nutr The 4(1): 012-017. DOI: 10.17352/jfsnt.000015During this study the viability of Saccharomyces Boulardii Bld-3 during passage through the upper GIT under fed and fasted conditions was evaluated. For this purpose, a Simulator of the Human Intestinal Microbial Ecosystem (SHIME®) was used, which allows to re-create the physiological conditions that are representative of the human GIT. Furthermore, the aim of this study was also to study the modulatory effect of the Saccharomyces Boulardii Bld-3 on the colonic microbiota composition and activity after passage of the strain through the fed or fasted upper GIT or when delivered directly to the colon. For this purpose, four different types of short-term colonic batch experiments were performed that simulate the colonic fermentation process. The Saccharomyces Boulardii Bld-3 completely survived the stomach phase of the fed upper GIT, characterized with a long residence time and intermediate pH conditions and was capable to cope with the high concentrations of bile acids in the fed small intestine. Subtle effects were observed on SCFA, BCFA and ethanol production with a consistent significantly moderately higher acetate production in the present of Saccharomyces Boulardii Bld-3. This was also confirmed through determination of the concentrations of lactobacilli, bifidobacterial, and firmicutes at the end of the colonic incubations. Indeed, no statistical significant differences were observed between the control experiments and those performed with the Saccharomyces Boulardii Bld-3. Only higher concentrations of Bacteroidetes were present at the end of the experiments to which the Saccharomyces Boulardii Bld-3 was added (highest effect after fed and fasted upper GIT passage). This could explain the higher concentrations of BCFA during the colonic incubations with the Saccharomyces Boulardii Bld-3 since several members of the Bacteroidetes are known proteolytic bacteria.
Introduction
Saccharomyces Boulardii (S. boulardii), a patented yeast preparation, is the only yeast probiotic that has been proven effective in double-blind studies [1-3]. Controlled clinical trials have shown the efficacy of S. boulardii for the prevention and/or the treatment of various intestinal disorders including antibiotic-associated diarrhea [4,5], recurrent Clostridium difficile disease [6-8], Clostridium difficile associated enterocolopathies in infants [9-12], acute diarrhea in adults [13] and children [14], traveler’s diarrhea(Buts JP, 2004), diarrhea in critically ill tube-fed patients [15-17].
Although S. boulardii is a variety of S. cerevisiae, it differ from S. cerevisiae in several taxonomic, metabolic, and genetic properties [18]. S. boulardii is resistant to acidity, to proteases, and naturally to all antibacterial antibiotics [19]. After oral administration of S. boulardii, steady state concentrations of viable yeast cells are achieved within a mean of 3 days and the yeast is cleared from the stools 2–5 days after discontinuation [20]. The mechanisms of action of S. boulardii include protective effects toward various enteric pathogens and beneficial effects on the host intestinal mucosa [19].
The aim of this study was to evaluate the survival of a specific Saccharomyces Boulardii Bld-3 under simulated conditions for the human upper gastrointestinal tract (GIT). For this purpose, a Simulator of the Human Intestinal Microbial Ecosystem (SHIME®) was used, which allows to re-create the physiological conditions that are representative of the human GIT [21,22]. Secondly, the aim of this study was to study the modulatory effect of the Saccharomyces Boulardii Bld-3 on the colonic microbiota composition and activity after passage of the strain through the fed or fasted upper GIT or when delivered directly to the colon.
Materials and Methods
Simulator of the Human Intestinal Microbial Ecosystem(SHIME®)
The reactor setup was adapted from the SHIME, representing the gastrointestinal tract (GIT) of the adult human, as described by Molly et al. [21-23]. The SHIME consists of a succession of five reactors simulating the different parts of the human gastrointestinal tract. The first two reactors are of the fill-and-draw principle to simulate different steps in food uptake and digestion, with peristaltic pumps adding a defined amount of SHIME feed and pancreatic and bile liquid, respectively to the stomach and small intestine compartment and emptying the respective reactors after specified intervals. The last three compartments – continuously stirred reactors with constant volume and pH control – simulate the ascending, transverse and descending colon. Retention time and pH of the different vessels are chosen in order to resemble in vivo conditions in the different parts of the gastrointestinal tract.
In order to mimic fed or fasted conditions, a specific gastric suspension is added to the reactor. After this, a standardized enzyme and bile liquid is added to simulate the small intestinal condition. Incubation conditions (pH profiles, incubation times) are optimized in order to resemble in vivo conditions in the different regions of the gastrointestinal tract for fasted or fed conditions. The applied conditions are summarized below:
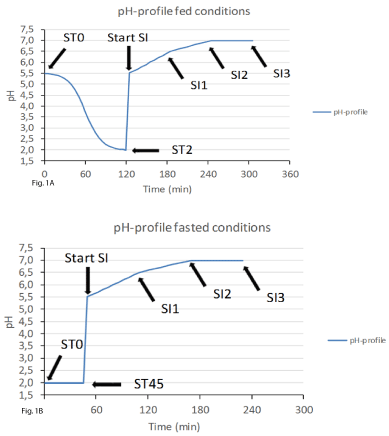
Gastric phase (fed state): Incubation during 2h at 37°C, while mixing via stirring, with sigmoidal decrease of the pH profile from 5.5 to 2.0 (Figure 1). Pepsin (Fiers Kuurne, Belgium) is supplied with the activity being standardized by measuring absorbance increase at 280nm of TCA-soluble products upon digestion of hemoglobin (reference protein). Addition of phosphatidylcholine (Carl Roth, Karlsruhe, Germany). Addition of the SHIME® nutritional medium (ProDigest, Zwijnaarde, Belgium) containing arabinogalactan, pectin, xylan, starch, glucose, yeast extract, peptone, mucin, and L-cystein-HCl. The salt levels recommended by the consensus method (NaCl and KCl) were implemented. Sampling: t = 0 and 2h; at these time points the pH of the medium was equal to 5.5 ± 0.05 and 1.99 ± 0.05, respectively.
Gastric phase (fasted state): Incubation during 45min at 37°C, while mixing via stirring, pH = 2.0 (Figure 1). Addition of 4-fold lower pepsin and phosphatidylcholine levels. As the background medium, only salts and mucins (Carl Roth, Karlsruhe, Germany) are supplied. Sampling: t = 0 and 45min; at these time points the pH of the medium was equal to 2.0 ± 0.1 and 2.0 ± 0.1, respectively.
Small intestinal phase (fed state): While mixing via stirring, the pH initially automatically increases from 2.0 to 5.5 within a period of 5 minutes after which a gradually increasing pH from 5.5 to 7.0 during an incubation of 3h at 37°C is controlled automatically by the software (Figure 1).Regarding pancreatic enzymes both a raw animal pancreatic extract (pancreatin from pocine pancreas; Sigma-Aldrich Corp., St. Louis MO, USA) containing all the relevant enzymes in a specific ratio as well as defined ratios of the different enzymes can be used. Regarding bile salts, 10mM bovine bile extract (Oxgall; BD Biosciences, Erembodegem, Belgium) is generally supplemented. Sampling: t = 1h, 2h, and 3h; at these time points the pH of the medium was equal to 6.5 ± 0.05, 7.0 ± 0.05, and 7.0 ± 0.1, respectively.
Small intestinal phase (fasted state): Following parameters were adapted as compared to the fed protocol: Pancreatic enzyme levels should decrease with a factor 5, Bile salts should decrease with a factor 3.
Inoculation of S. Boularddi into the SHIME reactor
The S. Boularddi Bld-3 was tested to assess its survival, while passing through the stomach and small intestine. To each stomach reactor (simulating half a human) 1 gram of S. Boularddi Bld-3 powder(Angel Yeast Co., Ltd., YiChang, China) was added. All experiments were performed in biological triplicate to account for biological variability.
Quantification of colony forming units
Samples were collected at different stages of the experiment for stomach and small intestine to determine the number of colony forming units of the yeast tested by spread plating. The number of colony-forming units of the Saccharomyces Boulardii Bld-3 contained in the dry powder (product) were also determined. Ten-fold dilution series were prepared from these samples in phosphate buffered saline (PBS) and subsequently transferred to petri dishes containing glucose-yeast extract (GY) agar medium. Glucose was obtained from Merck (Merck, Darmstadt, Germany)and yeast extract was obtained from Oxoid (Oxoid Ltd., Basingstoke, United Kingdom). Results are reported as average log (CFU) ± stdev (n = 3).
Impact on the colonic microbiota and its metabolic activity
Short-term colonic batch incubations were performed using a representative colon medium containing host -and diet-derived compounds. All colonic batch incubations were inoculated with a fresh fecal inoculum that contains a viable and metabolically active colonic microbiota. The inoculation of the reactor was done as described by Molly et al. [22,23], using human faecal material and supernatant of Western diet suspension. Absorption of metabolites and water was not simulated in the SHIME reactor, and adhesion to reactor vessels and tubings was of negligible importance.
Different types of colonic incubations were performed. The CONTROL experiments consisted of colonic incubations to which the test product was not added but were inoculated with the fecal background microbiota. These experiments were performed to assess the overall metabolic activity, composition, and growth of the fecal background microbiota. During the FED experiments, a part of the small intestinal liquid phase (after passage of the Saccharomyces Boulardii Bld-3 under fed conditions) was transferred to colonic reactors containing the colon medium and a fecal background microbiota. During the FASTED experiments, a part of the small intestinal liquid phase (after passage of the Saccharomyces Boulardii Bld-3 under fasted conditions) was transferred to colonic reactors containing his colon medium and fecal background microbiota. During the COLON DOSE experiments, 0.1 gram of yeast powder was added to the colonic incubations containing the colon medium and fecal background microbiota. These three types of experiments allowed to elucidate the impact of the Saccharomyces Boulardii Bld-3 on the colonic fermentation process when the Saccharomyces Boulardii Bld-3 reaches the colon after fed or fasted upper GIT passage or when delivered directly to the colon.
All bottles were incubated for 48h at 37°C under anaerobic conditions. Samples were taken at the start of the incubations (0h) and after 24h and 48h of incubation. The following parameters were determined at these sampling points.
pH
The pH of the samples was measured with an external pH meter (Senseline F410; ProSense, Oosterhout, The Netherlands).
Gas Production: The colon incubations were performed in closed incubation systems. The gas pressure in the headspace of the incubations was determined with a pressure meter (Hand-held pressure indicator CPH6200; Wika, Echt, The Netherlands).
Ethanol, SCFA (short chain fatty acid) and BCFA (branch chain fatty acid): A chromatographic method was developed and optimized to determine the concentrations of ethanol, SCFA, and BCFA. The concentrations of ethanol SCFA and BCFA in the liquid samples were determined by performing a solvent extraction using diethytether on the samples followed by quantification through gas chromatography with flame ionization detection (GC-RD; Shimadzu, Jette, Belgium).
Microbial position: Microbial cell pellets were generated from the samples taken at 0h and 48h of incubation. DNA was extracted from these cell pellets. The extracted DNA was subsequently used during qPCR with primers specific for bifidobacterial, lactobacilli, firmicutes and bacteroidetes to determine the growth of these bacterial groups during the incubations. All qPCR reactions were performed in technical triplicate. Results are reported as average log (16S copies/mL) ±stdev of the three independent biological replicates.
Statistics
Statistical analysis was performed in SPSS Statistics 25. Normality of data and equality of the variances were determined with a Shapiro-Wilk test and a Levene’s test, respectively. In terms of statistics, the differences for all data discussed and indicated by “p < 0.05” were significant with a confidence interval of 95 %. Statistical differences in between experiments were made clear by assigning different letters to the bars in the figures.
Results and Discussion
Viability of the saccharomyces boulardii
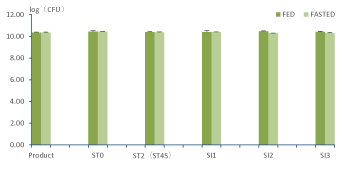
During passage of the Saccharomyces Boulardii Bld-3 through the upper GIT under fed conditions the number of culturable yeast cells at the start of the stomach was comparable with the number of CFU obtained through spread plating of the pure product. (Figure 2). Following the stomach incubation phase, the yeast cells enter the small intestinal incubation phase. This coincides with a sharp increase in the pH of the gastrointestinal fluids till 5,5 which subsequently increases towards a pH value of 7 at the end of the small intestinal incubation. On the contrary, pancreatic and gall bladder secretions in the small intestine deliver high concentrations of pancreatic enzymes and bileacids, respectively. Especially, the high concentrations of bile acids present in the small intestine could have a large impact on cell survival. However, as is evident from the obtained results for CFU determination, the number of culturable yeast cells remained constant during the passage through the small intestine. This indicated that the Saccharomyces Boulardii Bld-3 was not sensitive to the bile acids in the small intestine.
These results indicated that no statistical significant decrease in culturable yeast cells occurred during the stomach incubation phase. Furthermore, no significant decrease in CFU occurred during the small intestinal incubation phase. Hence, during the overall passage of the Saccharomyces Boulardii Bld-3 through upper GIT under fed conditions no loss in culturability occurred.
Impact on the pH and gas production
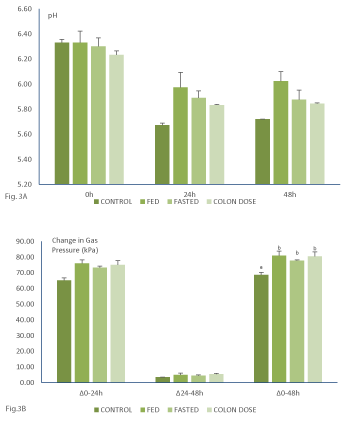
During all experiments the pH of the medium decreases over the course of the incubations indicating the presence of active colonic fermentation. The main microbial activity occurred within the first 24h of incubation where after, the pH remained fairly constant till the end of the experiments. During the control experiments, the pH value of the medium decreased more as compared to the experiments to which the Saccharomyces Boulardii Bld-3 was added (Figure 3). Determination of the gas pressure during the experiments revealed that the main microbial fermentative activity occurred within the first 24h of incubation. Comparison of the different colonic incubations revealed that statistically significant more gas was produced during the experiments with the Saccharomyces Boulardii Bld-3 as compared to the control experiments (Figure 3).
Production of SCFA, BCFA and ethanol
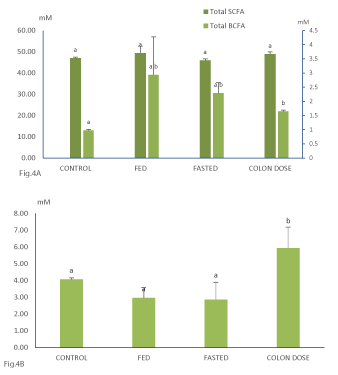
Supplementation of the Saccharomyces Boulardii Bld-3 to the colonic incubations didn’t result in statistically significantly more short chain fatty acids (SCFA) and Ethanol production as compared to the control incubations (Figure 4). Notwithstanding these subtle effects, it can be concluded that the addition of the Saccharomyces Boulardii Bld-3 to the colonic background microbiota did not result in a major change in short chain fatty acids and ethanol production.
During all experiments, BCFA were mainly produced during the first 24h of incubation with only a minor fraction being produced after 24h. Addition of the Saccharomyces Boulardii Bld-3 to the colonic incubation experiments resulted in statistically higher concentrations of BCFA produced (Figure 4). This could be due to the proteolytic activity of the Saccharomyces Boulardii Bld-3s itself. However, the increased consumption of the saccharides present in the colon medium by the Saccharomyces Boulardii Bld-3 could have resulted in lower carbohydrate concentration which necessitated the bacteria of the colonic background microbiota to switch to increased proteolytic fermentation.
Impact on the Composition of the colonic microbiota
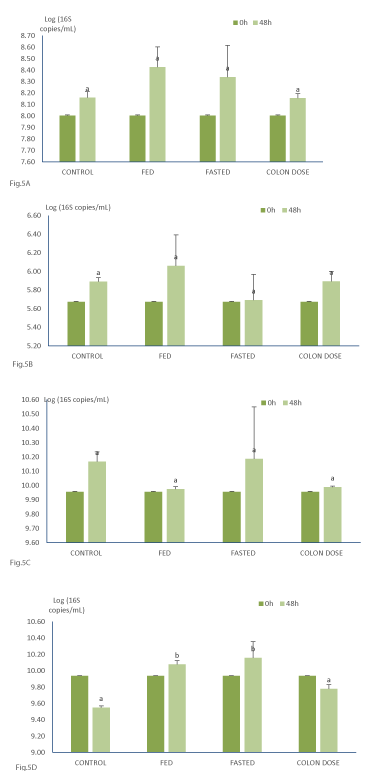
The influence of the addition of the saccharolytic Saccharomyces Boulardii Bld-3 on the composition of major saccharolytic bacterial groups was evaluated. Addition of the Saccharomyces Boulardii Bld-3 did not result in statistically significant differences in the concentrations of bifidobacterial, lactobacilli, and firmicutes at the end of the colonic incubations (Figure 5). Also, the mode of delivery namely after passage through the upper GIT under fed or fasted conditions or directly to the colon did not result in any effects. Addition of the Saccharomyces Boulardii Bld-3 after passage through the upper GIT under both fed and fasted conditions resulted in statistically significant higher concentrations of Bacteroidetes at the end of the colonic incubations as compared to the control experiments and those performed with a direct delivery of the Saccharomyces Boulardii Bld-3 (Figure 5). Notwithstanding these subtle effects, it can be concluded that the addition of the Saccharomyces Boulardii Bld-3 to the colonic background microbiota did not result in a major change in the bacterial composition at least for the major saccharolytic bacterial groups.
Conclusion
During this study the viability of a Saccharomyces Boulardii Bld-3 during passage through the upper GIT under fed and fasted conditions was evaluated. Determination of the number of culturable yeast cells through spread plating and the number of viable yeast cells through flow cytometry demonstrated the outstanding survival of this Saccharomyces Boulardii Bld-3 during upper GIT passage. The Saccharomyces Boulardii Bld-3 completely survived the stomach phase of the fed upper GIT, characterized with a long residence time and intermediate pH conditions and was capable to cope with the high concentrations of bile acids in the fed small intestine. Furthermore, the strain completely survived the constantly harsh low pH conditions of the fasted stomach. As such, no decrease in culturable and viable cells occurred during the overall passage of the upper GIT under both fed and fasted conditions. Furthermore, during both the fed and fasted experiments, the number of culturable cells, determined through spread plating, was nearly equal to the number of total viable yeast cells, determined by flow cytometry. This indicated that the yeast cells were both viable and metabolically active. Hence, the complete orally administered dose of the Saccharomyces Boulardii Bld-3 reached the colon and this in a fully metabolically active state. In the colon, the administration of the Saccharomyces Boulardii Bld-3 resulted in a significant different pH decrease of the medium and significantly higher gas production as compared to the control experiment indicating that the Saccharomyces Boulardii Bld-3 was capable to be metabolically active under colonic conditions in the presence of a background microbiota. This effect was observed for all delivery modes to the colon, namely after passage through the fed and fasted upper GIT or when the Saccharomyces Boulardii Bld-3 was directly delivered to the colon. This result in not surprising considering the complete survival of the Saccharomyces Boulardii Bld-3 after both the passage through the fed and fasted upper GIT. Subtle effects were observed on SCFA and ethanol production with a consistent significantly moderately higher acetate production in the present of the Saccharomyces Boulardii Bld-3. This minor effect on SCFA production revealed that the Saccharomyces Boulardii Bld-3 did not outcompete the colonic background microbiota and allowed this microbiota to produce a normal blend of the health-promoting SCFA acetate, propionate, and butyrate. This was also confirmed through determination of the concentrations of lactobacilli, bifidobacterial, and firmicutes at the end of the colonic incubations. Indeed, no statistical significant differences were observed between the control experiments and those performed with the Saccharomyces Boulardii Bld-3. Only higher concentrations of Bacteroidetes were present at the end of the experiments to which the Saccharomyces Boulardii Bld-3 was added (highest effect after fed and fasted upper GIT passage). This could explain the higher concentrations of BCFA and ammonium produced during the colonic incubations with the Saccharomyces Boulardii Bld-3 since several members of the Bacteroidetes are known proteolytic bacteria.
The project was finically supported by Hubei Natural Science Foundation No.2018CFB612.
- Adam P (1976) Essais cliniques controlés endouble insu de l’ultra-levure lyophilisée (étude multicentrique par 25 médecinsde 388 cas). Méd Chir Dig 5: 401–406. Link: https://tinyurl.com/yby2ojlt
- Blehaut H, Massot J, Elmer GW, Levy RM (1989) Disposition kinetics of Saccharomyces boulardii in man and rat. Biopharm Drug Dispos 10:353–364. Link: https://tinyurl.com/ya6ppy8x
- Bleichner G, Blehaut H, Mentec H,Moyse D (1997) Sacchromyces boulardii prevents diarrhoea in critically ill tube-fed patients. A multicenter, randomized, double-blind–placebo-controlled trial. Intensive Care Med 23: 517–523. Link: https://tinyurl.com/y853vs8o
- Buts JP (1999) Mechanisms of action of biotherapeutic agents. In Elmer GW, McFarland LV, Surawicz CM (eds) Biotherapeutic agents and infectious disease. Humana Pres, Totowa, NJ 27–46. Link: https://tinyurl.com/y98t2a7a
- Buts JP, Dekeyser N, Stilmant C, Delem E, Smets F, et al. (2006) Saccharomyces boulardii produces in rat small intestine a novel protein phosphatase that inhibits Escherichia coli endotoxin by dephosphorylation. Pediatric Res 60: 24–29. Link: https://tinyurl.com/ycs8dfp6
- Cardinali G, Martini A (1994) Electrophoretic karyotypes of authentic strains of the sensu stricto group of the genus Saccharomyces. Int J Syst Bacteriol 44: 791–797. Link: https://tinyurl.com/ycodvwvs
- Cetina-Sauri G, Sierra Basto G (1994) Evaluation th´erapeutique de Saccharomyces boulardii chez des enfants souffrant de diarrh´ee aigu¨e. Ann Pediatr 41:397–400.
- Guslandi M, Mezzi G, Testoni PA (2000) Saccharomyces boulardii in maintenance treatment of Crohn’s disease. Dig Dis Sci 45: 1462–1464. Link: https://tinyurl.com/y8se3787
- Hennequin C, Thierry A, Richard GF, et al. (2001) Microsatellite typing as a new tool for identification of Saccharomyces cerevisiae strains. J Clin Microbiol 39: 551–559. Link: https://tinyurl.com/y937d28y
- H¨ochter W, Chase D, Hegenhoff G (1990) Saccharomyces boulardii in treatment of acute adult diarrhoea. Efficacy and tolerance of treatment. M¨unch Med Wochen 132: 188–192.
- McFarland LV, Surawicz CM, Greenberg RN, et al. (1995) Prevention of b-lactam-associated diarrhea by Saccharomyces boulardii compared with placebo. Am J Gastroenterol 90: 439–448. Link: https://tinyurl.com/ycv4bfw2
- Kollaritsch H, Holst H, Grobara P, Wiedermann G (1993) Prophylaxis of traveller’s diarrhoea with Saccharomyces boulardii : results of aplacebo-controlled double-blind study. Fortschr Med 111: 152–156. Link: https://tinyurl.com/y7ugaxko
- Kurugol Z, Koturoglu G (2005) Effects of Saccharomyces boulardii in children with acute diarrhea. Acta Pediatr 94: 44–47. Link: https://tinyurl.com/ydcbkqcd
- McFarland L (2007) Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel Med Infect Dis 5: 97–105. Link: https://tinyurl.com/y8jla2ey
- McFarland LV (1998) Epidemiology, risk factors and treatments for antibiotic-associated diarrhea. Dig Dis 16: 292–307. Link: https://tinyurl.com/ycno3xev
- Molly K, De Smet I, Nollet L, Woestyne MV, Verstraete W (1996) Effect of Lactobacilli on the ecology of the gastro- intestinal microbiota cultured in the SHIME reactor. Microb. Ecol. Health Dis 9: 79–89. Link: https://tinyurl.com/ycwbqcnn
- Molly K, Woestyne MV, Verstraete W (1993) Development of a 5-step multichamber reactor as a simulation of the human intestinal microbial ecosystem. Applied Microbiology and Biotechnology 39: 254-258. Link: https://tinyurl.com/yd2s64h5
- Molly K, Vande Woestyne M, De Smet I, Verstraete,W (1994) Validation of the simulator of the human intestinal microbial ecosystem (SHIME) reactor using microorganism-associated activities. Microb. Ecol. Health Dis 7: 191–200. Link: https://tinyurl.com/ybk6ja8y
- Molly K, De Smet I, Nollet L, Vande Woestyne M, Verstraete W (1996) Effect of Lactobacilli on the ecology of the gastro- intestinal microbiota cultured in the SHIME reactor. Microb. Ecol. Health Dis 9: 79–89. Link: https://tinyurl.com/ya53fg5l
- Plein K, Hotz J (1993) Therapeutic effects ofSaccharom yces boulardii on mild residual symptoms in a stable phase of Crohn’s disease with special respect to chronic diarrhea – a pilot study. Z Gastroenterol 31: 129–34. Link: https://tinyurl.com/ybwvxhhv
- Sazawal S, Hiremath G, Dhingra U, Malik P, Deb S, et al. (2006) Efficacy of probiotics in prevention of acute diarrhoea: a meta-analysis of masked, randomised, placebo-controlled trials. Lancet Infect Dis 6: 374–382. Link: https://tinyurl.com/y7pc24eb
- Surawicz CM, McFarland LV, Greenberg RN, Rubin M, Fekety R, et al. (2000) The search for a better treatment for recurrent Clostridium difficile disease: use of high-dose vancomycin combined with Saccharomyces boulardii. Clin Infect Dis 31: 1012–1017. Link: https://tinyurl.com/ybu4rnby
- Szajewska H, Ruszczynski M, Radzikowski M (2006) Probiotics in the prevention of antibiotic-associated diarrhea in children: a meta-analysis of randomized trials. J Pediatr 149: 367–372. Link: https://tinyurl.com/ybo63zjq
- Vaughan-Martini A (2003) Reflections on the classification of yeasts for different endusers in biotechnology, ecology and medicine. Int Microbiol 6: 175–182. Link: https://tinyurl.com/y7zudpsz
- Villarruel G, Rubio DM, Lopez F, Cintioni J, Gurevech R, et al. (2007) Saccharomyces boulardii in acute childhood diarrhoea: a randomized, placebo-controlled study. cta Pediatr 96: 538–541. Link: https://tinyurl.com/ybn5d4qp
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
