Journal of Dental Problems and Solutions
The study on material surface on the effect of the Low-Intensity Pulsed Ultrasound (LIPUS) for Osseointegration of the Hydroxyapatite coating dental implant
Masanori Kobayashi*
Cite this as
Kobayashi M (2020) The study on material surface on the effect of the Low-Intensity Pulsed Ultrasound (LIPUS) for Osseointegration of the Hydroxyapatite coating dental implant. J Dent Probl Solut 7(2): 079-083. DOI: 10.17352/2394-8418.000089Excellent osseointegration of biomaterial is very important for the stability of dental implants in clinical field. Much has been learned about this concept and significant improvements on the design and surface modification of implants have been done in the implant dentistry.
Recently, some clinical studies have also reported that low-intensity pulsed ultrasound Irradiation (LIPUS) might enhance the osseointegration (bone-bonding) by means of X-ray radiographic evaluation.
We have already reported that the LIPUS could accelerate the bone-like hydroxyapatite precipitation on the bioactive material surface, which corresponds to osseointgration function. In this review, the mechanism of osseointegration enhanced by LIPUS was presented by the data of our in vitro and in vivo study on hydroxyapatite surface using a Scanning electron microscope and X-ray diffraction analysis. This review suggested that the formation of bone-like apatite on material surface is very important for rapid and firm osseointegration success, which might depend on the initial epitaxial nucleation and crystal growth of apatite on the surface, and LIPUS could activate this crystallization process.
Introduction
Nowadays, the favorable clinical performance of dental implants has been attributed to their firm “osseointegration” as a direct contact between living bone and the surface of a load-carrying implant at the histological level introduced by Brånemark [1,2]. Especially, Hydroxyapatite (HA; Ca10(PO4)6(OH)2) has an excellent biocompatibility and osseointegration properties, therefore have been widely used as dental implants coating material.
Considering the clinical view, more early bonding between dental implant and maxilla bone will accelerate the onset of activity of patients soon after an operation, with subsequent good long-term results, therefore, some approach has been tried to accelerate the osseointegration or the bone bonding to HA material [3-5].
Against these backgrounds, to enhance the osseointegration of dental implant to bone tissue, Low-Intensity Pulsed Ultrasound (LIPUS) stimulation has been focused [6-9], some clinical studies have reported the LIPUS might enhance the bone-bonding [10,11]. However, as these data of reports have been evaluated by means of indirect analysis such as X-ray radiography measures of bone tissue surround implant. Therefore, it is still unclear whether the LIPUS can promote the direct attachment between bone and implant surface defined as an osseointegration though the reduce of dead space of bone around dental implant is certificated.
We have also already reported that the osseointegration of some bioactive materials was enhanced by the pulsed ultrasound radiation [12-14], the present review demonstrates the fact that LIPUS could accelerate the osseointegration (direct bone-bonding ability) of implant surface side due to the induce of bone-like hydroxyapatite formation on the surface.
Method
To evaluate the enhancement of osseointegration of the HA material by LIPUS irradiation, two experiments, in vitro simulated experiment and in vivo tests using rabbits.
Simulated body fluid soaking test
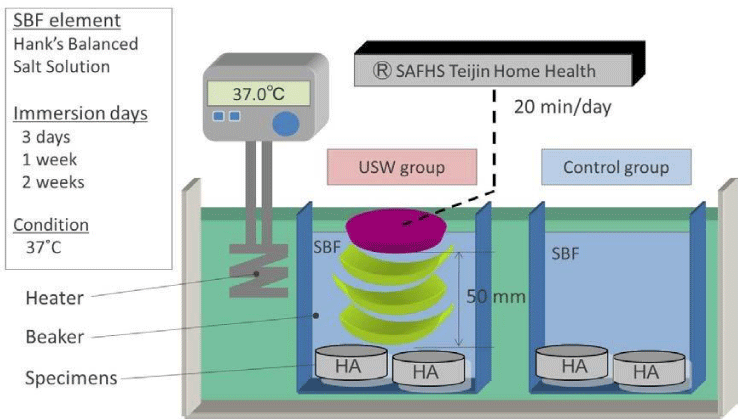
As a basic bone-bonding ability test , the Simulated Body Fluid (SBF) soaking method was performed as a simulation experiment according to Kokubo’s studies in order to evaluate the apatite-forming ability of bio-active titanium and Bio-glass ceramics [15-17].
Hank’s balanced solution (®Lonza; USA) was used as a SBF solution, and maintained at pH over 7.0 and 37 °C and replaced every two days.
Ultrasound radiation was applied by using Sonic Accelerated Fracture Healing System (SAFHS; Smith & Nephew, Memphis, TN, USA ; Teijin Pharma, Tokyo, Japan). The treatment head module delivered ultrasound waves with 1.5 Hz sine waves, 200 μsec signal term, and spatial average intensity of 30 mW/cm2 .
As for a hydroxyapatite specimen, commercial HA pellet (®CELLYARD, HOYA, Japan; 99.9% HA: Ø 5mm ×2mm) was used. Each samples were soaked in SBF and subjected to ultrasound stimulation for 20 min daily during the operation term for three days, one week, and two weeks, respectively. As a control, the same samples were left in SBF without ultrasound radiation under the same experimental conditions (Figure 1).
After the above-mentioned processes were completed, the HA surface was subjected to Scanning Electron Microscopy (SEM) and X-ray diffraction (XRD), and these analyses were focused on the top surfaces, on which the ultrasound wave was directly radiated.
Furthermore, in order to compare with the effect of ultrasound waves, the change of the sample’s mass before/after the SBF treatment was measured. The sample mass were measured periodically, the value obtained by dividing the increased mass by the surface area was defined as the amount of precipitates per unit material surface area. Statistics analysis of the data was examined through t-test and the evaluation and comparison between two groups and the level of significance was determined to be at p <0.05.
In vivo experiment (Animal experiment)
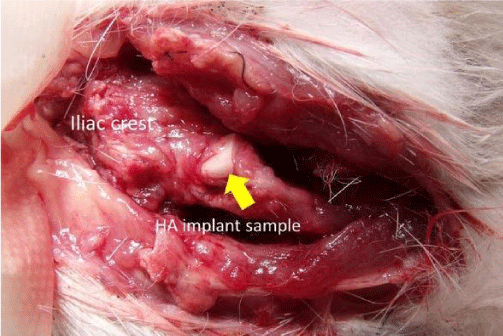
As an implant sample for animal experiment, the HA pallets (Ø 5mm ×2mm ) were cut into the 3 mm ×3 mm ×2 mm cuboid dice, and implanted in the bilateral iliac region of eight mature female rabbits.
After operation, one side of iliac region of rabbits was radiated with the ultrasound waving by using SAFHS as a LIPUS group, another side was not radiated as a control group. All rabbits were reared in cages with no postoperative immobilization, and sacrificed at four weeks after operation, HA samples were resected from rabbits, the bone contact surface was examined by SEM and energy dispersive spectroscopy (EDS).
All the above manipulations in the experimental process were in conformity with the Guideline for Animal Experiments, Institute for Frontier Medical Sciences, Kyoto University of Japan (Approval No.2821).
Results
In vitro simulated body fluid soaking test
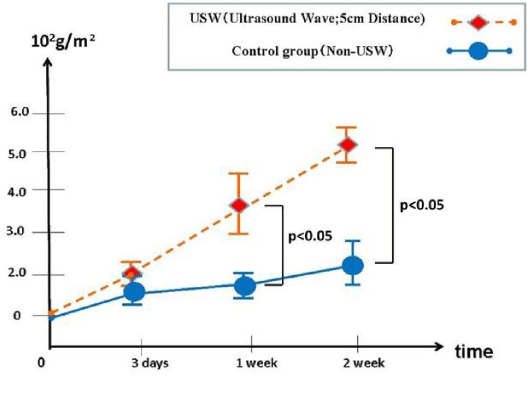
Measurement of the mass of Ca-P crystallization: Figure 2 shows the measured sample mass change as a hydroxyapatite-like precipitation (calcium phosphate crystalline) before/after the SBF soaking. In ultrasound waving group, this bone-like crystalline showed significant increases as compared with that in control group in one week and two weeks soaking in SBF.
SEM observation in vitro test
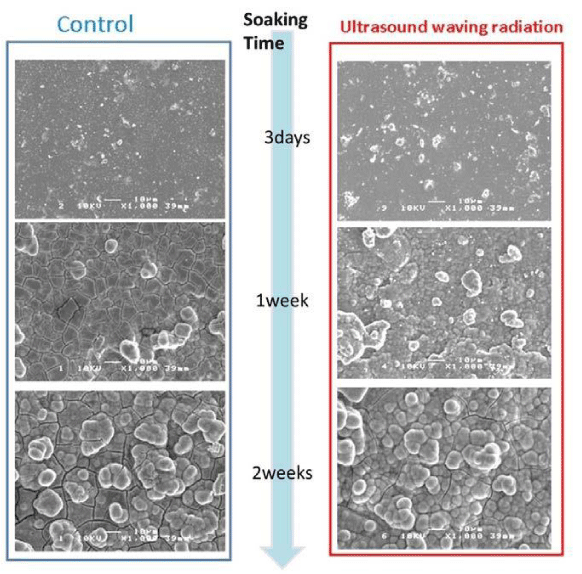
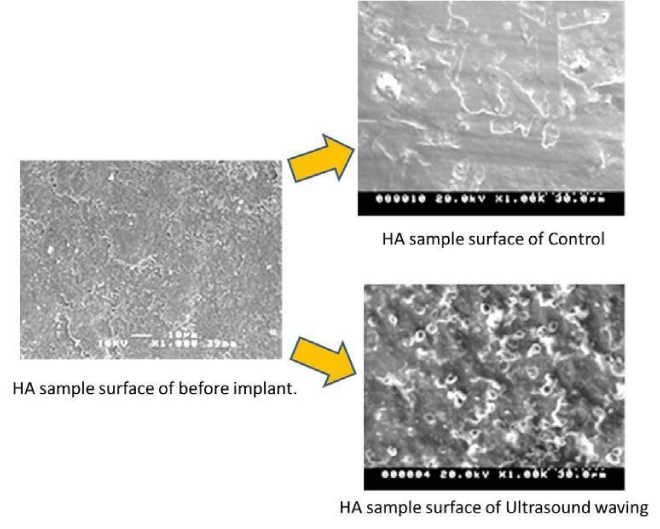
Figure 3 shows the SEM images of the texture on HA specimen surface of the LIPUS and control group in the immersion experiments for 3 days, one week and two weeks.
In both groups, morphological change of the crystalline on the substrate surface have showed according to the soaking time. These crystallization change on HA surface of both groups have demonstrated more active progress and growth in the LIPUS group.
XRD ( X-ray diffraction) analysis
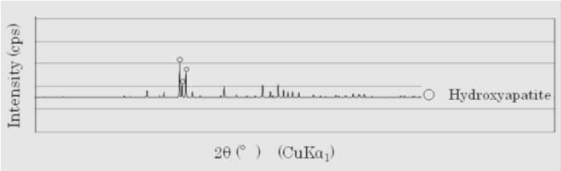
These compounds on HA surface have also identified by XRD . The XRD patterns of the layer on the HA surface after one week of control groups is shown in Figure 4. Though the typical XRD pattern of hydroxyapatite was not observed, some peaks of hydroxyapatite were observed.
In vivo experiment (Animal experiment)
Macro appearance of the animal specimens: Figure 5 shows the macro-appearance of HA implant on rabbits iliac crest of LIPUS group postoperative 4 weeks. Neither inflammation nor infection sign was observed in this area.
SEM observation of the implant surfaces: Figure 6 shows the micro-appearance of the surface of HA sample implanted in rabbits by SEM. As well as shown in SBF soaking test, the morphological change of the crystalline on the HA surface was observed. This finding indicated the precipitation and growth of bone-like hydroxyapatite on HA surface, the crystalline change on substrate showed more active progress and growth in the LIPUS group.
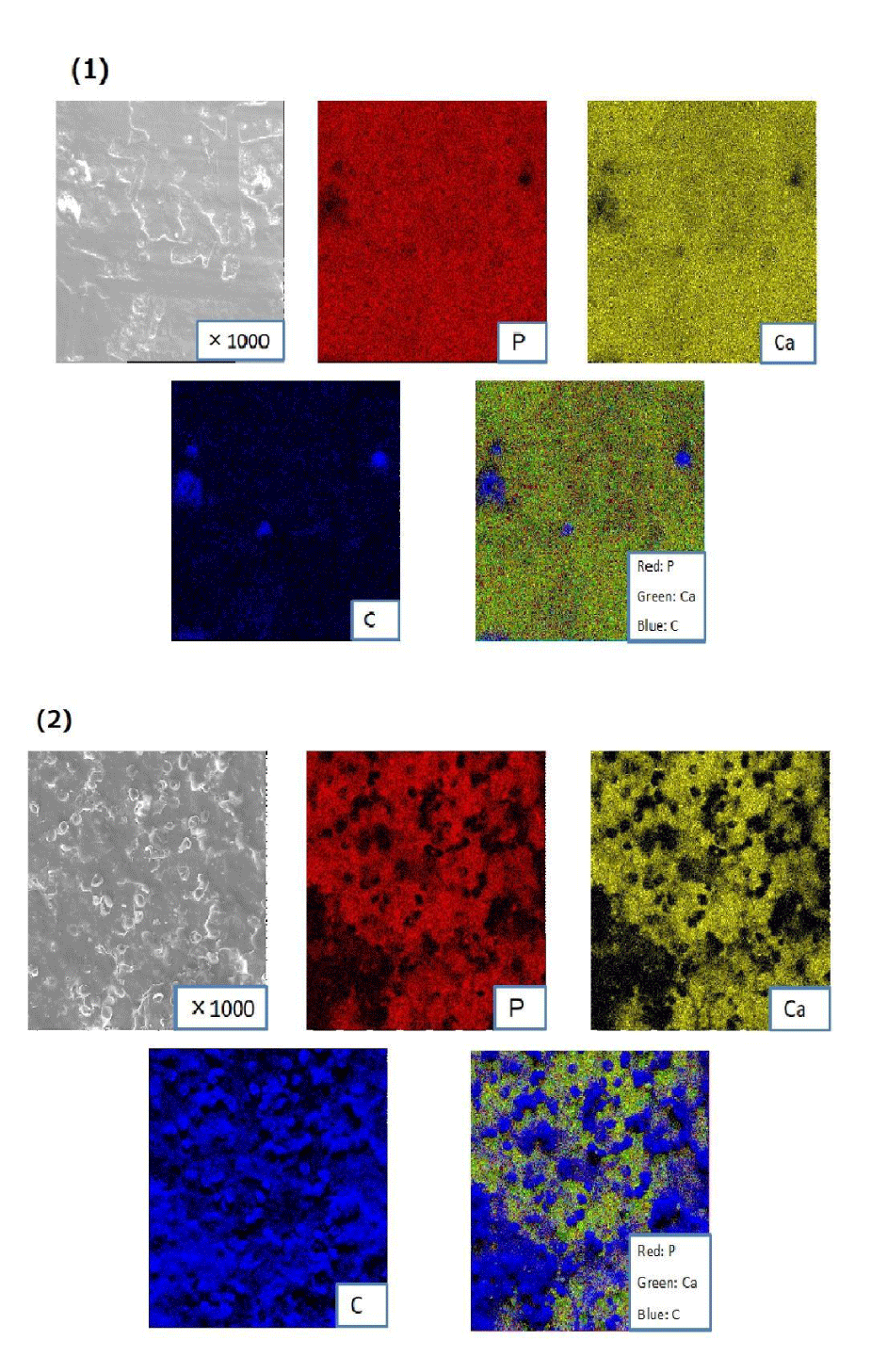
EDS analysis of the implant surfaces: Figure 7 shows the EDS images of implanted HA sample of LIPUS and control group. As shown in the color distribution in figures, the precipitation indicated the composition of rich phosphate (P: red) and calcium (C: green) as well as bone tissue. The carbon (C: blue) in EDS images represented a composition of organic substance such as collagen fiber etc. from bone tissue, which suggested a biological reaction to osteo-conductive biomaterial in vivo.
These findings indicated the good bonding condition between the HA surface and bone tissue, the existing of more rich calcium-phosphate crystal layer in the specimen of LIPUS group could associate with the enhancement of osseointegration by ultrasound waving.
Discussion
The simulation method using SBF soaking in this study is well known as a in vitro assay for evaluation of bone-bonding ability of biomaterials, and has been already used widely for the study regarding the osteoconductivity [18-21] .
According to Kokubo who have developed SBF, the osseointegration of biomaterial mainly due to some step mechanisms, at first the calcium-phosphate precipitate and grows on implant material surface as bone-like apatite, this apatite layer secondary progress the bonding to the bone tissue side grown by the biological mechanism in vivo, and the further biological bone modeling of surround implant follows. Therefore, the in vivo successful bone-bonding of bio-active material is depend on the first apatite formation step on the surface.
Since LIPUS therapy have been shown to enhance the osteogenesis and are widely used as clinical treatment for complicated bone fracture in the orthopaedics surgery field, it is often speculated that the enhancement of osseointegration of HA implant using LIPUS might be mainly due to the bone ingrowth of bone tissue surround implant. However, as shown our results in SBF soaking test , the non-biological bone-like apatite formation on the material is also enhanced by LIPUS.
This fact indicated that the partial concentration and interatomic potential of the Ca and PO4 ions in SBF can be changed by ultrasound micro vibration, and accelerated to the nucleation and deposition of ions, resulting in the crystallization of calcium phosphate on specimen surface. Cavitation by ultrasound waves might also contribute to formation of the apatite layer by means of the microfracture of calcium-phosphate and the circulation of new Ca and PO4 ions of SBF on the titanium surface. These environmental changes will accelerate further changes of interatomic potential of surrounding atoms, and deteriorate the thermal stability of calcium-phosphate phase versus Ca and PO4 ions.
Following the SBF simulation tests, some analysis in animal experiments also showed more rich apatite formation on surface in LIPUS group. That also supports other clinical reports, and strongly suggests the possibility of the enhancement of osseointegration by LIPUS. In addition, our data indicated the enhance mechanism of the osseointegration by low-intensive ultrasound irradiation could progress through not only the biological pathway for osteogenesis but also crystallographic bone-like apatite precipitation on the implant surface. This finding suggested the possibility of modification and characterizing of implant surface as the approach to improving the osseointgration. From a clinical point of view, the enhancement for bone-material bonding of LIPUS would be expected as not only the promotion of dental implant placement or bone grafting but preventing from postoperative loosening of dental implant.
This study is basic biomaterial research, just suggested the possibility of the enhancement of osseointegrarion by LIPUS.
Therapeutic condition for LIPUS for osseointegrarion was still unclear. Although further assessment regarding the Therapeutic condition for clinical application of LIPUS therapy for ossointegration will be necessary, the LIPUS therapy might have a potential as useful clinical application for the osseointegration between the HA coating implant and maxilla bone in dental surgery.
Conclusion
This study suggested that the clinical application of Low Intensity Ultrasound (LIPUS) irradiation has a great potential for enhancement of osseointegration of hydroxyapatite dental implant through the promoting mechanism of the nucleation and crystallization of bone-like apatite on implant surfaces.
- Brånemark PI, Hansson BO, Adell R, Breine U, Lindström J, et al. (1977) Implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand J lPlastic Reconstr Surg Suppl 16: 1-132.
- Brånemark PI (1983) Osseointegration and its experimental background. J Pros Dentistry 50: 399-410. Link: https://bit.ly/3jVq1hh
- Smeets R, Stadlinger B, Schwarz F, Beck-Broichsitter B, Jung O, et al. (2016) Impact of Dental Implant Surface Modifications on Osseointegration Bio Med Res Inter 1-16. Link: https://bit.ly/329ilSt
- Thomas KA, Kay JF, Cook SD, Jarcho M (1987) The effect of surface macrotexture and hydroxyapatite coating on the mechanical strength and histologic profiles of titanium implant materials. J Biomed Mater Res 21: 1395-1406. Link: https://bit.ly/3bCMa0V
- De Andrade MD, Sader MS, Filgueiras MRT, Ogasawara T (2000) Microstructure of ceramic coating on titanium surface as a result of hydrothermal treatment. J. Mater Sci Mater Med 11: 751-755. Link: https://bit.ly/2R4vKVF
- Heckman JD, Ryaby JP, McCabe J, Frey JJ, Kilcoyne RF (1994) Acceleration of tibial fracture-healing by non-invasive, low intensity pulsed ultrasound. J Bone Joint Surg 76: 26-34. Link: https://bit.ly/2GC6tjD
- Kristiansen TK, Ryaby JP, McCabe J, Frey JJ, Roe LR (1997) Accelerated healing of distal radial fractures with the use of specific, low-intensity ultrasound. (1997) A multicenter, prospective, randomaized, double-blind, placebo-controlled study. J Bone Joint Surg 79: 961-973. Link: https://bit.ly/3lZS6ps
- Tanzer M, Kantor S, Bobyn JD (2001) Enhancement of bone growth into porous intramedullary implants using non-invasive low intensity ultrasound. J Orthop Res 19 : 195-199. Link: https://bit.ly/3hacReo
- Rego EB, Takata T, Tanne K, Tanaka E (2012) Current status of low intensity pulsed ultrasound for dental purposes. Open Dent J 6: 220-225. Link: https://bit.ly/3jU43v8
- Abdulhameed EA, Enezei HH, Komori OM, Sugita Y, Hegazy FA, et al. (2017) The Effect of Low Intensity Pulsed Ultrasound Therapy on Osseointegration and Marginal Bone Loss Around Dental Implants. J Hard Tissue Bio 26: 323-330. Link: https://bit.ly/2F1Rv6i
- Khomich I, Rubnikovich S, Khomich S (2015) Enhancing osseointegration and new bone formation around dental implants using low intensity pulsed ultrasound. Int J Oral Maxillo Surg 44: Octover Supplement 1. e-243. Link: https://bit.ly/33bGg3f
- Kobayashi M, Noda K, Tatematsu N (2010) Preliminary in vitro study on Enhancement of Bone-like Hydoxyapatite formation on bioactive titanium alloy by low-intensity pulsed ultrasound waving for early bone bonding. J Biomech Sci Eng 5: 449-460. Link: https://bit.ly/2R41hHd
- Kobayashi M (2017) Study on enhancement of Osseointegration of the bio-active titanium implant by low intensive Ultrasound wave. (Part1: Simulated body fluid soaking test). Int J Biomed Eng Sci (IJBES) 4: 1-10. Link: https://bit.ly/3bITb0f
- Kobayashi M, Shiraishi M, Kinoshita M (2018) Study on enhancement of Osseointegration of the bio-active titanium implant by low intensive Ultrasound wave. (Part2: In vivo Experiment). Int J Biomed Eng Sci (IJBES) 1: 1-10. Link: https://bit.ly/328lzWx
- Kokubo T, Takamada H (2006) How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 27: 2907-2915. Link: https://bit.ly/3bA8ice
- Kokubo T, Miyaji F, Kim HM, Nakamura T (1996) Spontaneous formation of bonelike apatite layer on chemically treated titanium metals. J Am Cerami Soc 79: 1127-1129. Link: https://bit.ly/35j1fDI
- Kokubo T, Yamaguchi S (2010) Novel Bioactive Titanate Layers Formed on Ti Metal and Its Alloy by Chemical Treatments. Materials 3: 48-63. Link: https://bit.ly/339Xeie
- Yamaguchi S, Akeda K, Murata K, Takegami N, Goto M, et al. (2015) Chemical and Heat Treatments for Inducing Bone-Bonding Ability of Ti-6Al-4V Pedicle Screw. Key Eng Mate 631: 225-230. Link: https://bit.ly/2F8BLOL
- Mukai H, Kobayashi M (2011) The Effect of Low-intensive Pulsed Ultrasound Waving on Hydroxyapatite in Simulated Body Fluid. Proceed of IEEE 11th Inter. Confer. on Bioinf. Bioeng (BIBE) IEEE Computer Society Taipei 125-128. Link: https://bit.ly/3icTzGE
- Sato T, Shimizu Y, Odashima K, Sano Y, Yamamoto A, et al. (2018 ) In vitro and in vivo analysis of the biodegradable behavior of a magnesium alloy for biomedical applications. Dent Mate J 1: 1-11. Link: https://bit.ly/2Fdn2BN
- ZhaoW, Michalik D, Ferguson S, Hofstetter W, Lemaître J, et al. ( 2019 ) Rapid evaluation of bioactive Ti-based surfaces using an in vitro titration method. Nature commun 10 : 2062. Link: https://go.nature.com/329jKZf
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
