Journal of Clinical Research and Ophthalmology
Congenital left hemidiaphragm agenesis and use of glatiramer acetate
Hossein Kalanie1, Ali Amini Harandi2*, Manije Farajpoor1 and Milad Moradi2
2Brain Mapping Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Cite this as
Kalanie H, Harandi AA, Farajpoor M, Moradi M (2020) Congenital left hemidiaphragm agenesis and use of glatiramer acetate. J Clin Res Ophthalmol. 7(2): 091-093. DOI: 10.17352/2455-1414.000079Congenital Diaphragmatic Hernia (CDH) is a congenital defect of the diaphragm through which intestine and other viscera herniate in to the chest. In extreme form of diaphragmatic maldevelopment, there might be a complete agenesis of diaphragm. Neonates with CDH present postnatally with respiratory distress and a characteristic absence of breath sounds in the ipsilateral chest. Here we present a 26-year-old gravida 1, para 1 woman with Multiple Sclerosis (MS) on glatiramer acetate who was admitted for elective caesarean section at 38 weeks of gestation. Abdominal ultrasonography had been performed for mother monthly from month 3 and all were reported normal. A baby girl (3300g) was born. Shortly after birth neonate became cyanotic with heavy shallow respiration. Chest x-ray showed massive gas filled intestinal herniation in left thoracic cavity along with right sided mediastinal shift and right sided pneumothorax. Orogastric tube was placed, intubation was performed and she was placed immediately on a ventilator but in the way to operating room she was expired.
Introduction
Congenital Diaphragmatic Hernia (CDH) occurs 1 in 3000 live births, accounting for 8% of all birth defects and 1-2% of infant mortality, making it one of the most prevalent and lethal congenital anomalies [1-3]. The diaphragm develops during the 4th-8th weeks of gestation, and the hernia is thought to occur when the pleuroperitoneal folds and septum transversum fail to converge and fuse. Posterior lateral hernias (Bodaleck) account for >95% of neonatal diagnoses with 85% occurring on the left side [1,4]. Anterior retrosternal CDH (Morgagni) account for around 2% of all CDH cases. Other rare types of hernias include an anterior one and a central hernia. Diaphragmatic eventration is also included within the spectrum of CDH. Approximately 50-80% of CDHs are diagnosed in the prenatal period when the liver and intestines are visualized in the chest with a malpositioned heart. CDH may occur as an isolated defect, but about 40% of cases are non-isolated and have at least one additional anomaly [5,6], the most common one being CHD which is present in —20% of patients [4,6]. Outcome of CDH are largely dependent on the severity of the defect and the appropriate timing of treatment. A small percentage of cases go unrecognized in to adulthood [7].
Case presentation
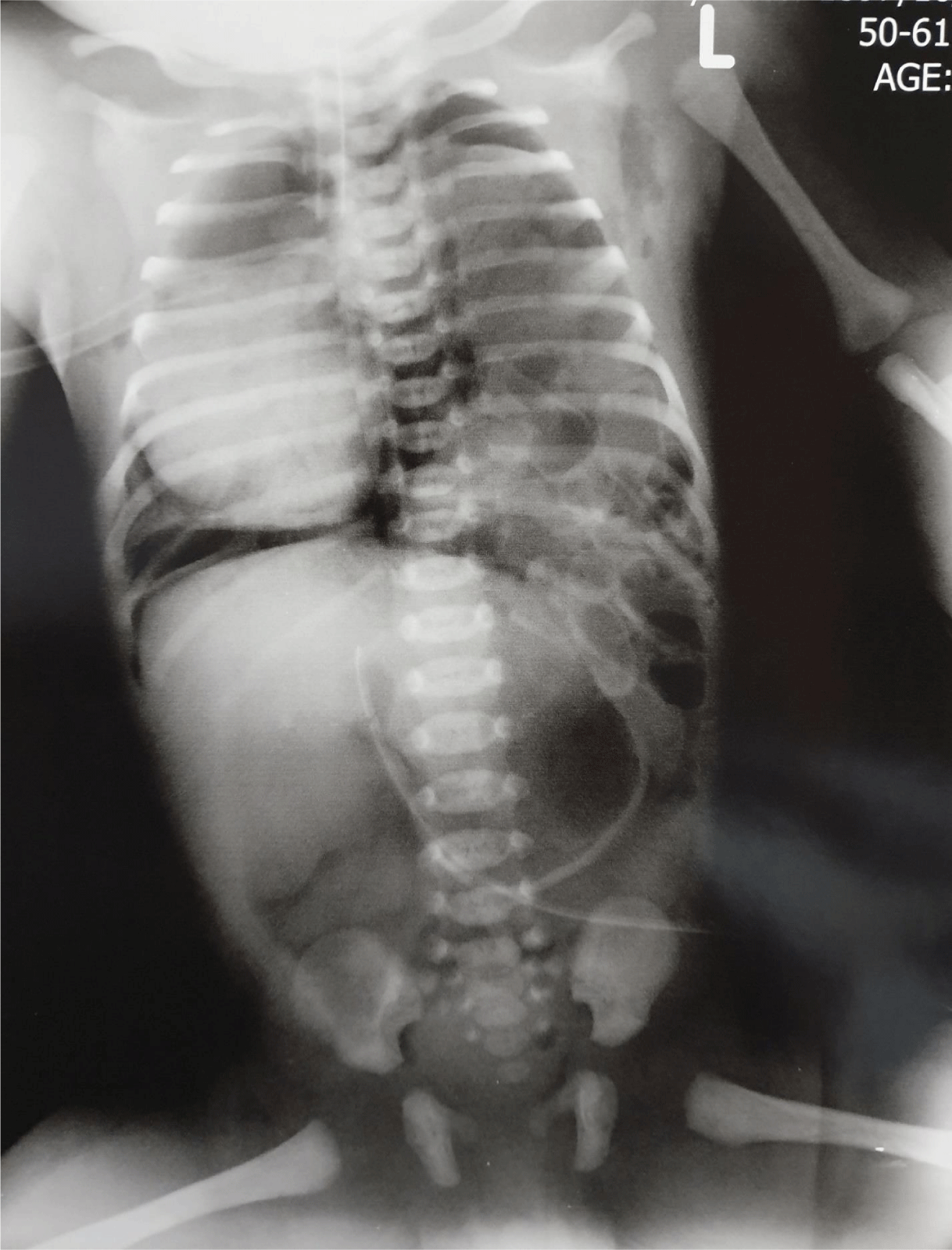
At January 8, 2019 a 29-year-old gravida 1, para 1 woman at 38 weeks of gestation was admitted for an elective caesarean section following an uncomplicated pregnancy. From month 3 she underwent abdominal ultrasonography, all were reported as normal (Figure 1). She had a history of MS for the past 6 years for which she was being treated with interferon b1a, and two months prior to decision for pregnancy the drug was switched to glatiramer acetate 3 sc injection per week. The father was 33 years old and there was no consanguinity. There was no family history of birth defects, any antenatal infection or exposure to any other medications, alcohol, smoking, or X-ray. Next day she was operated and a baby girl (3300g) was born with an APGAR of 8. About an hour after birth she became cyanotic and showed signs of respiratory distress with a scaphoid abdomen and auscultation of bowel sounds in the left chest. She was intubated and transferred to NICU. A chest X-ray (Figure 2) showed air-filled intestine herniated in to the left chest with shift of mediastinal structure to right side with right sided pneumothorax. Orogastric tube was placed, intubation was performed and she immediately was placed on a ventilator but in the way to operating room she was expired.
Discussion
Congenital Diaphragmatic Agenesis (CDA)is considered as one of the rare congenital malformations of diaphragm and reported to be in 6% of all CDH. Unilateral diaphragmatic agenesis is common on left side as compared to the right side. The etiology of CDA is largely based on assumption and speculation, and not well defined to date [8]. No cases of CDH in human have been unequivocally attributed to teratogenic or environmental exposure. Recently a potential association between one syndromic case of CDH (Fryns syndrome-like phenotype) and the immunosuppressive drug Mycophenolate Mofetil (MMF) has been made. MMF may also be associated with diaphragmatic hernia in developing rabbits [9]. The mechanism by which MMF could cause diaphragmatic defect is unknown. One retrospective questionnaire study has reported association of CDH with maternal alcohol use [9], and there is one case report following usage of lithium carbonate [10]. It has been well established that disruption of the retinoic acid signaling pathway is associated with diaphragmatic and other defects. There is new evidence that retinoic acid and vitamin A play an important role in human diaphragmatic development as well as its established role in other aspects of organogenesis. Mutation of STRA6, a membrane receptor for vitamin A retinol binding protein, is associated with Matthew-Wood syndrome. Since retinoic acid and vitamin A affect many aspects of development, it will be difficult to determine how they could be involved in case of isolated CDH. One small study showed decreased levels of retinol in newborns with CDH compared to control [9].
Another main etiologic factor for CDH is hereditary sources and chromosomal abnormalities. About 10% of all individuals with CDH have a chromosomal abnormality. The most common abnormalities are trisomy 18 and isochromosome 12p. Although many additional abnormalities have been reported. Several small rearrangements have been found in unrelated individuals, suggesting that one or more genes important for normal diaphragm development reside in these critical regions [9]. Congenital anomalies do not appear to be associated with MS [11]. In the past, it was generally advised that no DMTs (Disease- modifying treatment) for MS be taken during pregnancy as none was approved for use and still none of them is approved for use during pregnancy in the USA. However, there is some evidence from ongoing pregnancy registries that certain DMTs including glatiramer acetate (GA) are relatively safer for the developing fetus [12]. GA is now approved in the European Union for use during pregnancy in patients with RRMS [13]. The medical management of MS during pregnancy and the postpartum period is challenging given the risks of medication exposure to the fetus in utero and to the infant through breast milk by drugs with at most 30% effectiveness. Indeed, we cannot accuse the drug by a case report, but we prefer not to treat patients who has less sever MS from the beginning had no new attacks within the past 2 years prior to conceive. Ideally, clinician and their patients with MS should discuss family planning and different scenario of treating or not treating the patient as early as possible. This way patients may make informed decision about their medication choices during pregnancy while maintaining optimal disease protection throughout pregnancy and postpartum period.
- Doyle NM, Lally KP (2004) The CDH Study Group and advances in the clinical care of the patient with congenital diaphragmatic hernia. Semin Perinatol 28: 174-184. Link: https://bit.ly/2DFbI0K
- Tonks A, Wyldes M, Somerset DA, Dent K, Abhyankar A, et al. (2004) Congenital malformations of the diaphragm: findings of the West Midlands Congenital Anomaly Register 1995 to 2000. Prenat Diagn 24: 596-604. Link: https://bit.ly/2DECJ4t
- Dott MM, Wong LY, Rasmussen SA (2003) Population-based study of congenital diaphragmatic hernia: risk factors and survival in Metropolitan Atlanta, 1968-1999. Birth Defects Res A Clin Mol Teratol 67: 261-267. Link: https://bit.ly/3a8jQms
- Wynn J, Krishnan U, Aspelund G, Zhang Y, Duong J, et al. (2013) Outcomes of congenital diaphragmatic hernia in the modern era of management. J Pediatr 163: 114-119.e1. Link: https://bit.ly/2DMeFMQ
- Colvin J, Bower C, Dickinson JE, Sokol J (2005) Outcomes of congenital diaphragmatic hernia: a population-based study in Western Australia. Pediatrics 116: e356-e363. Link: https://bit.ly/3ad7TM5
- Zaiss I, Kehl S, Link K, Neff W, Schaible T, et al. (2011) Associated malformations in congenital diaphragmatic hernia. Am J Perinatol 28: 211-218. Link: https://bit.ly/3iCbg2v
- Swain JM, Klaus A, Achem SR, Hinder RA (2001) Congenital diaphragmatic hernia in adults. Semin Laparosc Surg 8: 246-255.
- Mirza B, Bashir Z, Sheikh A (2012) Congenital right hemidiaphragmatic agenesis. Lung India 29: 53-55. Link: https://bit.ly/3fGpeyo
- Longoni M, Pober BR, High FA (2006) Congenital Diaphragmatic Hernia Overview. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2020. Link: https://bit.ly/2XKJ1Xo
- Hosseini SH, Mousavi SA, Rashidi H (2010) Congenital diaphragmatic hernia following usage of lithium carbonate; is lithium a teratogen? Iran J Pediatr 20: 127-130. Link: https://bit.ly/3kvR4AL
- Ramagopalan SV, Guimond C, Criscuoli M, Dyment DA, Orton SM, et al. (2010) Congenital abnormalities and multiple sclerosis. BMC Neurol 10: 115. Link: https://bit.ly/2PGlJNS
- Houtchens MK, Kolb CM (2013) Multiple sclerosis and pregnancy: therapeutic considerations. J Neurol 260: 1202-1214. Link: https://bit.ly/33HpYke
- Voskuhl R, Momtazee C (2017) Pregnancy: Effect on Multiple Sclerosis, TreatmentConsiderations, and Breastfeeding. Neurotherapeutics 14: 974-984. Link: https://bit.ly/3gNJkrK

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
