Journal of Clinical Research and Ophthalmology
Evaluation of corneal morphology among newly detected patients with primary open angle glaucoma
Nagalakshmi Narayana-Swamy1* and Alhaj Farhath Tasneem2
2Professor, Department of Ophthalmology, Vydehi Institute of Medical Sciences and Research Center, Bangalore, India
Cite this as
Swamy NN, Tasneem AF (2020) Evaluation of corneal morphology among newly detected patients with primary open angle glaucoma. J Clin Res Ophthalmol 7(1): 008-014. DOI: 10.17352/2455-1414.000064Introduction: Glaucoma is an insidious blinding disease that may present in a variety of clinical scenarios. While Intraocular pressure is a major risk factor in its pathophysiology, patients have the disease without any documented elevation in pressure. To avoid missing a diagnosis of glaucoma, a thorough and careful evaluation of all the structural and functional components involved in glaucoma, should be performed. Although, most studies done so far have only focused on the Endothelial cell density among glaucoma patients, we highlighted its importance in the newly detected glaucoma patients.
Materials and methods: The present prospective case control study was conducted at Vydehi Institute of Medical Sciences and Research Center between June 2019 to August 2019. Study subjects included cases (subjects) who are newly detected primary open glaucomatous patients and controls as healthy subjects.
Results: During the period of study, 60 newly diagnosed primary open angle glaucoma's and 80 controls presented to us. The age of our patients varied from 21 to 60. In our study we found that there was endothelial cell loss among the case groups with 11.66% in newly detected cases of glaucoma. As the disease progressed, we saw a significant difference between the two study groups (glaucoma suspects and newly detected cases of glaucoma) with respect to mean Corrected Intraocular Pressure (c IOP), Cup Disc Ratio (CDR), Central Corneal Thickness (CCT). cIOP and CDR are indirectly proportional Endothelial Cell Density (ECD) whereas, CCT is directly proportional to ECD. On correlating the above finding, we saw statistical significance between reducing corneal ECD and increased central corneal thickness with larger CDR newly detected glaucoma patients.
Conclusion: There is an association between ECD and mean IOP in newly detected cases of primary glaucoma and glaucoma suspect. With increase in IOP there is loss of endothelial cells. Also There is a correlation between reducing corneal ECD and minimal changes in central corneal thickness with and larger CDR, newly detected glaucoma patients. Corneal ECD and CCT can be used as predictable risk factors among glaucoma suspects.
Introduction
Glaucoma is the leading cause of irreversible blindness worldwide. Primary open angle glaucoma is the most common form of glaucoma worldwide and accounts to an incidence of 0.5%-8.8% [1]. Although the Aravind Comphrensive Eye Survey [2], study conducted among the southern rural population and the Andhra Pradesh eye disease study conducted among urban population showed a prevalence of 1.7% and 2.6% respectively in individuals aged 40years and older.
Glaucoma is characterized by progressive death of ganglion cells with optic disc cupping and functionally noted with visual field detioration. All these changes are common but not always associated with raised IOP [3]. Numerous hypothesis have been noted eluding the pathophysiology of glaucoma including the mechanical theory of elevated IOP, vascular theory implicating chronic hypoxia, excitotoxicity and neurodegeneration secondary to autoimmune mechanism and inflammatory cytokine induced cell death [4]. However the scleral lamina of Optic Nerve Head (ONH) and inner retina are the primary sites of axonal injury [5]. The hallmark of glaucomatous optic neuropathy is the cup enlargement with progressive thinning of neuroretinal rim [6]. The cup disc ratio depends on the size of the cup but a ratio greater than 0.65 is found in less than 5% of the individuals [7]. A difference of less than or equal to 0.2 is seen in more than 96% of health subjects [8]. Therefore when significant asymmetry is noted between eyes possibility of glaucoma should be considered.
The ocular risk factors include thinner Central corneal thickness, myopia, disc hemorrhage, increased Cup Disc Ratio and asymmetric cupping. Non ocular risk factor includes older age, African race, positive family history, adult onset Diabetes mellitus, female sex, peripheral vasospasm, smoking and alcoholism [9-12].
Because of the wide variation in appearance of the disc, it is difficult to ascertain the glaucomatous damage. Hence Visual fields are better predictors of a progressive disease, requiring a mandatory evaluation of the glaucoma. In general clinically recognizable disc changes precede detectable field loss [13] and the presence or absence of glaucomatous filed defects can also be predicted based on appearance of ONH [14,15].
The effect of Central Corneal Thickness on the Intraocular Pressure readings by conventional tonometer’s was first acknowledged by Goldmann. Various studies have shown variation of central corneal thickness with ethnicity, age, type of glaucoma and anti-glaucoma medication’s [16], with advancing age decline in Central Corneal Thickness is noted. This decrease is accelerated in glaucoma [17]. Meta-analysis and reviews have been found the normal mean Central Corneal Thickness of 544μm and 95% confidence intervals noted to be 477-610μm [18]. Patients with ocular hypertension have been found to have higher Central Corneal Thickness by 50μm higher than glaucomatous patients. And normotensive glaucomatous patients were shown to have thinner corneas [19,20]. Few studies have published that thinner Central Corneal Thickness is associated with severity of glaucoma [21]. Also it is noted that thinner Central Corneal Thickness have higher risk of progression from ocular hypertension to Primary open angle glaucoma.
The study of endothelial cell morphology among glaucomatous patients had set in a great interest in field of glaucoma. Endothelial cell density is often reduced in patients with glaucoma. The pre-existing factor includes elevated Intraocular pressure, congenital abnormalities, ocular surgery and trauma [22]. Knorr, et al., [23], reported 31% reduction in endothelial cell density in Primary open angle glaucoma group in comparison to normal group in contrast to this Korrey, et al., showed no comparison of changes with Endothelial cell density in glaucomatous patients and ocular hypertensives [24].
In our present study we evaluated the relationship between corneal morphology and Central corneal thickness among newly detected glaucomatous patients in comparison with healthy subjects.
Materials and methods
The present prospective case control study was conducted at Vydehi Institute of Medical Sciences and Research Center between June 2019 to August 2019. Study subjects included cases (subjects) who are newly detected primary open glaucomatous patients and controls as healthy subjects.
A pre-structured Performa was used to collect the baseline data and an informed written consent was obtained after explaining about the need of the study and the procedures that were to be performed for the collection of data. Detailed history was taken and examination (ocular and systemic) was performed as per the proforma for those who satisfied the inclusion and exclusion criteria .
Inclusion criteria
Newly detected primary open angle glaucomatous patients.
Exclusion criteria
• Those with media opacities (like Dense lenticular changes, vitreous changes)
• Previous ocular surgeries
• Ocular inflammation or trauma
• Unstable patients/non-ambulatory patients
Sampling method
Consecutive sampling technique.
Statistical analysis was done using Microsoft excel and SPSS Software.
Investigations
The following investigations and interventions were done on newly detected primary glaucoma patients and glaucoma suspects, in case group and participants on control group:
• Visual acuity test (with and without pinhole) as assessed by Snellen’s chart.
• Best corrected Visual acuity
• Slit lamp examination of the anterior segment of eye
• Direct and Indirect Ophthalmoscopy and 78 D lens to view the posterior segment.
• Goldman’s applanation tonometry
• Specular Microscopy for measuring endothelial cell density and central corneal thickness using Model-Cellchek SL Premier Endothelial Analytics.
Results
The present study titled "TO CORNEAL MORPHOLOGY AND CENTRAL CORNEAL THICKNESS IN NEWLY DETECTED PRIMARY GLAUCOMA PATIENTS "was conducted in Vydehi Institute Of Medical Sciences and Research Centre, Whitefield, Bangalore (VIMS & RC) on the subjects who visited the outpatient department of Ophthalmology at VIMS & RC from MARCH 2019 to AUGUST 2019. This was a duration based, case-control study of diagnosed patients of primary open glaucoma and controls which took into consideration a sample of sixty for the case group and a sample of eighty participants as the control group, all cases satisfied the inclusion and exclusion criteria Table 1, Figure 1.
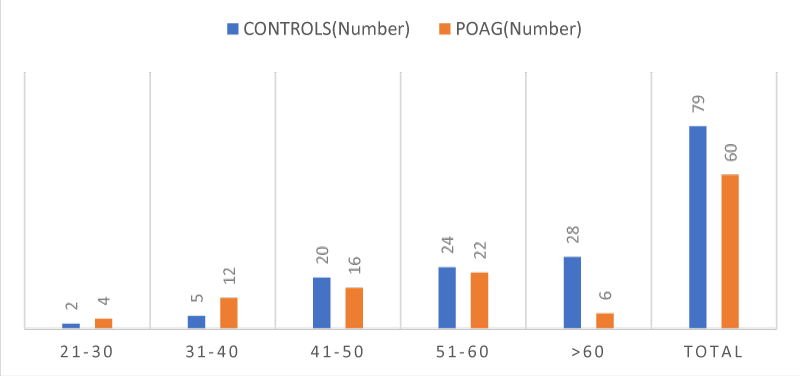
The age wise distribution of patients as analyzed showed a maximum number of patients (35.44%) in control group were above 60 years, and (36.6%) in POAG group were 51-60 years of age Table 2, Figure 2.
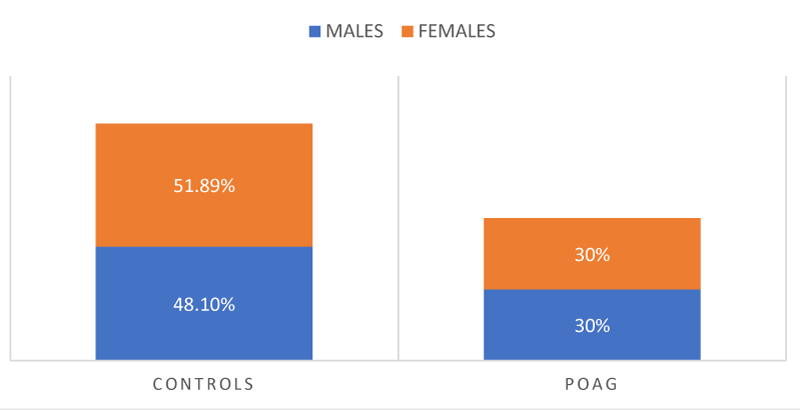
A gender wise distribution of patients as analyzed showed thirty-eight patients (48.1%) males and forty one (51.89%) females in control group, and eighteen(30%) males and forty two(30%) females in POAG group. Only the case study group 3 patients (5%) in POAG group. The rest had no significant family history of glaucoma. No significant difference in gender or family history was detected between the two groups Figure 3, Table 3.
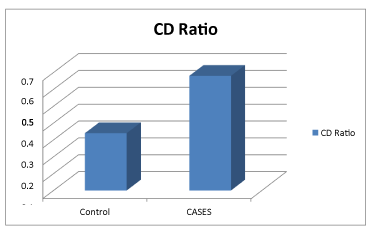
In our control group the range of Cup-Disc Ratio (CDR) was 0.20-0.50 with a mean of 0.34±0.08 and between 0.40-0.90 with a mean of 0.68±0.10 among POAG was noted. There was a significant difference between the CDRs among the two groups with POAG more than the controls Figure 4.
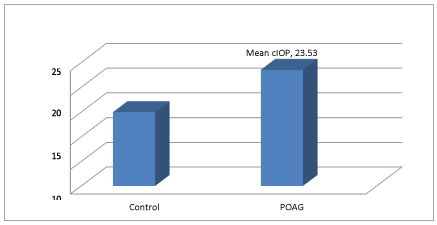
In our study, we observed the mean corrected IOP in control group was 14.99±2.06 with a minimum IOP of 11mm Hg. The Mean cIOP between glaucoma suspects and POAG was also found to be statistically significant (p<0.001) Table 4, Figure 5.
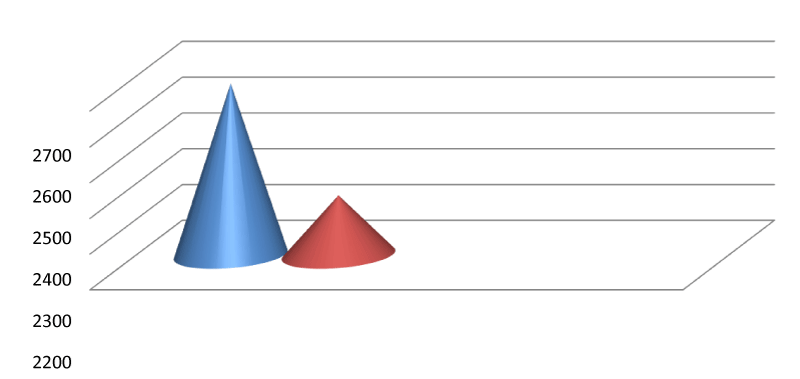
The mean endothelial cell density among the controls, and POAG was 2679.75±306.64 and 2367.23±261.31 respectively. We can see a significant difference between the ECD among the two study groups i.e. POAG in comparison to their control group Tables 5,6.
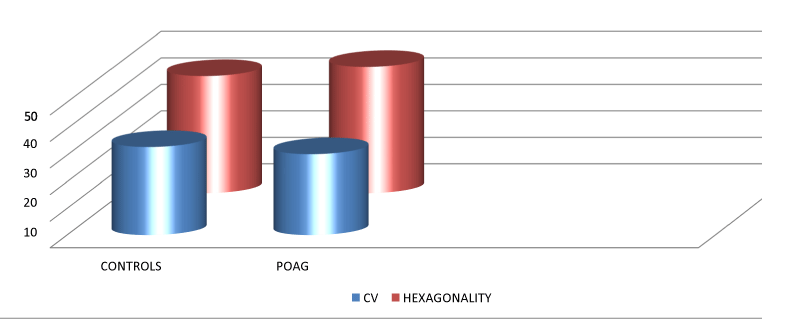
The mean Coefficient of variation among the controls was 33.09±6.9 and 30.38±9.07 among controls and Primary open angle glaucoma patients belonging to our study with their respective ranges as shown in the table above. The mean H among the controls was 44.16±7.74 and 47.61±10.42 among controls and Primary open angle glaucoma patients with their respective ranges as shown in the table above. In our study a statistical significance was seen for the Hexagonality of the endothelial cells and for the coefficient of variation of these cells Table 7, Figure 6.
The mean Central Corneal Thickness among the controls and POAG was 527.04±38.89 and 531.42±35.82 respectively, with statistical significance between the groups Table 8.
The mean age in the control group was 56.15 with a standard deviation of 10.62 and newly detected POAG patients in 50.97±11.52 years, which was statistically significant. The mean Endothelial cell density in the control group was 2679.75 with a standard deviation of 306.64 and newly detected POAG patients in 2367.23±204.64, which was statistically insignificant. The mean coefficient of variation in the control group was 33.09 with a standard deviation of 6.98 and newly detected Primary open angle glaucoma patients in 30.38±9.07, which was statistically significant.
The mean H in the control group was 44.16 with a standard deviation of 7.74 and newly detected Primary open angle glaucoma patients in 47.61±10.42, which was statistically significant. The mean IOP in the control group was 14.99 with a standard deviation of 2.06and newly detected POAG patients in 23.53±2.99, which was statistically significant. The mean Central corneal thickness in the control group was 527.04 with a standard deviation of 38.89 and newly detected Primary open angle glaucoma patients in 531.42±35.82, which was statistically significant. The mean Cup disc ratio in the control group was 0.34 with a standard deviation of 0.08 and newly detected POAG patients in 0.68±0.10, which was statistically significant Table 9.
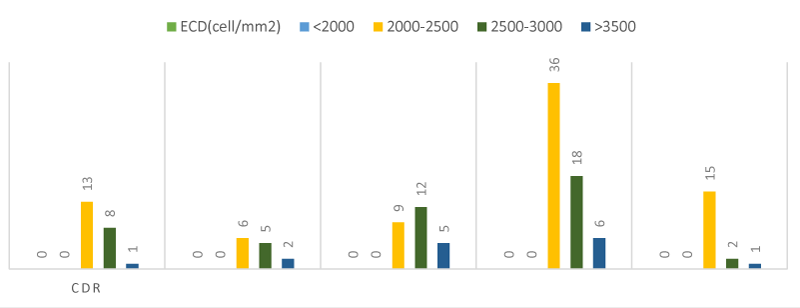
A statistical analysis was made to find the association between Endothelial cell density and Cup disc ratio among controls and newly detected cases of Primary open angle glaucoma. The analysis revealed that majority of the patients with a CDR less than 0.5 had an Endothelial cell density between 2000-2500. Even with a Cup disc ratio of 0.6-0.7, we encountered patients with Endothelial cell density of 2000-2500.As the Cup disc ratio increased beyond 0.6-0.7, (11.3%) of them showed an Endothelial cell density between 2000-2500 cells/mm2 and (18.9%) showed similar Endothelial cell density with a CDR >0.7 Figure 7, Table 10.
The above table and graph shows the association between Endothelial cell density and Intraocular pressure. It was seen that the patients with 10-15mm Hg ,have an Endothelial cell density of 2500-3000 cells/mm2. With an increasing Intraocular pressure beyond of 20-25mm Hg, we saw the loss of Endothelial cell density making it a total of 2000-2500 cells/mm2 (39%).
Discussion
This study was primarily aimed at correlating the corneal morphology, central corneal thickness among newly detected primary glaucoma patients.
The demographic data both the groups are summarized, there was no statistical significance in age (Figure 1, Table 1), gender (Figure 2, Table 2) among the two groups. There is a considerable amount of literature on low ECD among patients who fall into the category of incipient or terminal glaucoma like ocular hypertensive glaucoma/normal tension glaucoma/early primary glaucoma, established glaucoma or angle closure glaucoma, thus emphasizing the vital role of endothelium in glaucoma. Gagon et al reported a 13.0% reduction in ECD in POAG patients, and a 11.9% reduction in NTG (n=5) patients who were on glaucoma medication, but we in our study excluded patients on glaucoma medication [25]. Sung Woo Cho et al showed no significant reduction in corneal endothelial cell density in normal tension glaucoma patients, unlike the POAG patients who showed 13% reduction compared to normal. ECD was significantly lower in patients with glaucoma (2514+/-419 cells/mm2) compared with controls (2560+/-306 cells/mm2) [26], Despite this interest, to the best of our knowledge it is not yet known whether glaucoma suspects have early changes in their endothelial count.
In this context, we evaluated incipient primary glaucoma patients and we found that the ECD was significantly reduced (p<0.001). The mean endothelial cell density among the controls and newly detected cases of POAG was 2679.75±306.64 and 2367.2 ±204.64 respectively (Table 5, Figure 5).
The mean ECD was significantly low among the newly detected cases of primary glaucoma. The POAG patients showed a mean difference of 92.4cells (3.44%) and 312.52 cells (11.66%) compared with the normal group respectively. A modest increase in IOP(<40mm Hg) for a prolonged period of time is essential to have even a minute effect on the corneal endothelium and corneal thickness. Patients with an healthy endothelium rarely develop changes, unless there is an acute and long lasting elevation in IOP [27].
Surprisingly, our analysis showed significant difference in Coefficient of Variation (CV) and Hexagonality (H) among the groups(Figure 6, Table 6). This is in complete disagreement with previous findings in the literature as changes in CV and Hexagonality indicate acute corneal endothelial damage [28-30]. Justifying our finding is a study which states that an elevated or abnormal rate of polymegathism is usually the first sign of endothelial damage. This finding indicates physiological stress to the corneal endothelium and an overactive wound repair mechanism [31].
This lends support to the previous findings in the study on, role of CCT in diagnosis of glaucoma which also found to have significantly higher CCT in ocular hypertensive than in those of glaucoma and the normals [32].
The Ocular Hypertension Treatment Study (OHTS) has now recognized lower Central Corneal Thickness (CCT) as an independent risk factor for the development of POAG in eyes with ocular hypertension (OH) [33]. We need not sound a note of caution as both our study groups having CCTs within normal limit and we also considered CCTs of every patient by correcting their IOP using modified Ehler's correction factor.
The mean score for cIOP among newly detected primary glaucoma patients (23.53mm Hg) (Figure 4, Table 4) was found to be statistically significant (p<0.001). Although ,there was only a minute difference in IOP between the two case groups,corneal endothelial cell density was reduced by 11.66 % in the newly detected glaucoma patients [34], who found a similar difference in IOP , had a 13 % decrease in ECD among POAG patients and thus inferred that there are two mechanisms which account for the decrease in ECD induced by a small IOP rise. Firstly, a small IOP rise of long duration may affect the barrier function of the corneal endothelium and secondly IOP fluctuation [35], reported that POAG eyes that experience IOP >21mm Hg show more clear-cut IOP-dependence than do NTG eyes and thus concluded that a small rise in IOP over a long period contributes significantly to glaucoma progression, and it may also cause a decrease in corneal endothelial cell density. However, we need to evaluate the time period required for this small rise in IOP to cause reduction in corneal endothelial cell density, leading to disease progression [36]. confirmed that endothelial cell counts in glaucoma groups were inversely proportional to the means of IOP .
Our results share similarities to the above mentioned fact by showing a decrease in endothelial cell count to 2000-2500 cells/mm2 when there was a surge in IOP more than 20mm Hg.
The significant difference in the CDR between glaucomatous (0.68±0.10) and healthy eyes (0.34±0.08) was expected, and agrees with the principles of glaucomatous diagnostic criteria. Although our results differ slightly from George Tomais (2008), which showed that CDR (Figure 3, Table 3) was highest in POAG than the normal, but this difference wasn't significant [37].
Our study provides considerable insight about the relationship between ECD, CCT and CDR which are derivatives of neural crest. We observed that with ECD and CCT are inversely proportional to CDR (Figure 7, Table 9). This phenomenon could be related with the underestimation of IOP measurement in eyes with thin corneas and probably with the high tolerance of lamina cribrosa in eyes with thick corneas. Based on that remark, we could consider that the coexistence of thin cornea and big C/D area ratio in a glaucoma suspect, may contribute to glaucoma progression. Pakaravam and colleagues (2007) confirmed that CDR was inversely correlated with CCT in patients with POAG [38].
Hence it is important that understanding the relationship with corneal morphology and CCT in glaucomatous patients is vital in understanding the disease pathology and to aid treatment. Further studies in glaucoma, which take ECD into account, will need to be performed by classifying glaucoma suspects into five groups i.e; Type I-Normal IOP, no damage, Type II-Normal IOP, possible damage, Type III-High IOP, no damage, Type IV-High IOP, possible damage and Type V-Possible glaucomatous damage .We are confident that this may further improve the knowledge about the role of ECD in glaucoma suspects.
- Cho HK, Kee C (2014) Population-based glaucoma prevalence studies in Asians. Surv Ophthalmol 59: 434-447. Link: http://bit.ly/2ScgSoy
- Ramakrishnan R, Nirmalan PK, Krishnadas R, Thulasiraj RD, Tielsch JM, et al. (2003) Glaucoma in a rural population of southern India: the Aravind comprehensive eye survey. Ophthalmology 110: 1484-1490. Link: http://bit.ly/2RKO0EP
- Dandona L, Dandona R, Srinivas M, Mandal P, John RK, et al. (2000) Open-angle glaucoma in an urban population in southern india: The Andhra Pradesh eye disease study. Ophthalmology 107: 1702-1709. Link: http://bit.ly/2vyCIut
- Fechtner RD, Weinreb RN (1994) Mechanisms of optic nerve damage in primary open angle glaucoma. Surb Ophthalmol 339: 23-42. Link: http://bit.ly/2uRVAEo
- Quigley HA, Addicks EM, Green WR, Maumenee AE (1981) Optic nerve damage in human glaucoma-II. The site of injury and susceptibiltiy to damage. Arch Ophthalmol 99: 635-649. Link: http://bit.ly/2Sj4cfF
- Jonas JB, Budde WM (2000) Diagnosis and pathogenesis of glaucomatous optic neuropathy:morphological aspects. Prog Retin Eye Res 19: 1-40. Link: http://bit.ly/36Gp8lH
- Varma R, Tielsch JM, Quigley HA, Hilton SC, Katz J, et al. (1994) Race, age, gender and refractive error related differences in the normal optic disc. Arch Ophthalmol 112: 1068-1076. Link: http://bit.ly/38ZB5Ev
- Jonas JB, Gusek GC, Naumann GO (1988) Optic disc,cup and neuroretinal rim size,configuration and correlation in normal eyes. Invest Ophthalmol Vis Sci 29: 1151-1158. Link: http://bit.ly/2vvzkk3
- Leske MC, Connell AM, Wu SY, Hyman LG, Schachat AP (1995) Risk factors for open angle glaucoma. The Barbados Eye Study. Arch Ophthalmol 113: 918-924. Link: http://bit.ly/2OgjgJL
- Klein BE, Klein R, Sponsel WE, Franke T, Cantor LB, et al. (1992) Prevalence of glaucoma. The Beaver Dam Eye Study. Ophthalmology 99: 1499-1504. Link: http://bit.ly/2RNfN7H
- Brandt JD (2004) Corneal thickness in glaucoma screening, diagnosis and management. Curr Opin Ophthalmol 15: 85-89. Link: http://bit.ly/2UeXV7k
- (2005) American Academy of Opahtlamology: Primary open angle glaucoma preferred practice pattern. San Francisco. The Academy. Link:
- Zeyen TG, Caprioli J (1993) Progression of disc and field damage in early glaucoma. Arch Ophthalmol 111: 62-65. Link: http://bit.ly/2tVreB3
- Drance SM (1976) Correlation between optic disc changes and visual field defects in chronic open angle glaucoma. Trans Am Acad Ophthalmol Otolaryngol 81: 224-226. Link: http://bit.ly/36Knuj4
- Hitchings RA, Spaeth GL (1977) The optic disc in glaucoma II: correlation of the appearance of the optic disc with the visual field. Br J Ophthalmol 61: 107-113. Link: http://bit.ly/37KgNi5
- Nemesure B, Wu S, Hennis A, Leske MC (2003) Corneal thickness and intraocular pressure in the Barbados eye studies. Arch Ophthalmol 121: 240-244. Link: http://bit.ly/391GKKB
- Brandt JD, Beiser JA, Kass MA, Gordon MO (2001) Central corneal thickness in the ocular hypertension treatment study (OHTS). Ophthalmology 108: 1779-1888. Link: http://bit.ly/3b1wUtG
- Weizer JS, Stinett SS, Herndon LW (2006) Longitudinal changes in central corneal thickness and their relation to glaucoma status: an 8 year follow up study. Br J Ophthalmol 990: 732-736. Link: http://bit.ly/38PqpZ8
- Doughty MJ, Zaman ML (2000) Human corneal thickness and its impact on intraocular pressure measures:a review and metaanalysis approach. Surv Ophthalmol 44: 367-408. Link: http://bit.ly/31asEUi
- Herndon LW, Choudhri SA, Cox T, Damji KF, Shields MB, et al. (1997) Central corneal thickness in normal, glaucomatous and ocular hypertensive eyes. Arch Ophthalmol 115: 1137-1141. Link: http://bit.ly/3b1Y2sz
- Copt RP, Thomas R, Mermoud A (1999) Corneal thickness in ocular hypertensionprimary open-angle glaucoma and normal tension glaucoma. Arch Ophthalmol 117: 14-16. Link: http://bit.ly/2OcP95B
- Herdon LW, Weizer JS, Stinett SS (2004) Central corneal thickness as a risk factor for advanced glaucoma damage. Arch Ophthalmol 122: 17- 21. Link: http://bit.ly/2GHeSz2
- Knorr HL, Handel A, Naumann GO (1991) Morphometric and qualitative changes in corneal endothelium in primary chronic open angle glaucoma. Fortschr Ophthalmol 88: 118-120. Link: http://bit.ly/2UaUgXQ
- Korey M (1982) ECD and CCT in ocular hypertension and POAG. Am J Ophthalmol.
- Cho SW, Kim JM, Choi CY, Park KH (2009) Changes in corneal endothelial cell density in patients with NTG. Jpn J Ophthalmol 53: 569-573. Link: http://bit.ly/31dR0wE
- Boisjoly H, Mortazavi A, Brunette I, Charest M, Amyot M (1996) Corneal endothelial cell anomalies in glaucoma suspects with ocular hypertension. Acta Ophthalmol 74: 477-484. Link: http://bit.ly/2RHsYH5
- Bourne WM, Mc Laren JW (2004) Clinical responses of the corneal endothelium. Exp Eye Res 78: 561-572. Link: http://bit.ly/38Wfz3w
- Matsuda M, Suda T, Manabe R (1984) Serial alterations in endothelial cell shape and pattern after intraocular surgery. Am J Ophthalmol 98: 313-319. Link: http://bit.ly/2RJkTS3
- Kim JH, Kim CS (2006) The change in corneal endothelial cells after glaucoma valve implantation. J Korean Ophthalmol Soc 47: 1972-1980.
- Phillips C, Laing R, Yee R (2005) Specular Microscopy. In: Krachmer JH, Mannis MJ, Holland EJ (eds). Cornea, 2nd ed.Philadelphia: Elsevier Mosby 261-277.
- Thomas R, Korah S Muliyil J (2000) The role of central corneal thickness in the diagnosis of glaucoma. Indian J Ophthalmol 48: 107-111. Link: http://bit.ly/2GCEugF
- Brandt JD, Beiser JA, Kass MA, Gordon MO (2001) Central corneal thickness in the Ocular Hypertension Treatment Study (OHTS). Ophthalmology 108: 1779-1788. Link: http://bit.ly/3b1wUtG
- Cho SW, Kim JM, Choi CY, Park KH (2009) Changes in corneal endothelial cell density in patients with NTG. Jpn J Ophthalmol 53: 569-573. Link: http://bit.ly/2tjHBXM
- Sihota R, Saxena R, Gogoi M, Sood A, Gulati V, et al. (2005) A comparison of the circadian rhythm of intraocular pressure in primary chronic angle closure glaucoma, primary open angle glaucoma and normal eyes. Indian J Ophthalmol 53: 243-247. Link: http://bit.ly/2uMx2wO
- Inatani M, Iwao K, Inoue T, Awai M, Muto T, et al. (2008) Long-term relationship between intraocular pressure and visual field loss in primary open-angle glaucoma. J Glaucoma 17: 275-279. Link: http://bit.ly/2uPQsRr
- Gagnon MM, Boisjoly HM, Brunette I, Charest M, Amyot M (1997) Corneal endothelial cell density in glaucoma. Cornea 16: 314-318. Link: http://bit.ly/2uNBtaw
- Tomais G, Georgopoulos G, Koutsandrea C, Moschos M (2008) Correlation of central corneal thickness and axial length to the optic disc and peripapillary atrophy among healthy individuals, glaucoma and ocular hypertension patients. Clin Ophthalmol 2: 981-988. Link: http://bit.ly/2tfficS
- Pakravan M, Parsa A, Sanagou M, Parsa CF (2007) Central corneal thickness and correlation to optic disc size: A potential link for susceptibility to glaucoma. Br J Ophthalmol 91: 26-28. Link: http://bit.ly/36G3SN7

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
