Journal of Cardiovascular Medicine and Cardiology
The possibility of catching severe COVID-19 disease with cardio-renal manifestation after the first dose of the BNT-162b2 vaccine: A case report
Randa Tabbah1*, Nagi Azzi2 and Rachoin Rachoin1
2Departement of Cardiology, Lebanese American University, Risk Hospital, PO Box 11-3288, Beirut Lebanon
Cite this as
Tabbah R, Azzi N, Rachoin R (2021) The possibility of catching severe COVID-19 disease with cardio-renal manifestation after the first dose of the BNT-162b2 vaccine: A case report. J Cardiovasc Med Cardiol 8(3): 059-062. DOI: 10.17352/2455-2976.000171Introduction: Since November 2019, coronavirus disease 2019 (COVID-19) has continued to spread across the world in an unpredictable way. In December 2020, the US Food and Drug Administration (FDA) issued Emergency Use Authorizations for two mRNA-based vaccines for prevention of COVID-19 including the BNT-162b2 or Pfizer-BioNTech vaccine. The window period of the vaccine efficacy and baseline patients’ characteristics remain factors to be taken into consideration in all cases.
Clinical presentation: A 92-years old lady known to have a history of severe Aortic Stenosis (AS), bedridden years ago received her first BNT-162b2 shot as per country protocol. Five-days later, a routine lab-test revealed hypereosinophilia. Ten-days after vaccination, patient developed a low-grade fever 38.2 with cough, tachycardia of 160bpm and SpO2= 95% on 2 liters of oxygen. She was dehydrated and had oliguria. CT-scan revealed a bilateral pleural effusion with no other relevant findings and EKG was in favor of rapid atrial fibrillation (AFib) of 160 bpm, back to sinus after digoxin 0.25mg IV. COVID-PCR came back positive, and patient was discharged home by the COVID-19 team for symptomatic treatment and follow up. Two-days later, patient became sicker with shortness of breath, loss of appetite and higher fever. She was admitted then, and lab-tests revealed very high levels of hepatic and pancreatic enzymes in addition to high levels of cardiac-enzymes revealing cardiac injury. Moreover, inflammatory labs were high and same for her creatinine levels revealing a cardiorenal syndrome. Patient was on corticosteroids and hydration for several days with improvement of her vital signs and lab-tests with hepatic enzymes close to normal values but an increase in WBC with CRP levels remaining low. Neurologic status was poor. CT brain revealed a small ischemic stroke on the left side with weakness and hemiparesis on the right. Echocardiography revealed a decrease in LV function with global hypokinesis. After 1week, the neurologic status of the patient declined, and invasive ventilation was needed. 3 days after intubation patient died.
Conclusion: Cardiorenal syndrome is possible after a first dose of covid-19 vaccine. That is why masking and social distancing should be maintained to prevent fully immunized subjects from acting as human vectors to non-immunized ones. No protection for patients after first shot of vaccine within the window of 12 day.
Learning objectives
- Masking and social distancing should be maintained to prevent contracting COVID-19 and thereafter to prevent immunized subjects from acting as human vectors
- The presence of eosinophilia right after a COVID-19 vaccination should rather be attributed to the vaccine than to the disease, because of the demonstrated protective role of that eosinophilia.
- There is no protection before the 12th day of the first vaccine dose, with a gradual improvement thereafter before reaching 70% protection on the 21st day.
- The cardiovascular manifestations of COVID-19 include atrial arrhythmias as being the most frequent, with atrial fibrillation as the most common and ominous for the patient’s outcome because it denotes an extensive myocardial injury.
Introduction
Since November 2019, coronavirus disease 2019 (COVID-19) has continued to spread across the world following a trajectory that is difficult to predict. In December 2020, the US Food and Drug Administration (FDA) issued the Emergency Use Authorizations for two mRNA-based vaccines for prevention of COVID-19 including the BNT-162b2 or Pfizer-BioNTech vaccine. A two-dose formulation of BNT162b2 in subjects 16 years or older was 100% effective against severe disease, and 95.3% effective against COVID-19 as defined by the FDA [1], with a favorable safety and tolerability profile. The window period of the vaccine efficacy and baseline patient’s characteristics remain factors to be taken into consideration.
Case presentation
A 92-years old lady known to have a history of severe Aortic Stenosis (AS) who previously refused any TAVI procedure, chronic bilateral pleural effusion treated with diuretics, Parkinson disease and bedridden many years ago received her first BNT-162b2 shot as per country protocol. Five-days later, a routine lab-test revealed normal White Blood Count (WBC) but hypereosinophilia (9.5%; N=1-4%), normal creatinine clearance, electrolytes, and CRP =11.2mg/l. Chest X-ray showed only a moderate bilateral pleural effusion. Furosemide dosage was increased for 2-3 days from 1 tablet 40mg to 2 tablets daily.
Ten-days after vaccination, patient had a low-grade fever 38.2 with cough (known to have recurrent aspiration pneumonia). She presented to the ER the same day with dyspnea, tachycardia of 160bpm and SpO2= 95% on 2 liters of oxygen. On physical examination, patient was dehydrated and oliguric. She had bilateral crackles on lung auscultation and a systolic murmur radiating to the carotid.
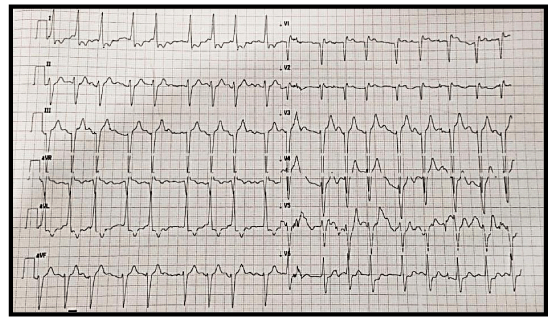
A CT scan was done revealing a bilateral pleural effusion with no other relevant findings. The most suspected diagnosis was sepsis from aspiration pneumonia or a urosepsis because patient had chronic urinary infection with ESBL. On lab test, acute renal failure was noticed with creatinine= 2mg/dl and CRP= 20mg/l. On Electrocardiogram, patient was in rapid Atrial Fibrillation (AF) of 160 bpm, back to sinus after digoxin 0.25mg IV [Figure 1].
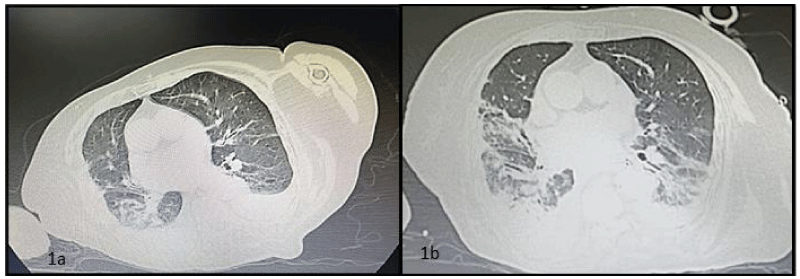
COVID-19 PCR came back positive, and patient was discharged home after stabilization of her vital signs by the COVID-19 team for symptomatic treatment and follow up. Two-days later, patient became sicker with shortness of breath, loss of appetite and higher fever. Chest CT scan was done revealing bilateral pleural effusion with pulmonary congestion [Figure 2-1a] with a normal sinus rhythm.
She was admitted then to the covid floor and lab tests revealed very high levels of hepatic and pancreatic enzymes in addition to high levels of cardiac enzymes revealing cardiac injury and probably a multiorgan failure due to the virus. WBC= 24100 103/UL, Creatinine= 4,03 mg/dl, SGOT= 6726 U/L, SGPT= 1419 U/L, LDH= 7426 U/L, creatine phosphokinase(CPK)= 2353 U/L, CKMB= 190U/L, Troponin T > 10000 ng/L, CRP= 53 mg/L. Patient was on corticosteroids methylprednisolone 40mg daily and hydration with 1L of saline with furosemide 80 mg/ day IV for several days with improvement of her vital signs and lab tests with hepatic enzymes close to normal values as well as a decrease in troponin levels, CPK and an increase in WBC (maybe due to steroids) with CRP levels remaining low, but neurologic status remained poor [Table 1]. Abdominal ultrasound was done to rule out any hepatic or biliary duct issues and reveled only a mild congestion of the liver. In addition, she had some short runs of atrial fibrillation but was on enoxaparin for secondary prevention.
CT brain revealed a small ischemic stroke on the left side with weakness and hemiparesis on the right. Echocardiography was done revealing a decrease in LV function with global hypokinesis and an EF= 40% on top of her severe AS and a mildly congested liver. After 1week, the neurologic status of the patient declined (Glasgow 4-5) and invasive ventilation was needed. CT scan was repeated with no major decline in pulmonary function or damage to the parenchyma (30% involvement) [Figure 2-1b]. 3 days after intubation patient died after proper cardiorespiratory resuscitation.
Discussion
This is an unusual case in its context, patient’s comorbidities, and clinical evolution since she received BNT-162b2 vaccine, then contracting COVID-19, until her death after a presenting a cardiorenal syndrome. All these events were secondary to COVID-19 infection associated with recent immunization.
First, it is well established that vaccinated subjects may play the role of human vectors who transmit SARS-CoV2 to non-vaccinated contacts, so masking and social distancing should be maintained [2]. A person is fully immunized 2 weeks after the second BNT-162b2 dose, before this time frame, infection with SARS-CoV2 is possible.
Moreover, concerning vaccination in patients on palliative care, CDC had already stated that the risk for severe disease with COVID-19 increases with age, and with underlying medical conditions. Their current recommendation is to prioritize this subpopulation.
The presence of eosinophilia early during this patient’s illness was intriguing, because despite its “protective role” patient had a fatal outcome. Note that previous patient’s lab tests did not reveal any eosinophilia. Consequently, this eosinophilia may be a reaction to the vaccination per se and probably preceded contraction of COVID-19. To support this, Arun Nair et al. saw that patients with eosinophilia had a lower level of CRP, milder clinical course and better disease outcomes compared to those without eosinophilia, which was not the patient’s case.
They mentioned a “protective role of eosinophils in mitigating the severity of inflammatory diseases through an inhibitory mechanism”. However, these findings need to be confirmed by further prospective studies [3]. In addition, it is notable that pulmonary eosinophilia is not part of the lung pathology attributed to SARS-CoV-2 [4].
Moreover, there’s considerable concern about whether SARS-CoV-2 exposure postvaccination would cause eosinophil-associated lung pathology through immunopotentiation. However, this does not apply to our patient.
Furthermore, only minimal lung involvement was seen in this patient, who rather presented with a cardiorenal syndrome, with a progression of the neurologic status leading to death. Was there a delay in lung involvement due to vaccination? In fact, it has been shown that there was no evidence of protection until about 12 days after the first dose, but afterwards the incidence of COVID-19 gets lower with time. Therefore, our patient probably contracted COVID-19 when she still had no protection from the vaccine itself and what she had was a specific course of the disease itself. [5]. Also, Siren study did not find a significant protection before 21days after BNT162b2 first dose [6]. So, we would say that there is no protection within 12 days after the first dose, with a gradual improvement thereafter before reaching 70% protection on the 21st day.
On top of the patient status, she also had severe cardiovascular involvement from COVID-19. Indeed, arrhythmias have been reported in COVID-19 cases (7 to 16.7% of hospitalized patients) [7]. Atrial arrhythmias were the most frequent (35%), with AF the most common one (21%). Ventricular tachycardia and fibrillation were the least common (4.8-5.6%) [8].
New-onset arrhythmias in COVID patients appear to be associated with higher WBC, CPK and troponin levels, suggesting an inflammatory stress causing myocardial injury.
A higher mortality was then observed among hospitalized patients (~50%) [9], as arrhythmias increase by 2-folds the all-cause mortality [7].
Moreover, the AF with elevation of cardiac enzymes, with this clinical presentation are in favor of secondary ischemia or Type 2-MI following a mismatch between myocardial oxygen supply and demand over a severe AS, with a potential underlying Coronary Artery Disease (CAD). The rapid decrease in troponin, CPK and with CRP levels remaining low, add in favor of this diagnosis, rather than a COVID-19 induced myocarditis [10]. Although coronary thrombosis is common in patients with severe COVID-19, but the clinical course, electrocardiogram and echocardiography make it even more unlikely than myocarditis in this patient. Cardiac MRI is the gold standard for differentiating myocarditis from myocardial ischemia, but the rapidly deteriorating clinical picture made its realization of sole diagnostic interest and it was not available in the hospital.
In the end, vaccine-induced susceptibility to virus infection have been documented for infections with different virus families. Mechanisms are poorly understood. For vaccine-induced enhanced susceptibility to infection with certain viruses has shown that Antibody-Dependent Enhancement (ADE) plays an important role. There may be a delicate balance between the induction of protective immunity and the induction of enhanced susceptibility [11].
Conclusion
Cardiorenal syndrome with mild or no lung involvement could be a possible outcome after a first dose of covid-19 vaccine. That is why masking and social distancing should be maintained to prevent fully immunized subjects from acting as human vectors to non-immunized ones.
To all Health Workers who struggled during this pandemic
- Cobb S, Anderson F, Bauer W (1953) Length of life and cause of death in rheumatoid arthritis. N Engl J Med 249: 553–556. Link: https://bit.ly/3wK2GGe
- Chen J, Norling LV, Cooper D (2021) Cardiac Dysfunction in Rheumatoid Arthritis: The Role of Inflammation. Cells 10: 881. Link: https://bit.ly/3gQPmJd .
- Castañeda S, Nurmohamed MT, González-Gay MA (2016) Cardiovascular disease in inflammatory rheumatic diseases. Best Pract Res Clin Rheumatol 30: 851-869. Link: https://bit.ly/3qlu5vS
- Sharifzadeh B, Kalbasi R, Jahangiri M, Toghraie D, Karimipour A (2020) Computer modeling of pulsatile blood flow in elastic artery using a software program for application in biomedical engineering. Computer Methods and Programs in Biomedicine 192: 105442. Link: https://bit.ly/3wNU5Cy
- Charles-Schoeman C, Lee YY, Grijalva V, Amjadi S, FitzGerald J, et al. (2012) Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Annals of the Rheumatic Diseases 71: 1157–1162. Link: https://bit.ly/3zL33T0
- Wållberg-Jonsson S, Caidahl K, Klintland N, Nyberg G, Rantapää-Dahlqvist S (2008) Increased arterial stiffness and indication of endothelial dysfunction in long-standing rheumatoid arthritis. Scandinavian Journal of Rheumatology 37: 1–5. Link: https://bit.ly/3xHsBP3
- He M, Liang X, He L, Wen W, Zhao S, et al. (2013) Endothelial dysfunction in rheumatoid arthritis: the role of monocyte chemotactic protein-1-induced protein. Arteriosclerosis Thrombosis and Vascular Biology 33: 1384–1391. Link: https://bit.ly/3vKwfG8
- Sun HJ, Wu ZY, Nie XW, Bian JS (2020) Role of Endothelial Dysfunction in Cardiovascular Diseases: The Link Between Inflammation and Hydrogen Sulfide. Frontiers Pharmacology 10: 1568. Link: https://bit.ly/3vJ7L04
- WHO (2000) Cardiovascular diseases. Link: https://bit.ly/3zHEoyr
- van den Hoek J, Boshuizen HC, Roorda LD, Tijhuis GJ, Nurmohamed MT, et al. (2017) Mortality in patients with rheumatoid arthritis: a 15-year prospective cohort study. Rheumatology international 37: 487–493. Link: https://bit.ly/3vH63MD
- Widdifield J, Bernatsky S, Paterson JM, Tomlinson G, Tu K, et al. (2015) Trends in Excess Mortality Among Patients With Rheumatoid Arthritis in Ontario, Canada. Arthritis care & research 67: 1047–1053. Link: https://bit.ly/2SGwAwr
- Chodara AM, Wattiaux A, Bartel CM (2017) Managing Cardiovascular Disease Risk in Rheumatoid Arthritis: Clinical Updates and Three Strategic Approaches. Current Rheumatology Reports 19: 16. Link: https://bit.ly/3vSWvP3
- Bissell LA, Erhayiem B, Fent G, Hensor E, Burska A, et al. (2018) Carotid artery volumetric measures associate with clinical ten-year cardiovascular (CV) risk scores and individual traditional CV risk factors in rheumatoid arthritis; a carotid-MRI feasibility study. Arthritis Research & Therapy 20: 266. Link: https://bit.ly/3wFLDoG
- Bradham WS, Ormseth MJ, Oeser A, Solus JF, Gebretsadik T, et al. (2014) Insulin resistance is associated with increased concentrations of NT-proBNP in rheumatoid arthritis: IL-6 as a potential mediator. Inflammation 37: 801–808. Link: https://bit.ly/3gGuTbq
- Urman A, Taklalsingh N, Sorrento C, McFarlane IM (2018) Inflammation beyond the Joints: Rheumatoid Arthritis and Cardiovascular Disease. Scifed J Cardiol 2: 1000019. Link: https://bit.ly/2TOuryX
- Bernatsky S, Hudson M, Suissa S (2005) Anti-rheumatic drug use and risk of hospitalization for congestive heart failure in rheumatoid arthritis. Rheumatology (Oxford, England) 44: 677–680. Link: https://bit.ly/2UgP9HH
- Marks JL, Edwards CJ (2012) Protective effect of methotrexate in patients with rheumatoid arthritis and cardiovascular comorbidity. Therapeutic Advances in Musculoskeletal Disease 4: 149–157. Link: https://bit.ly/3gRRZdM
- Deyab G, Hokstad I, Whist JE, Smastuen MC, Agewall S, et al. (2017) Methotrexate and anti-tumor necrosis factor treatment improves endothelial function in patients with inflammatory arthritis. Arthritis Research & Therapy 19: 232. Link: https://bit.ly/3zKnizV
- Solomon DH, Reed GW, Kremer JM, Curtis JR, Farkouh ME, et al. (2015) Disease activity in rheumatoid arthritis and the risk of cardiovascular events. Arthritis & Rheumatology (Hoboken, N.J.) 67: 1449-1455. Link: https://bit.ly/3ja6NYn
- Barnabe C, Martin BJ, Ghali WA (2011) Systematic review and meta-analysis: anti-tumor necrosis factor α therapy and cardiovascular events in rheumatoid arthritis. Arthritis Care Res 63: 522–529. Link: https://bit.ly/3xtoFRM
- Galarraga B, Khan F, Kumar P, Pullar T, Belch JJ (2009) Etanercept improves inflammation-associated arterial stiffness in rheumatoid arthritis. Rheumatology (Oxford, England) 48: 1418–1423. Link: https://bit.ly/3zENZWN
- Kotani K, Miyamoto M, Ando H (2017) The Effect of Treatments for Rheumatoid Arthritis on Endothelial Dysfunction Evaluated by Flow-Mediated Vasodilation in Patients with Rheumatoid Arthritis. Current Vascular Pharmacology 15: 10–18. Link: https://bit.ly/3gIlwIn
- Hoffman E, Rahat MA, Feld J, Elias M, Rosner I, et al. (2019) Effects of Tocilizumab, an Anti-Interleukin-6 Receptor Antibody, on Serum Lipid and Adipokine Levels in Patients with Rheumatoid Arthritis. Int J Mol Sci 20: 4633. Link: https://bit.ly/3vHDRcD
- Yokoe I, Kobayashi H, Kobayashi Y, Giles JT, Yoneyama K, et al. (2018) Impact of tocilizumab on N-terminal pro-brain natriuretic peptide levels in patients with active rheumatoid arthritis without cardiac symptoms. Scand J Rheumatol 47: 364-370. Link: https://bit.ly/3zHjIXq
- Ikonomidis I, Pavlidis G, Katsimbri P, Lambadiari V, Parissis J, et al. (2020) Tocilizumab improves oxidative stress and endothelial glycocalyx: A mechanism that may explain the effects of biological treatment on COVID-19. Food and Chemical Toxicology 145: 111694. Link: https://bit.ly/3iVSaYh
- Ikonomidis I, Pavlidis G, Katsimbri P, Andreadou I, Triantafyllidi H, et al. (2019) Differential effects of inhibition of interleukin 1 and 6 on myocardial, coronary and vascular function. Clin Res Cardiol 108: 1093–1101. Link: https://bit.ly/3gSx0I9
- Kang EH, Jin Y, Brill G, Lewey J, Patorno E, et al. (2018) Comparative Cardiovascular Risk of Abatacept and Tumor Necrosis Factor Inhibitors in Patients With Rheumatoid Arthritis With and Without Diabetes Mellitus: A Multidatabase Cohort Study. Journal of the American Heart Association 7: e007393. Link: https://bit.ly/3gMN7qn
- Matsumoto T, Sasaki N, Yamashita T, Emoto T, Kasahara K, et al. (2016) Overexpression of Cytotoxic T-Lymphocyte-Associated Antigen-4 Prevents Atherosclerosis in Mice. Arteriosclerosis, thrombosis, and vascular biology 36: 1141–1151. Link: https://bit.ly/3cXFQ6a
- Pérez-Baos S, Barrasa JI, Gratal P, Larrañaga-Vera A, Prieto-Potin I, et al. (2017) Tofacitinib restores the inhibition of reverse cholesterol transport induced by inflammation: understanding the lipid paradox associated with rheumatoid arthritis. British Journal Pharmacology 174: 3018–3031. Link: https://bit.ly/3cYJpsJ
- Wolk R, Armstrong EJ, Hansen PR, Thiers B, Lan S, et al. (2017) Effect of tofacitinib on lipid levels and lipid-related parameters in patients with moderate to severe psoriasis. J Clin Lipidol 11: 1243-1256. Link: https://bit.ly/35EQlXX
- Kume K, Amano K, Yamada S, Kanazawa T, Ohta H, et al. (2017) Tofacitinib improves atherosclerosis despite up-regulating serum cholesterol in patients with active rheumatoid arthritis: a cohort study. Rheumatology International 37: 2079–2085. Link: https://bit.ly/3xCR488
- Yang X, Jia J, Yu Z, Duanmu Z, He H, et al. (2020) Inhibition of JAK2/STAT3/SOCS3 signaling attenuates atherosclerosis in rabbit. BMC cardiovascular disorders 20: 133. Link: https://bit.ly/3xtpkme
- Novikova D, Kirillova I, Markelova E, Udachkina H, Gerasimova H, et al. (2019) The first report of significantly improvement of NT-proBNP level in rheumatoid arthritis patients treated with tofacitinib during 12-month follow-up, European Heart Journal 40: ehz745.0836. Link: https://bit.ly/3xAiD1z
- Syngle A, Garg N, Chauhan K (2021) POS0205 Amelioration of endothelial dysfunction with JAK inhibition in rheumatoid arthritis: JAK CV-Risk reduction study. Annals of the Rheumatic Diseases 80: 318-319. Link: https://bit.ly/35GZGP8
- Charles-Schoeman C, et al. (2019) Treatment with upadactinib is associated with improvements in reverse cholesterol transport in patients with rheumatoid arthritis: Correlation with changes in inflammation and HDL levels. Annals of the Rheumatic Diseases. 78: 356.2-357. Link: https://bit.ly/3cWPKVL
- Giles JT (2015) Cardiovascular disease in rheumatoid arthritis: Current perspectives on assessing and mitigating risk in clinical practice. Best practice & research. Clinical Rheumatology 29: 597–613. Link: https://bit.ly/3xE9FRg
- Ghosh-Swaby OR, Kuriya B (2019) Awareness and perceived risk of cardiovascular disease among individuals living with rheumatoid arthritis is low: results of a systematic literature review. Arthritis Research & Therapy 21: 33. Link: https://bit.ly/3wJC0pd
- Barber CE, Esdaile JM, Martin LO, Faris P, Barnabe C, et al. (2016) Gaps in Addressing Cardiovascular Risk in Rheumatoid Arthritis: Assessing Performance Using Cardiovascular Quality Indicators. Journal of Rheumatology 43: 1965–1973. Link: https://bit.ly/3qeEBVv
- Frølund JC, Primdahl J (2015) Patients' Experiences of Nurse-Led Screening for Cardiovascular Risk in Rheumatoid Arthritis. Musculoskeletal care 13: 236–247.
- Boo S, Oh H, Froelicher ES, Suh CH (2017) Knowledge and perception of cardiovascular disease risk among patients with rheumatoid arthritis. PloS One 12: e0176291. Link: https://bit.ly/3zJm1ZW
- Alm-Roijer C, Stagmo M, Udén G, Erhardt L (2004) Better knowledge improves adherence to lifestyle changes and medication in patients with coronary heart disease. European journal of cardiovascular nursing : journal of the Working Group on Cardiovascular Nursing of the European Society of Cardiology 3: 321-330. Link: https://bit.ly/3wJ2mYo
- Kaczorowski J, Chambers LW, Dolovich L, Paterson JM, Karwalajtys T, et al. (2011) Improving cardiovascular health at population level: 39 community cluster randomised trial of Cardiovascular Health Awareness Program (CHAP). BMJ (Clinical research ed.), 342.
- Singh JA, Saag KG, Bridges SL, Akl EA, Bannuru RR, et al. (2016) 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care & Research 68: 1-25. Link: https://bit.ly/3cXcvsl
- Smolen JS, Landewé R, Bijlsma J, Burmester GR, Dougados M, et al. (2020) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 79: 685–699. Link: https://bit.ly/3vErdLn
- Arts EE, Fransen J, Den Broeder AA, van Riel P, Popa CD (2017) Low disease activity (DAS28≤3.2) reduces the risk of first cardiovascular event in rheumatoid arthritis: a time-dependent Cox regression analysis in a large cohort study. Ann Rheum Dis 76: 1693–1699. Link: https://bit.ly/3zJrLDd
- Roodenrijs N, Kedves M, Hamar A, Nagy G, van Laar JM, et al. (2021) Diagnostic issues in difficult-to-treat rheumatoid arthritis: a systematic literature review informing the EULAR recommendations for the management of difficult-to-treat rheumatoid arthritis. RMD Open 7: e001511. Link: https://bit.ly/3gQnsNA

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
