Journal of Cardiovascular Medicine and Cardiology
ACEi to ARNi: “The Switch” An Evidence Based Review
Yusuf Hassan1*, Zahra Hassan2, Ayesha Hassan3, Asad A Saleem4 and Waqas Ahmed2
2Shifa College of Medicine, Islamabad, Pakistan
3West Virginia University Medical Center, Morgan Town, USA
4Shifa International Hospital, Islamabad, Pakistan
Cite this as
Hassan Y, Hassan Z, Hassan A, Saleem AA, Ahmed W (2020) ACEi to ARNi: “The Switch” An Evidence Based Review. J Cardiovasc Med Cardiol 7(4): 288-292. DOI: 10.17352/2455-2976.000154Heart failure affects approximately 6.5 million adults in the United States, and the cost to healthcare system is tremendous [1]. Effective treatment for this very important disease is constantly evolving. The most significant change in the recent past has been the development of ARNI (Sacubitril/ Valsartan) for treatment of heart failure [2].
Recently ACC, AHA and HFSA, have recommended switching NYHA class II or class III patients to ARNI from ACE-I or ARB if they are symptomatic, (Class I recommendation) giving it level of evidence B-R. (Moderate quality evidence from 1 or more RCTs, or Moderate quality meta-analysis) [3].
With these recommendation in place and data to show that ARNIs are useful in treatment of NYHA Class II, and Class III patients with reduced Left Ventricular Ejection Fraction (35- 40% or less) and elevated BNP levels, there has been a significant increase the use of ARNIs in patients with heart failure, and practitioners are switching patient from ACE inhibitors to ARNI.
This however needs to be reviewed in light of previous data showing a very significant benefit from ACE inhibitors in patient with class IV heart failure; a 32% reduction in mortality [4]. Similar effect of Enalapril was further emphasized in SOLVD trial which showed that in class II and III patients there was survival benefit (16% relative risk reduction in mortality) and decreased hospital admission due to heart failure (26% risk reduction in death or hospital admission) [5]. Remarkably there was a 26% reduction in the rate of fatal myocardial infarction in the Enalapril group (40 with Enalapril vs 53 with placebo). There was also a wide dose range over which Enalapril could be used effectively (2.5 mg twice daily to 10 mg twice daily). And this effect was most significant in patients with lowest ejection fraction, in other words, the sickest patients benefitted the most from Enalapril [6].
In addition there has been evidence of improvement in hemodynamics and symptoms with use of ACE inhibitors in patients with congestive heart failure [5,7].
The use of ACE inhibitors in secondary prevention of ischemic heart disease is well established, and the effect is independent of the benefit seen in patients with reduced left ventricular ejection (<40%), and includes not only significant mortality benefit but also reduced rates of stroke, myocardial infarction, congestive heart failure and angina [8].
There are additional benefits of using ACE inhibitors in patients following acute myocardial infarction. This benefit is most significant in the sickest STEMI patients, including patients with pump failure and reduced ejection fraction, 50 lives saved per 1000 patients treated [9]
The beneficial effect of ACE inhibitors in diabetic patient population goes beyond their usefulness in heart failure or ischemic heart disease, hazards ratio of 0.49 with a confidence interval of 0.4-0.61 [10].
The American College of Cardiology/American Heart Association guidelines recommend the use of ACE inhibitors over ARBs in patients with heart failure, but notes that ARBs may be used in patients who cannot tolerate ACE inhibitors, and data has indicated equivalence (with a few exceptions [11]) of ARBs with ACE inhibitors [12].
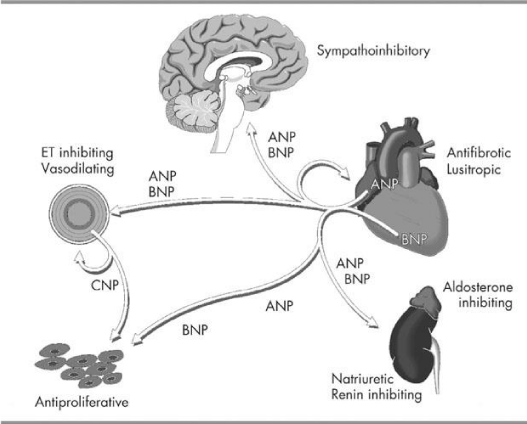
Excitement for using natriuretic factor for patient with heart failure had been building since the introduction of BNP as an effective diagnostic test for heart failure [13]. Despite the understanding that BNP, secreted in response to volume overload, and its action as powerful diuretic, natriuretic, and vascular smooth muscle relaxing agent, its intravenous use as a therapeutic agent was largely disappointing [14]. Even though an increase in cGMP levels, a suppression in renin/ aldosterone was noted, but the overall benefit was limited [15,16].
[15]. BNP Consensus Panel 2004.
A trial using Neprilysin inhibitor Ecadotril in patients with NYHA class II and III, demonstrated dose dependent increase in serum and urinary cGMP without any improvement in clinical symptoms or a positive effect on serum renin, angiotensin II or endothelin activity [17].
A drug which inhibited Neprilysin and ACE; Omapatrilat, showed dose dependent increase in serum ANP and BNP levels with decrease in plasma ACE activity but was no more effective than ACE monotherapy, and was associated with higher incidence of angioedema [18].
Despite dramatic increase in circulating Natriuretic Peptides (NP) concentration as CHF progresses, their effect becomes blunted. Increase in diuresis, natriuresis, and vasodilation after administration of exogenous atrial (ANP) or brain (BNP) natriuretic peptides is attenuated in patients with advanced CHF compared with controls. This reduced effectiveness is believed to be due to reduction in active forms of NPs, decreased target organ responsiveness and counter regulatory effect of RAAS, sympathetic overstimulation and enhanced activity of endothelin I [19]. It has also been observed that administration of Neprilysin Inhibitors increase BNP levels and through a negative feedback reduce the levels of NT-Pro BNP, which was demonstrated in phase II trials testing Sacubitril and confirmed in PIONEER-HF trial [20].
Decreased vasodilatory response to ANP and BNP infusion in patients with heart failure has been noted in trials assessing local blood flow differences in forearm blood flow [21].
As Cody et al, showed in their experiment that in patients with advanced heart failure ANP infusion resulted in little or no increase in urine output or natriuresis compared to healthy volunteers [22]. Similar results were demonstrated in other experiments, with additional data confirming no effect of intravenous ANP on cGMP levels in the urine of patients with heart failure but significant increase in healthy volunteers. They also showed that BNP resulted in a better response in patients with heart failure however the response was still significantly less than the healthy volunteers [23].
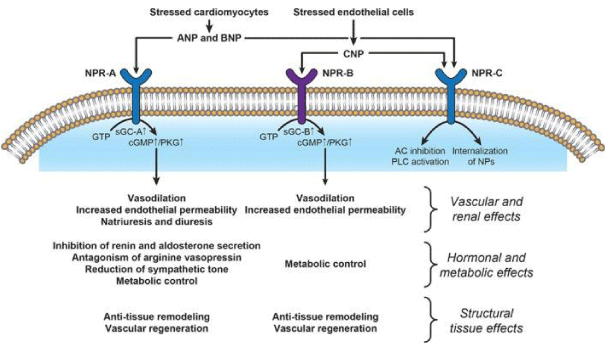
Mechanisms of action and main effects of natriuretic peptides (NPs). AC, adenylyl cyclase; ANP, atrial natriuretic peptide; AVP, arginine–vasopressin; BNP, brain natriuretic peptide; cGMP, cyclic guanosine monophosphate; CNP, C-type natriuretic peptide; GTP, guanosine triphosphate; NPR, natriuretic peptide receptor; PKG, protein kinase G; PLC, phospholipase C; sGC, soluble guanylate cyclase [19].
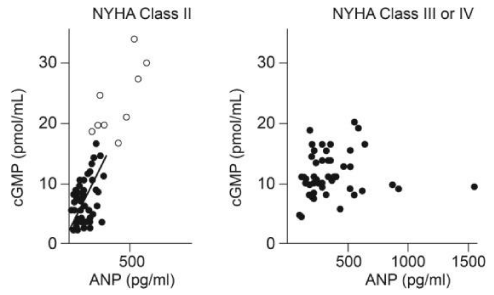
Also the cyclic GMP response to ANP is correlated almost perfectly in patients with Class II heart failure with a coefficient r=075, as opposed to patient with class III and IV heart failure where the effect was diminished and unpredictable [23].
The current recommendation for switching patients from ACEI to ARNI is based on PARADIGM-HF. This data was used to give Sacubitril/Valsartan class I recommendation with a level of evidence B-R indication for replacing ACE inhibitors in patients with Class II-III heart failure.
The trial recruited mainly Class II (approx. 70%), and Class III (approx. 24%) patients with less than 1% Class IV patients. Only patients able to tolerate 10 mg twice daily of oral Enalapril for two weeks were randomized, in a blinded fashion, to the study treatment.
Rate of the composite primary end point was assumed to be 14.5% and the rate of death from cardiovascular causes was estimated at 7.0% in the Enalapril group. However the actual rate of death in the trail from cardiovascular causes was significantly greater; 13.3% and 16.5% in LCZ696 and Enalapril groups respectively. The observed rate is 8% (8 months mortality, annual mortality of 12%) in a similar data set with 15% Class IV patients [24].
The other concerning aspect of this trial is the lack of benefit in the patients with predominantly NYHA Class III symptoms [2], and this lack of benefit was consistent in both primary outcomes. This however is not entirely surprising given previous observations, namely ineffectiveness of NP in patients with more advanced heart failure [19-25], and was also borne out in the trials using injectable Nisiritide [14], and some of the trials using oral agents with similar dynamics [17,18].
Novartis has used the recommendations provided by ACCF/AHA in their 2017 update (1), and modified them to include Class IV heart failure patients, and their insert reads, “ENTRESTO® is indicated to reduce the risk of cardiovascular death and hospitalization for heart failure in patients with chronic heart failure (NYHA Class II-IV) and reduced ejection fraction.
ENTRESTO is usually administered in conjunction with other heart failure therapies, in place of an ACE inhibitor or other ARB”.
The company is using PIONEER-HF to extend the scope of ARNI use, suggesting that reduction in NT-Pro BNP is equivalent to improvement in mortally and hospital admission rates.
It should also be pointed out that ARNI is a combination medication. There is data from previous randomized trial showing significant benefit in mortality in patients older than 65 year of age with use of ARB over an ACE-I. Incidentally this trail also randomized patients with LVEF less than 40%, and like the PARADIGM-HF trial had similar distribution of Class II and III patients. Unlike PARADIGM-HF, ELITE was not powered for finding statistical significance in the Hospital admission and Mortality despite 4% difference in favor of Losartan (9.4% in Losartan vs 13.2% in Captopril group) [11].
Beyond the effectiveness of ACE inhibition in advanced heart failure, and added benefit of symptomatic and hemodynamic improvement in these patients [7], there is significant additional benefit in patients with ischemic heart disease and ischemic cardiomyopathy [8,9]. Majority of the patients with heart failure have underlying ischemic etiology [26], and by replacing ACE inhibitors with a drug without a proven advantage in this setting may not be very prudent.
Also patients with diabetes [10], and underlying chronic kidney disease [27], as well as kidney failure patients without diabetes, benefit significantly from the use of ACE inhibitors. This benefit is also noted in patients with hypertension and proteinuria, and goes beyond the effect of lowering blood pressure [28-32].
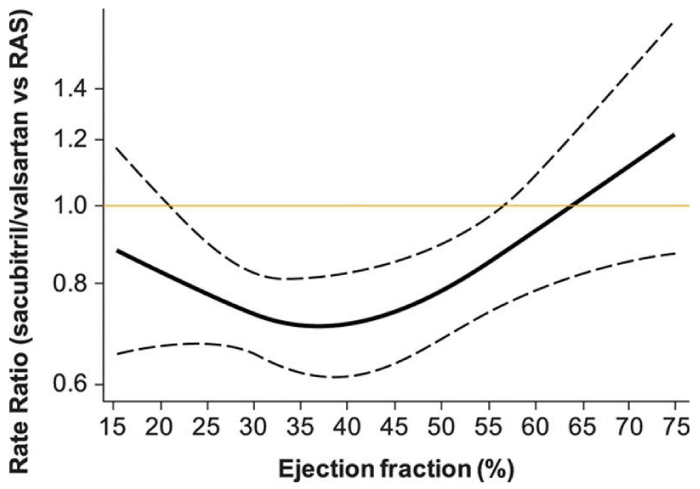
More recently, ARNI has been tested in patients with heart failure with preserved ejection fraction (HFpEF) [33]. The PARAGON-HF trial concluded, “Sacubitril–valsartan did not result in a significantly lower rate of total hospitalizations for heart failure and death from cardiovascular causes among patients with heart failure and an ejection fraction of 45% or higher”. Subsequently Solomon et al combined data from PARADIGM-HF and PARAGON-HF trials and analyzed the two data sets (13195 patients from two trials) to assess the variability of treatment effect based on LVEF [34]. In addition to using LVEF as a continuous variable, they also used restricted cubic splines with four knots to modify the extrapolation of missing data, LVEF 40% - 45%, not selected in either trial. Although, unlike the other two trials, they did not use NYHA Class as selection criteria, and their conclusion has all the limitations that accompany a post-hoc analysis, the overall benefit of ARNI, not surprisingly, was more consistent in the moderately reduced LVEF range. This further supports our basic premise of Natriuretic Peptides being less effective in more advanced heart failure state.
Treatment effects of sacubitril/valsartan vs active comparator (either enalapril or valsartan) across a range of ejection fraction for the composite of total heart failure hospitalization and cardiovascular death. Estimated rate ratios and 95% confidence intervals obtained from negative binomial regression models with ejection fraction expressed via restricted cubic spline. RAS indicates renin-angiotensin-aldosterone–system inhibitor [34].
Unfortunately enrollment in a trial designed to exclusive test ARNI in severe heart failure patients with LVEF less than 35%, and NYHA Class IV symptoms was stopped due to COVID-19 pandemic [35-37].
While the data does support use of ARNIs in class II patients, due to the analysis given above, their use in class III and, without question, Class IV patients is an extrapolation. Therefore making this change in patients admitted to the hospital for acute decompensation (invariably class III or IV patients) is not supported by data at this time.
In the office setting, it is imperative that the clinician takes into account other variables which should be carefully weighed before stopping ACE-I or ARB on patients who have been stable on these medications for years.
The flexibility of dose adjustment provided by a single agent especially in patients who may already have borderline low blood pressure is another aspect which may not be available with a combination therapy that makes up ARNI. This will become increasingly relevant as we start introducing SGLT2 inhibitors to our treatment regimen for heart failure.
Thus it is important to individualize this decision for your patients. We feel that to lose the additional benefits in the patients with ischemic heart disease, diabetes, chronic kidney failure, and hypertension, and particularly in patients with proteinuria has to be weighed against any potential benefit that may be incurred.
ACE Inhibitors have decades of data, consistently emphasizing their safety and efficacy in both randomized trials and registry data base. And even though current ACC/AHA guidelines recommend switching patients in class-II and class-III heart failure to ARNI and class-II to class IV heart failure as recommended by the manufacturers of Sacubitril/Valsartan, caution is needed before making “the switch”.
- Mentzer G, Hsich EM (2019) Heart Failure with Reduced Ejection Fraction in Women. Heart Fail Clin 15: 19-27. Link: https://bit.ly/2HJBlzs
- McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, et al. (2014) Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. N Engl J Med 371: 993-1004. Link: https://bit.ly/31VwSkt
- Yancy CW, Jessup CM, Bozkurt B, Butler J, Casey DE, et al. (2017) 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 136: e137-e161. Link: https://bit.ly/37V6ZVA
- Consensus Trial Study Group (1987) Effects of Enalapril on Mortality in Severe Congestive Heart Failure. N Engl J Med 316: 1429-1435. Link: https://bit.ly/3jGTTO7
- Kramer BL, Massie BM, Topic N (1983) Controlled Trial of Captopril in Chronic Heart Failure: A Rest and Exercise Hemodynamic Study. Circulation 67: 807-816. Link: https://bit.ly/37UPsgo
- SOLVD Investigators, Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN, (1991) Effect of Enalapril on Survival in Patients with Reduced Left Ejection Fraction And Congestive Heart Failure. NEJM 35: 293-302. Link: https://bit.ly/3kHsCfE
- Garg R, Yusuf S (1995) Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. JAMA 273: 1450-1456. Link: https://bit.ly/31Vz4Z6
- Bangalore S, Fakheri R, Wandel S, Toklu B, Wandel J, et al. (2017) Renin angiotensin system inhibitors for patients with stable coronary artery disease without heart failure: systematic review and meta-analysis of randomized trials. BMJ Clin Res 356: j4. Link: https://bit.ly/37RHLr1
- (1998) Indications for ACE inhibitors in the early treatment of acute myocardial infarction: systematic overview of individual data from 100,000 patients in randomized trials. ACE Inhibitor Myocardial Infarction Collaborative Group. 97: 2202. Link: https://bit.ly/2TEb20g
- Eurich DT, Majumdar SR, Tsuyuki RT, Johnson JA (2004) Reduced Mortality Associated With the Use of ACE Inhibitors in Patients With Type 2 Diabetes Diabetes Care 27: 1330-1334. Link: https://bit.ly/31UfMTX
- Pitt B, Segal R, Martinez FA, Cowley AJ, Meurers G, et al. (1997) Randomised trial of losartan versus captopril in patients over 65 with heart failure (Evaluation of Losartan in the Elderly Study, ELITE). Lancet 349: 747-752. Link: https://bit.ly/35LlGYG
- Lee VC, Rhew DC, Dylan M, Badamgarav E, Braunstein GD, et al. (2004) Meta-analysis: angiotensin-receptor blockers in chronic heart failure and high-risk acute myocardial infarction. Ann Intern Med 141: 693-704. Link: https://bit.ly/2GaKxfE
- Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, et al. (2002) Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. NEJM 347: 161-167. Link: https://bit.ly/34FxDQl
- Shatsky M (2006) Nesiritide (Natrecor) for Acute Decompensated Heart Failure. Am Fam Physician 73: 687-688. Link: https://bit.ly/3mynsmG
- Boomama F, Van der Meiracker AH (2001) Plasma A‐ and B‐type natriuretic peptides: physiology, methodology and clinical use. Cardiovasc Res 51 :442-449. Link: https://bit.ly/3eg5Itl
- Chen HH, Glockner JF, Schirger JA, Cataliotti A, Redfield MM, et al. (2012) Novel protein therapeutics for systolic heart failure: chronic subcutaneous B‐type natriuretic peptide. J Am Coll Cardiol 60: 2305-2312. Link: https://bit.ly/2HKe7ZR
- Cleland JG, Swedberg K (1998) Lack of efficacy of neutral endopeptidase inhibitor ecadotril in heart failure. The International Ecadotril Multi-centre Dose-ranging Study Investigators. Lancet (London, England) 351: 1657-1658. Link: https://bit.ly/3kIAHR5
- Packer M, Califf RM, Konstam MA, Krum H, McMurray JJ, et al. (2002) Comparison of omapatrilat and enalapril in patients with chronic heart failure: the Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE). Circulation 106: 920-926. Link: https://bit.ly/3e8vHmt
- Diez J (2017) Chronic heart failure as a state of reduced effectiveness of the natriuretic peptide system: implications for therapy. Eur J Heart Fail 19: 167-176. Link: https://bit.ly/3jKN91n
- Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, et al. (2019) Angiotensin–Neprilysin Inhibition in Acute Decompensated Heart Failure List of authors. N Engl J Med 380: 539-548. Link: https://bit.ly/3jFu4xG
- Nakamura M, Arakawa N, Yoshida H, Makita S, Niinuma H, et al. (1998) Vasodilatory effects of B‐type natriuretic peptide are impaired in patients with chronic heart failure. Am Heart J 135: 414-420. Link: https://bit.ly/35Qn8sE
- Cody RJ, Atlas SA, Laragh JH, Kubo SH, Covit AB, et al. (1986) Atrial natriuretic factor in normal subjects and heart failure patients. Plasma levels and renal, hormonal, and hemodynamic responses to peptide infusion. J Clin Invest 78: 1362-1374. Link: https://bit.ly/2Jk0XUh
- Eiskjaer H, Bagger JP, Danielsen H, Jensen JD, Jespersen B, et al. (1991) Attenuated renal excretory response to atrial natriuretic peptide in congestive heart failure in man. Int J Cardiol 33: 61-74. Link: https://bit.ly/37Ul1Xq
- Mozaffarian D, Anker SD, Anand I, Linker DT, Sullivan MD, et al. (2007) Prediction of Mode of Death in Heart Failure The Seattle Heart Failure. Circulation 116: 392-398. Link: https://bit.ly/2HPXvjR
- Tsutamoto T, Kanamori T, Morigami N, Sugimoto Y, Yamaoka O, et al. (1993) Possibility of downregulation of atrial natriuretic peptide receptor coupled to guanylate cyclase in peripheral vascular beds of patients with chronic severe heart failure. Circulation 87: 70-75. Link: https://bit.ly/34CiFdQ
- Ziaeian B, Fonarow GC (2016) Epidemiology and aetiology of heart failure. Nat Rev Cardiol 13: 368-378. Link: https://bit.ly/3e6OMFI
- Lewis EJ, Hunsicker LG, Bain RP, Rohde RD (1993) The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy: the Collaborative Study Group. N Engl J Med 329: 1456-1462. Link: https://bit.ly/3kBOqJz
- The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet 349: 1857-1863. Link: https://bit.ly/3oFKH08
- Ruggenenti P, Perna A, Gherardi G, Garini G, Zoccali C, et al. (1999) Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet 354: 359-364. Link: https://bit.ly/3eh5pyA
- Maschio G, Alberti D, Janin G, et al, and the Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group. Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. N Engl J Med . 1996; 334: 939–94
- Sarafidis PA, Khosla N, Bakris GL (2007) Antihypertensive therapy in the presence of proteinuria. Am J Kidney Dis 49: 12-26. Link: https://bit.ly/34He0qQ
- Chiurchiu, C, Remuzzi G, Ruggenenti P (2005) Angiotensin-Converting Enzyme Inhibition and Renal Protection in Nondiabetic Patients: The Data of the Meta-Analyses. 16: S58-S63. Link: https://bit.ly/37SgpkL
- Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, et al. (2019) Angiotensin–Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N Engl J Med 381: 1609-1620. Link: https://bit.ly/3jHUyi0
- Solomon SD, McMurray JJV, Claggett BL, Packer M, Zile M, et al. (2019) Sacubitril/Valsartan Across the Spectrum of Ejection Fraction in Heart Failure. Circulation 141: 352-361. Link: https://bit.ly/2JnF1Yx
- Mann DL, Greene SJ, Givertz MM, Vader JM, Starling RC, et al. (2020) Sacubitril/Valsartan in Advanced Heart Failure With Reduced Ejection Fraction: Rationale and Design of the LIFE Trial. JACC Heart Fail 8: 789-799. Link: https://bit.ly/2JkUkB6
- Pfeffer MA, McMurray JJ, Velazquez EJ, Rouleau JL, Køber L, (2003) Valsartan, Captopril, or Both in Myocardial Infarction Complicated by Heart Failure, Left Ventricular dysfunction or Both, Valsartan in Acute Myocardial Infarction Trial Investigators. *N Engl J Med 349: 1893-1906. Link: https://bit.ly/3mBooqA
- Silver MA, Maisel A, Yancy CW, McCullough PA¸ Burnett JC, et al. (2004) BNP Consensus Panel 2004: A Clinical Approach for the Diagnostic, Prognostic, Screening, Treatment Monitoring, and therapeutic Roles of Natriuretic Peptides in Cardiovascular Diseases. Congest Heart Fail 10: 1-30. Link: https://bit.ly/31UWyxy

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
