Journal of Cardiovascular Medicine and Cardiology
The impact of partial blood replacement on postoperative outcome for pediatric patients with cyanotic heart disease
Mohammad Saleh*
Cite this as
Saleh M (2020) The impact of partial blood replacement on postoperative outcome for pediatric patients with cyanotic heart disease. J Cardiovasc Med Cardiol 7(2): 167-188. DOI: 10.17352/2455-2976.000134Background: Pediatric patients with cyanotic congenital heart disease who underwent open heart corrective surgery, were unfortianetly suffering from postoperative clinical deterioration which may leads to morbidity and mortality. Most of blood transfusion indications are now well managed exclusively with blood component therapy, however concerns about logistics, safety, and relative efficacy making the blood transfusion a debating procedure in many cardiac centers around the world. The research aimed to investigate the partial blood replacement process for cyanotic pediatric patients by healthy RBCs (red blood cells), solving their postoperative clinical deterioration and proving that the RBCs membrane biomechanical characteristics alterations is the main cause of the adverse effects of prolonged hypoxia on the normal physiological functions of RBCs in oxygen transport and body tissue’s perfusion.
Methods: 450 Pediatric patients with congenital heart disease were divided into three equal groups, group I acyanotic pediatric patients, group II cyanotic pediatric patients and group III cyanotic pediatric patients treated with the partial blood replacement process. Blood components biophysical characteristics and cardiovascular performance were investigated and the postoperative clinical course was estimated.
Results: The results showed the improvement of group III pediatrics, as there were insignificant decrease in blood components characteristics, cardiovascular performance and postoperative clinical course estimation compared to group I.
Conclusion: The partial blood replacement process after cardiopulmonary bypass procedure for pediatric patients with cyanotic congenital heart disease who undergo corrective congenital surgery, may help in improving their postoperative clinical course and outcomes.
Introduction
Certain congenital cardiac defects or pulmonary diseases cause severe arterial desaturation and chronic hypoxia, are visible as cyanosis [1,2]. Those cyanotic pediatric patients should undergo critical corrective surgical procedures, which return them back to the normal blood circulation and oxygenation [3,4]. Generally, the chronic hypoxia leads to clinical deterioration of cyanotic pediatric patients post-open heart surgery, where ischemic organs symptoms appear pre and even during ICU (Intensive Care Unit) period post total correction [5-7]. Many studies on cyanotic pediatric patients showed multi-organs dysfunction e.g. kidney impairment, glomerular nephropathy, acute renal failure [8-10], abnormal coagulation system and late postoperative homeostasis [11,12]. They have such a positive correlation of platelets micro-particles and negative correlation of platelet aggregation response with severity of cyanosis [13]. Unfortunately, postoperative morbidity and mortality had been recorded in those cyanotic pediatric patients at Cairo and Aswan university centers, due to severe deterioration of organs metabolic activities [14]. That was a big challenge in postoperative clinical managements for the last three decades. The secondary polycythemia due to prolonged hypoxia “cyanosis” leads to significant decrease of plasma components and volume and the increase of whole blood share stress causing to aggregated and fragile RBCs (red blood cells) that deteriorates the RBCs physiological role and impairing microcirculatory perfusion. The chronic impaired microcirculation causes organs hypo-perfusion and insufficient tissues oxygenation, which results in enhancement of overall tissue hypoxia and patient’s multi-organ failure [15,16]. The practical observations of urine hemolysis occurrence intra and postoperative revealed the fragility of cyanotic RBCs, which doesn’t happen to pediatric patients with acyanotic heart diseases passing with the same procedure of CPB (Cardiopulmonary Bypass) for ASD (Atrial Septal Defect) and VSD (Ventricular Septal Defect) corrections. The surgical corrections will treat the future hypoxia, but what about the current pediatric patient’s own blood and its capability for normal whole body oxygenation and hemostasis in ICU postoperative period, in order to overcome the critical metabolic situation of whole body organs. The critical issue here is the proper management of cyanotic blood components -especially RBCs characterized by acquired alterations under effect of prolonged hypoxia- in order to maximize the beneficial effect of surgical correction for the whole body organs, especially in the ICU critical period. There are two choices for such management; the first choice is to wait until the normal production process of mature RBCs after corrective surgery – around 3 months – and the second choice is to prepare successful healthy RBCs transfusion procedure, where basically in such critical ICU period those patients are in need for normal healthy RBCs to be transfused. However, because the patient’s critical clinical status, it is very risky to perform such maneuvers with patient’s blood during ICU period. It is logical practically and medically to include the healthy RBCs transfusion process into the CPB procedure in form of partial blood replacement for the cyanotic pediatric patient’s circulating blood after the conclusion of CPB procedure. The research aimed to evaluate the partial blood replacement process for cyanotic pediatric patients underwent corrective congenital surgery as intraoperative interventional procedure performed immediately post CPB termination.
So the present study is designed to investigate the blood components biophysical characteristics and the postoperative clinical course outcome for cyanotic pediatric patients treated with the partial blood replacement process. The results may help in the postoperative ICU management challenge of cyanotic pediatric patients underwent corrective surgeries and resolve their postoperative clinical deterioration.
Materials and methods
Ethics and population
The current study was performed according to the Helsinki Declaration and approved by the Institutional Review Board of Cairo and Aswan Universities, which exempted the specific patient’s consent considering the routine informed consent for cardiac surgery that obtained from the pediatric patient’s relatives to use the FWB (Fresh whole blood) as routine priming components in Cairo university and to use the PRBCs (Packed red blood cells) cells when needed intra-operative in Aswan university. 450 pediatric patients, underwent an elective corrective congenital heart surgery for repair a variety of congenital diseases, using CPB procedure from January 2009 to September 2016 at Cairo and Aswan university hospitals, Egypt. Pediatric patients were assigned equally into three groups, 150 patinets each (Table 1) group I acyanotic pediatric patients underwent corrective surgery for complicated VSD and ASD (acting as a control group to offset the effect of CPB procedure using roller pump on the patient’s RBC’s biophysical characteristics during open heart surgery), groups II and III were pediatric patients with severe cyanotic congenital heart disease accompanied with secondary polycythemia hematocrit range of 60±5% undergoing open heart corrective procedures (Tricuspid, Pulmonary and Aortic valves disorder, Coarctation or complete interruption of the aorta, Ebstein anomaly, Hypoplastic left heart syndrome, Tetralogy of Fallot, Total anomalous pulmonary venous return, Transposition of the great arteries and Truncus arteriosus) all of them underwent the standard routine corrective surgery for such congenital cyanotic diseases. Group II, to explain the fatal impact of RBC’s acquired alteration under effect of prolonged hypoxia with secondary polycythemia on postoperative course outcome of cyanotic pediatric patients, and group III underwent exactly the same corrective surgery times pattern of group II, beside treated at the conclusion of CPB with a partial blood replacement process using FWB or PRBCs. All cases received a successful surgical correction procedure and the same cardiac postoperative pediatric ICU caring with the same setting and equipment.
Demographic analysis and CPB data
The pediatric patients’ demographics and perioperative characteristics were represented in Table 1. There were significant decrease of body weight, body surface area, ejection fraction and platelets (PLTs) count, and significant increase in hemoglobin for group II and III compared with group I (*p<0.05), without statistically discernible in the other parameters.
Study meaning and exclusion criteria
This study is meant to identify and investigate the effect of partial blood replacement for severe cyanotic pediatric patients, those underwent surgical corrective procedure on their postoperative outcome, and to recognize what is the main factor in the cyanotic RBCs acquired alterations that causing their clinical outcome deterioration postoperative. Exclusion criteria; 1) Pediatric patients who experienced re-on bypass with re-cardiac arrest existence or not. 2) Complicated surgical procedures and unexpected blood loss. 3) Unexpected events impeding the completion of the partial blood replacement process. 4) Pediatric patients who required mechanical support postoperatively.
Anesthesia and anticoagulation
All patients received the standard general anesthesia management for pediatric cardiac surgery. Anticoagulation was established with an initial bolus of porcine heparin 300 IU/kg “Heparin, Leo Pharmaceutical Products”, if needed an additional heparin dose was administrated to maintain activated clotting time (ACT) values higher than 480 seconds, measured using Hemochron “Hemochron Jr ® “ITC, Edison, NJ”, Helena Laboratories, Australia”. At the end of CPB initial Protamine sulfate dose of 3–3.5 mg/kg was administered over 3–5 min, if necessary an extra dose was given for more heparin neutralization action and to achieve an ACT values equal or close to the baseline values at the beginning of heart cannulation. All patients received 30 mg/kg of corticosteroids (methylprednisolone) just before and after CPB, with 100 mg/kg tranexamic acid “as hemostatic drug” loaded during induction and another 100 mg/kg added to the pump prime, along with a steroid (dexamethasone) 1–10 mg/100 mL of CPB unit prime. Post CPB blood transfusion management was using FWB or RBCs for all groups.
CPB procedure
Based on the calculated patient’s blood flow, QUADROX-I Pediatric oxygenation systems (Maquet getting group, Sweden Hirrlingen, Germany) with customized arteriovenous loops 1/4”×3/8” and 1/4”×1/4” were utilized for the three studied groups I, II and III. The CPB circuits were primed with heparinized ringer lactate (Ringer’s Solution; Baxter, Utrecht, The Netherlands) solution of 5% human albumin concentration (Buminate Human Albumin 20%, Adamo healthcare, India), mannitol dose of 0.5 gm/kg (20% Mannitol Injection USP, B. Braun Medical Inc., USA) and FWB or PRBC’s was added if needed to reach 30% prime Hct with a total volume of 600±50 ml in all groups. CPB was conducting of target non-pulsatile calculated blood flow of 2.4–2.6 L/min/m2 utilizing a roller pump (Sorin, Stockert S3 and S5, respectively in Cairo and Aswan university hospitals) keeping mixed venous oxygen saturation values above 60% and the range of 30–60 mmHg mean arterial pressure with no drugs manipulations and only using blood flow at mild hypothermia of 28–32 °C. Arterial blood gas (alpha-stat strategy), venous saturation, electrolyte, glucose, lactate, hemoglobin (Hb) levels and ACT were continuously monitored. Myocardial preservation was achieved with intermittent cold blood cardioplegia composed of crystalloid cardioplegic solution mixed with oxygenated blood manually and delivered by anesthesia side, and at the ratio of 1:4, with an initial dose of 30 mL/kg followed by half initial dose every 20–30 min delivered using cardioplegia delivery device (MYOtherm XP Cardioplegia Delivery System, Medtronic Co., USA) in Cairo and Aswan university hospitals respectively (Figure 1).
The partial blood replacement process
Healthy RBCs preparation criteria; donated FWB, collected in the morning of surgery day in Cairo University, PRBCs processed in blood bank - Aswan health ministry, 1 day before scheduled surgical operation in Aswan university.
Replacement setting: 1) As part of the CPB circuit component setup, the replacement process tubing for group III, composed of two blood transfusion devices, six 3 way stopcock/luer lock connector, two 3/16” recirculation line of Medtronic pediatric custom tubing pack, two 1/4”×1/4” straight connectors and two 1/4” Y connectors with luer lock (Figure 2A). 2) The two synchronized roller pumps as the replacement heads, decided to be the main head and small suction pumps in Cairo University, and to be the cardioplegia blood head and another small suction pumps in Aswan University. As explained in diagram of replacement tubing setup (Figure 2B).
Replacement Procedures: 1) Priming the blood replacement tubing set accompanied with the CPB circuit components (Figure 3 A), then remove the two stopcocks connected to cardioplegia blood head input line and hang it to the blood replacement unit holder, during CPB (Figure 3B).
2) During rewarming stage, the perfusionist asks for the donated FWB or PRBCs unit in Cairo and Aswan university hospitals respectively, and incubates in water bath with 37 C° for about 20 min. 3) The link between the cardioplegia solution and blood heads (Figure 4A,B) has been stopped and switched to be between the cardioplegia blood head and the small suction head, which slaved 100% to the cardioplegia blood head (Figure 4C,D), so getting the cardioplegia blood head ready for warmed unit of FWB or PRBCs delivery and the small suction head ready for patient blood draining (near the conclusion of CPB). 4) Before conclusion of CPB, the arterial input side of cardioplegia blood head clamped, and connect the two stopcocks of primed replacement tubes side “which early connected to reservoir” to the input line of cardioplegia blood head, and to be sure one stopcock connection to the pressure line is opened (Figure 5). Connect the two stopcocks of the blood replacement tubes other side through the two delivery devices to the replacement blood unit (Figure 6). Close the arterial input of cardioplegia blood head, so got the carioplegia blood head ready to drain the replacement blood through the prepared replacement tubes and perfuse it to the right atrium through the cardioplegia delivery device and cardioplegia line switched to the venous cannula. And the small suction head inlet connected already primed and connected to the arterial line bifurcation to drain the replaced pediatric patient’s blood back to the reservoir (Figure 7). Unfortunately, at Cairo University there was no cardioplegia delivery device. In this situation we drain the replacement blood unit volume by the cardioplegia blood head through a prepared replacement tubing set. Then we perfuse the replacement blood by the cardioplegia blood head to the atrium through the Y connector witch connected to the venous line. The cardioplegia solution head which is slaved 100% to the crdioplegia blood head was used to drain the circulating pediatric patient’s blood back to the reservoir. The cardioplegia solution head was already prepared and connected to the arterial line bifurcation. 5) Immediately after CPB termination, the perfusionist used replacement process tubes to flash the heater cooler of cardioplegia delivery device then perfuse 25 mL of warmed FWB or PRBCs unit as test dose for the replacement process. 6) At patient’s hemodynamics stability and before the protamine dose infusion, the perfusionist start to delivers the warmed unit of FWB or PRBCs over 3 to 5 minutes targeted to replace the amount of patient’s blood ranged from 30% to 50% according the patient body volume (Figure 8). 7) The patient hemodynamics should be monitored seriously during replacement, the pressures of both delivery and draining lines should be also continually monitored to keep the pressure at maximum 25 mmhg and at minimum 5 mmhg respectively. 8) In some cases there is a need to maintain the patient systemic pressure to infuse of 30 to 50 mL per min as in (Figure 9). 9) Near blood replacement process termination (Figure 10), be ready to switch the slaved 100% link between the cardioplegia blood head and the small suction head to be between the cardioplegia solution and blood heads, in order getting ready in case to go back on by pass and deliver cardioplegia (Figure 11). Replacement limitations; blood reaction after test dose delivery or during replacement process and the negative or less than 5 mmHg of cardioplegia blood head input line pressure, which never happened during this study.
Investigated parameters
(I) Blood components biophysical characteristics investigation
(A) Whole blood rheological parameters
10 ml blood samples collected post-bypass and post-partial blood replacement from groups I, II and III respectively, in the purpose of blood rheological properties investigation as following;
Plasma and whole blood viscosity; were estimated at 25°C using Ostwald viscometer. Values were calculated according the following equation;
η sample / η water = t sample / t water
Where η and t are the plasma or whole blood viscosity value and time respectively.
The RBCs rheological parameters; were tested by estimation of red blood mean cell volume (MCV) µm3, mean corpuscular hemoglobin (MCH) (pg), mean corpuscular hemoglobin concentration (MCHC) g/dL and calculated surface to volume ratio (S/V ratio) using Automated Hematology Analyzer (Sysmex K - 1000, TOA manufactured by Medical Electronics Co., LTD. KOBE. in Japan).
(B) RBCs membrane biomechanical characteristics estimation
The membrane biomechanical characteristics investigated by solubilization and osmotic fragility tests.
Solubilization test: Testing the integrity of overall RBCs cellular membrane through solubilization test which performed using octylglucoside (60 mM) as nonionic detergent to solubilize washed RBCs samples, the detergent monomers distributed between cellular proteins then the lipid bilayer producing dissolve the RBCs membrane [5]. 2 mL of whole patient’s blood sample was collected on Ethylenediamine tetra-acetic acid disodium salt (EDTA) anticoagulant tubes. The preparation of washed RBCs was carried out according to Trivelli L., procedure as following; RBCs were isolated by centrifuged the whole blood sample at 1372 g. for 10 min. at 10 oC. Plasma was then removed and the top third of the sample volume to eliminate leukocytes contamination and collect only packed RBCs. Normal saline solution (0.9 % NaCl isotonic solution) of volume 5 times the volume of collected PRBCs were added and gently agitated for 2 min. then re-centrifuged at 1372 g. then the saline eliminated to separate the washed RBCs. This step was repeated 5 times [17]. The washed RBCs were then diluted using normal saline solution up to a concentration of 5×1000 RBCs /µL (counted automatically by automated hematology analyzer) which is the suitable range for detergent solubilization and give maximum absorbance following Beer Lambert’s low. As the turbidity of RBCs cell membrane was measured using UV visible spectrophotometer (Boehringer Mannheim Photometer 4010 Spectrophotometer * Vat Inc; manufactured by Behring Co in Germany “4010 DADE Behring, Germany”) at 620 nm, at this wavelength there is no absorption band for lipid, protein or detergent monomers which is necessary requirement.
The washed RBCs suspension was introduced to cuvette of 1.5 mL volume then using micropipette, 15 µL of detergent was added at repeated times in each time the total volume was mixed gently using micropipette and the turbidity reading of suspension was recorded, till turbidity reading become fixed and near zero. The detergent concentration is obtained by using the following equation;
DT (mM) = 60 (VA/VT)
When DT is the total detergent concentration in the cuvette (mM), VA is the total volume of detergent added to the cuvette in mL, VT is the total volume in the cuvette in mL, where VT = 1.5 + VA. The turbidity readings of RBCs suspension decreased directly with the decrease of the whole volume of the scattering cells, were plotted as a function of detergent concentration. These solubilization curves were differentiated converting the three break points limiting the four stages of every curve into three peaks donated as A, B and C, then peaks values were estimated (Figure 12).
Osmotic fragility test: Testing the changes in cellular membrane elasticity and permeability of overall RBCs through the osmotic fragility test, which performed according to parpart and co-workers method [18], as following; 1) the dilutions given in Table 2, of buffered sodium chloride were prepared, mixed well using parafilm to cover each tube while mixing and placed in the appropriately labeled test tubes. 2) 5 mL of each dilution is transferred to three separate sets of test tubes, labeled from 1 to 14. 3) 50 µL of heparinized whole blood is added to each of the 14 tubes. This step is done for blood samples from each of the three groups. 4) Each tube was immediately mixed by gentle inversion. 5) The tubes were allowed to stand at room temperature (15-25oC) for 30 min. 6) The tubes were remixed gently, and centrifuged at 1008 g for 5 min. 7) The supernatants were carefully transferred to cuvettes and the amount of hemolysis was determined spectroscopically using the spectrophotometer “4010 DADE Behring, Germany”, at wavelength of 550 nm. The optical density is set at zero using the supernatant in tube no. 1, which represents the blank or (0%) zero % hemolysis. 8) The hemolysis percentage was calculated for each supernatant, as in following equation; Hemolysis (%) = (optical density of supernatant / optical density of supernatant of 100 % hemolysis) × 100. 9) Drawing the osmotic fragility curves; the hemolysis (%) was plotted as a function of the sodium chloride 0.9% solution percentage, then the curves will be differentiated and plotted as a function of NaCl percentage (Figure 13). And the values of Cs% (the percentage of 0.9% buffered NaCl solution at which water can be pumped through the membrane (permeability)), Cp% (the percentage of 0.9% buffered NaCl solution at maximum transport of water inside cells), and the W hmax (the elastic range; width of the mean peak at half maximum) were estimated.
(C) Examination of the RBCs morphological alteration
Then RBCs morphological alterations were investigated using the blood film slides -which prepared from samples of pediatric patients- by light microscope (oil immersions lens). Then the blood film slides examined and imaged using image analyzer type SAMICA (scanning and measuring with automated image counter analyzer system) produced by ELBEK GmbH Germany), present at histology department faculty of medicine, Cairo University, and the RBCs morphological parameters (RBCs area, diameter, thickness, perimeter and roundness distribution) were estimated .
(D) Hemoglobin molecule structure investigations
The hemoglobin molecule structure characterizations investigated by estimation of dielectric properties, electrophoretic mobility and absorption spectrum for samples collected from groups I and II pediatric patients. Dielectric relaxation test; for hemoglobin molecules solution preparation; the same procedure of Trivelli L. to get 300 µL. of washed packed RBCs, then 20 mL of distilled water was add and kept overnight at - 20 0C, to insure the complete lyses of cells. The mixture was then centrifuged at 9500 g for 30 min. at 4 0C. The supernatant (hemoglobin solution) was taken and diluted by distilled water up to the absorbance of 0.5 at wavelength of 578 nm., then the solution used for the hemoglobin molecule structure investigation by dielectric relaxation test.
Pure hemoglobin solution as dielectric system of dipolar molecules with permanent dipole moment are purely randomly distributed, so the net average dipole moment is zero. Using external electric field will polarize the hemoglobin solution, the polarization defined as the limit of ∆p (the dielectric dipole) as ∆v (unit of volume). The average dipole moment will be calculated as
µd = µ2E/3kt = αE
Where α is the polarizability and E is the applied electric field and µ is the dipole moments of hemoglobin molecule which generally calculated using Oncley’s equation
µ = 9000 kTM/4πNb (∆Ɛ/c)
Where kT is the Boltzmann constant times the absolute temperature, M is the molecular weight, N is the number density per unit volume (cm3) of polar molecules in a pure polar liquid and given as NAρ/M (where NA is Avogadro’s constant, ρ the density (g/L) of the pure polar liquid), b is an empirical parameter taken to be equal to 5.8, c is the hemoglobin concentration in grams per liter and ∆Ɛ is the dielectric increment.
The ability of the hemoglobin molecule to orient in the applied electric filed determines its dielectric properties. The applied electric field producing displacement current in hemoglobin solution at low frequency “the dielectric loss is minimum or zero”, with increasing frequency joule heating of the solution occurs “the dielectric loss” and reflected as a in hemoglobin solution permittivity, where The dielectric constant is strongly frequency dependent with low-frequency (less than 100 Hz) values being up to 30% higher than those of high frequency (more than 100 Hz). At very high frequency “over the range of study” the displacement current has fallen to zero. The electric filed considering polarization expressed by Gauss law as following;
(Ɛo E + P) . n da = Q
In case of hemoglobin solution between two plates of area A and distance d, the capacitance (C) and conductance (G) of solution will be
C = Ɛs Ɛo A/d
G = σs A/d
Where Ɛs and σs are the static permittivity and conductivity respectively at very low frequency. Under effect of sinusoidal field with circular frequency ω, the complex conductance will be
G + j ω C = A/d (σs + j ω Ɛs Ɛo)
With the equation separation into complex conductivity and permittivity are respectively
σ* = σs + j ω Ɛs Ɛo
Ɛ* = Ɛr – j Ɛ′
Where Ɛs and σs are the dielectric parameters of hemoglobin solution, j = √-1, ω = 2πf, Ɛr and Ɛ′ are real part (dielectric constant) and imaginary part (dielectric loss) respectively.
The dielectric measurements were made for the hemoglobin solution in the frequency range from 0.1 to 10 MHz by a loss factor meter type 1033, R.F.T, Funkwerk Erfurt, (Germany), together with a sample cell (type PW 9510/60, manufactured by Philips). The sample cell has two squared platinum black electrodes each having an area of 0.8×0.8 cm with an inter-electrode distance of 1 cm. the cell with the hemoglobin solution sample was kept at 14±0.10C in temperature-controlled incubator type 2771 manufactured by Kottermann, Germany. The reading of each run were taken three times and the average was considered. For each sample the resonance position approached through connecting of the measuring-circuit inductivity (the build in system capacitance), and the test circuit with sample connected was resonated by adjustment of tuning capacitor, and the capacitance (C) was recorded. The circuit was again resonated without the sample by adjustment of tuning capacitor and the capacitance (Co) was recorded in farad. The capacitance C and resistance R for the samples were measured at different frequencies in the range of 0.1 to 10 MHz. The capacitance of the system at a particular frequency and temperature may be written as
C = Ɛs Ɛo K + Co
Where Ɛr is the relative permittivity “the dielectric constant measuring of the polarity of hemoglobin solution”, Ɛo is the permittivity of free space (8.85×10-12 f/m), K is the cell constant (a/d) that depends on the cell dimensions, and Co (farad) is the residual capacitance. The values of K and Co were found from measurements in air and distilled water and were checked by using methanol. Also the loss tangent, tan δ was obtained from the measured values of the resistance R (ohm), and C (Farad) as follows:
Tan δ = ½ π f RC = Ɛ′ / Ɛr
Consequently the dielectric loss Ɛ′ was calculated from the relation:
Ɛ′ = Ɛr Tan δ
The conductivity σ was then calculated from the relation:
σ = 2πf Ɛ′ Ɛo
Where f is the frequency in cycle/sec.
Relaxation theory; the kinetics of the displacement determines the frequency dependence of the hemoglobin solution. The total polarization is divided to P∞ (very fast time constant) indicates the polarization due to the electronic and atomic polarizabilities and Pdip (much longer time constant) indicates the polarization due to the orientation of permanent dipoles as illustrated for applied field (Figure 14A). The relaxational response of hemoglobin solution may be described by first order differential equations (pure hemoglobin molecules solution) characterized by a relaxation time (τ) as following
Pt = P∞ + (Pdip - P∞) (1- e -1/τ)
For hemoglobin solution under study, if considered as single relaxation time it will be expressed in form of frequency domain and converting the polarization into a dielectric constant using simple Debye equation as following
Ɛ*(ω) = Ɛ∞ + (Ɛs- Ɛ∞ / 1 + j ω τ)
The equation separated into
Ɛr (ω) = Ɛ ∞ + (Ɛs- Ɛ∞ / 1 + (ωτ)2)
Ɛ′ (ω) = (Ɛs- Ɛ∞) ωτ / (1 + (ωτ)2)
Cole-Cole devised an empirical method when a distribution of relaxation times exists, the width of the dispersion increases and introducing the parameter β which modifies the Debye equation as following
Ɛ* (ω) = Ɛ∞ + (Ɛs- Ɛ∞ / 1 + (j ω τ) β)
Where β is an empirical parameter ranges from 0 to 1. The plot of Ɛr against Ɛ′ gives a semicircle of radius (Ɛs - Ɛ∞) / 2, the center of semicircle lies below the abscissa and makes angle of β π / 2 as in (Figure 14B). In the interpretation of the relaxation time by Debye terms of rotation of hemoglobin molecule is a continuous solution with friction. He derived an equation utilizing Stockes law for rotating sphere.
τ = ξ/2 KT = 4 π a3 η / KT
Where ξ is Stockes’ law constant and a is the radius of sphere. The complex permittivity characterizes the linear response of hemoglobin solution to applied electric field, the Kramers-Kronig relations provide a necessary connection between the conductivity and permittivity (19). This implies that the permittivity of hemoglobin solution cannot vary independently with the frequency; any decrease with frequency in the permittivity must be accompanied by an increase in conductivity, this yields a consistency test for data.
In order to calculate the radius of hemoglobin macromolecule by using modified Debye equation (Cole-Cole)
Ɛ*(ω) = Ɛ∞ + (Ɛs- Ɛ∞ / 1 + (j ω τ) β)
τ = ξ/2 KT = 4 π a3 η / KT
Using the same method of plasma and whole blood viscosity measurements, the hemoglobin solution samples viscosity measurements were carried out by Ostwald viscometer using equation of
ηsample / ηwater = t sample / t water
Where η and t are the hemoglobin solution viscosity value and time respectively.
The variation of dielectric constant (Ɛr) and dielectric loss (Ɛ′) were plotted on one scale and the electrical conductivity (σ) plotted on another scale, as a function of the applied electric field in the range 0.1 to 10 MHz for the hemoglobin solution samples. The dielectric increment (∆Ɛr), electrical conductivity σ (s/m) at 10 MHz, relaxation time (τ) molecular radius (r) and Cole-Cole parameter (β) were calculated.
Electrophoretic mobility test: The electrophoresis at pH 8.4-8.6 using a cellulose acetate membrane as a substrate was measured using equipment type OPTISCAN manufactured by HELEMA Co Model 0280, Serial No. 12011 in France. (Involving electrophoresis tank and power pack, wicks of filter, blotting paper, applicators, cellulose acetate membrane and pH meter, Electrophoresis buffer solution Tris/EDETA/Borate (TEB), in a procedure as follows:
1) The same procedure of Trivelli L. to get 50 µL of washed packed RBCs, then add to 150 µL of lysing reagent. 2) Filling the compartments of the electrophoresis tank with TEB buffer. 3) Immersion of cellulose acetate membrane on buffer solution (TEB). 4) Applying small volume of sample into cellulose membrane near the end. 5) The test procedure done by starting the power supply at 250-350 V for 20 min. 6) After the end of automated procedure the hemoglobin bands scanned and converted to hemoglobin spectra, and the area under bands counted in percentage of hemoglobin sample. Absorption spectra test; the absorbance spectrum of the same hemoglobin solution samples prepared for dielectric measurements, were measured using JASCO V-570, UV/VIS/NIR spectrophotometer, at wavelengths between 200 to 700 nm, spectra peaks were identified and the ratio between A578 and A540 were calculated.
(II) Postoperative clinical course
(A) Cardiovascular system performance
Cardiac performance: Was estimated by measuring peak velocity (PV) and cardiac index (CI) using USCOM 1A advanced Doppler non-invasive hemodynamic monitor “Uscom Australian company Ltd” before CPB, immediately post CPB and at 24, 48 hours postoperative.
Circulation performance: Was estimated by measuring central venous pressure (CVP), heart rate (HR) and measuring mean blood pressure (MBP) before CPB and at 24, 48 hours postoperative. Counting the times of hypotension and using inotropic support intraoperative, and take the grad (nil, trace, + and ++) of hemoglobin in urine at the ICU first hour as assessment for the severity of intravascular RBCs destruction.
(B) The postoperative clinical outcome
The primary outcome: Was estimated by using the sequential organ failure assessment score (SOFA score) as following: Respiratory system (PaO2/FiO2 mmHg and oxygen saturation %), Nervous system (Glasgow coma score), Cardiovascular system (The inotropic support dose of dopamine and adrenaline), Liver (Bilirubin concentration), Coagulation profile (PLTs count using automated hematology analyzer (Sysmex K - 1000, TOA manufactured by Medical Electronics Co., “LTD. KOBE. Japan”) and Kidneys (creatinine concentration). The secondary outcome; was achieved by: Assessment of postoperative hemostasis (calculating the extubation and ICU stay times) and Estimation of postoperative complications (Recording the complications including need to re-intubation, chest and wands infections).
Although, performing such blood replacement process as a new technique, the perfusionist did not have the ability to change the routine of the other operation teams i.e. anesthesia and ICU teams during or post CPB, so the management of all groups involved blood transfusion was performed using WFB and RBCs at Cairo and Aswan universities respectively.
Results
(I) blood components biophysical characteristics investigation
(A) Whole blood rheological parameters
- The average values of plasma and whole blood viscosity (cP) for group II showed insignificant and significant (p<0.025) increase respectively, and insignificant increase for group III as compared to group I.
- the results of the average values of RBCs rheological parameters for samples from each group, showed significant decrease (p<0.05) of all parameters for group II as compared to group I in contrast with the insignificant decease for group III. Also the calculated surface to volume ratio (S/V) for group II was significant decrease (p<0.05) compared to group I in contrast the insignificant decrease for group III (Table 3).
(B) RBCs membrane biomechanical characteristics estimation
Solubilization test results: Figure 15 showed the variation of the turbidity of blood as a function of detergent concentration for samples collected from one patient of each group, the plots passes through four stages, it is clear from the figure the changes in the profile of the solubilization plots of groups II and III as compared to group I. The differential curves of the variation plots (in the right upper corner of the figure) showed the three apparent main peaks (A, B and C). The average values of peaks positions were considered for all patients from the three groups and represented with the standard deviation in Table 4. It is clear from table the shift of all considered characteristic peaks A, B and C positions towards lower detergent concentration values as compared to group I. However these shifts in peaks positions showed to be statistically significant for group II (p<0.05) and insignificant for group III.
Osmotic fragility test results; Figure 16 showed the variation of the RBCs hemolysis percentage as a function of 0.9% NaCl concentration percentage for a blood sample collected from one patient each group, the plots passes through one stage, it is clear from the figure the change in osmofragility plots of groups II and III as compared to group I. The differential curves of the osmofragility plots (in the left lower corner of the figure) passes through a one main peak. The values of CS%, CP% and Whmax were calculated and expressed as average values with standard deviation (Table 4). It is clear from table the shift of CS% and Cp% parameters towards higher 0.9% NaCl concentration percentage values, and Whmax parameter towards lower values compared to group I. However these parameters shifts proved to be statistically significant in CS% (p<0.05), Cp% (p<0.05) and Whmax (p< 0.01) for group II, and insignificant for group III.
(C) Examination of the RBCs morphological alteration
The results of RBCs morphological parameters measurements were represented as the mean with standard deviation values of the all blood films from the three group’s cases for every parameter (Table 5). It is clear from table, the significant decrease (P<0.05) in all parameters for groups II as compared to groups I. In contrast, as regards to these parameters, that decrease was insignificant in group III.
Figure 17 illustrates the image of blood film for a blood sample from group I. it showed normal shape of the RBCs with normal regular and smooth cell membrane with absence (or insignificant) Acanthocytes (looks like central pallor has 3 to 12 spikes or knobs) and the clear presence of healthy cell membrane of normal repulsion force between adjacent cells (marked by arrows). Figure 18 illustrates the image of blood film for a blood sample from group II. It indicates the significant presence of Acanthocytes, illustrates clearly the fusion of several neighboring cells to have common cellular membrane (marked by arrows), shows clear irregularity of the cellular membrane of the most of RBCs, however, the cells appear in stacked groups, a phenomena which never seen in group I samples. There were a lot of fragmentation due to destruction of some cells, acting as foreign bodies attached or sticked on the rest of RBCs membrane. Figure 19 illustrates the image of blood film for a blood sample from groups III. It illustrates the presence of normal healthy RBCs beside insignificant presence of abnormal forms of group II RBCs.
(D) Hemoglobin molecule structure investigations
Dielectric relaxation test: Figure 20 showed the variation of dielectric constant (Ɛr) and dielectric loss (Ɛ’) plotted on one scale and the electrical conductivity (σ) plotted on another scale, as a function of the applied electric field in the range 0.1 to 10 MHz for hemoglobin sample collected from one patient of groups I and II respectively. It is clear from the figure that, the dielectric relaxation of the hemoglobin molecule passes through a dispersion in the frequency range demonstrate. Moreover, it is clear from the figures that, the decrease of Ɛr with the increase of frequency is accompanied by an increase in the electric conductivity, which yields a consistency test for the data [19]. Figure 21 showed the Cole-Cole plot for hemoglobin samples collected from one patient of groups I and II respectively. The average values of the dielectric increment (∆Ɛr) electrical conductivity at 10 MHz (s/m) relaxation time (τ), molecular radius (r) and cole-cole parameter (β) were calculated from the dielectric relaxation curves and the average values for each group were considered and given in Table 6. It is clear from the data in the table that, there is no measurable difference between the measured parameters for the two groups I and II.
Electrophoretic mobility test: The patterns show that, hemoglobin from the two groups have molecular mobilities appeared in one main peak (A1) and shoulder (A2) (Figure 22). The area under each peak was measured and the average value was considered for each group as given in Table 6. The results indicate no considerable difference between the measured values for the two groups.
Absorption spectra test: Figure 23 illustrates the absorption spectra measured in the range 200 nm to 700 nm for hemoglobin solution of groups I and II. The results indicate the appearance of the well-known characteristic bands of hemoglobin. The absorbance ratios of A578 / A540 were calculated for the two groups and the average values with standard deviation were considered and given in Table 6. The results indicate no differences between the measured absorption spectra for the two groups I and II.
The investigations of hemoglobin molecule structure alterations using dielectric characteristics estimation, hemoglobin electrophoresis test and hemoglobin spectrophotometry did not show any significant changes between groups I and II.
(II) Postoperative clinical course
(A) Cardiovascular system performance
Cardiac performance: Compared to group I, the results showed significant decrease (p<0.05) for group II, in contrast, for group III it showed significant decrease before and immediately post CPB (p<0.05), and insignificant decrease 24 and 48 hours post CPB (Table 7).
Circulation performance: Compared to group I, the results showed significant increase (p<0.05) of CVP and HR for groups II and III before CPB, but at 24 and 48 hours post-bypass it was still significant for group II, and insignificant for group III. The results of MBP, compared to group I, showed significant decrease (p<0.05) for groups II and III before CPB, but at 24 and 48 hours post-bypass it was still significant for group II, and insignificant for group III. The times of hypotension intraoperative and use inotropic dose support for groups II and III was significantly increased compared to group I (p<0.01). The results of postoperative intravascular hemolysis assessment showed significant grade (p<0.01) for group II and insignificant for group III compared to group I, where the hemolysis started to occur in urine intraoperatively with 34% of group II pediatric patients (Table 7).
(B) The postoperative clinical outcome
The primary outcome: The sequential organ failure assessment parameters measured and the SOFA score calculated for 24 and 48 hours postoperative, and represented in Table 8, the table data showed significant increase (P<0.05) in score for group II and the insignificant increase for group III compared to group I. For cyanotic pediatric patients who received FWB the PLTs concentration found to be insignificantly increased compared to group I at 24 and 48 postoperative, in order to noticed that, the pediatric patients who received FWB and PRBCs recorded with their PLTs concentration measurements Table 8. The secondary outcome; the parameters of secondary outcome were calculated and presented in Table 8. As compared to group I, there was significant increase (p<0.05) in extubation time for Group II and insignificant increase for group III (who received RBCs), in time of insignificant decrease for group III (who received WFB) (noticed in table 8). The ICU stay data showed significant increase (P<0.05) for group II and insignificant increase for all group III pediatric patients compared to group I. All postoperative complications occurred only in group II.
Discussion
Cyanotic pediatrics show high ischemic symptoms for organs pre and postoperative corrective surgery [20]. There is a lot of factors contributed in postoperative clinical outcome of cyanotic pediatrics, one of them is the healthy RBCs which is able to perform its essential role in oxygen transport and body tissues perfusion during ICU critical period. The main objective of the present work is to evaluate the efficacy of the partial replacement of cyanotic patient’s blood with healthy RBCs immediately post bypass, and its impact on the biophysical characteristics of cyanotic patient’s blood and pediatric patient’s postoperative clinical outcome, and to recognize the main cause of the adverse effects of prolonged hypoxia on the normal physiological functions of RBCs “oxygen transport and body tissue’s perfusion”. The biophysical properties of blood components were achieved through the study of the whole blood and RBCs rheological parameters, the study of the biomechanical properties of the RBCs cellular membrane, also the study of RBCs morphological alteration and investigate the hemoglobin molecule structure. The patient’s clinical outcome estimation achieved by investigating the cyanotic patient’s cardiac and circulatory system performance, study of SOFA scour parameters, vand postoperative hemostasis and complications. Hemodilution in CPB practices; hemolilution has been widely applied for hypothermic CPB for many years primarily because of the belief of that hemolilution reduced viscosity would help in countering harmful effects of deep hypothermia. The bloodless cardiac surgery achieved in selected group of small children (> 18kg) with advanced heart lung machine and remote-mounted Perfusion System to reduce the priming volume [21]. But, hemodilution may impair oxygen delivery by shifting the oxygen dissociation curve to the left. Shin’oka T, et al. demonstrated that extreme hemodilution (HCT < 10%) causes inadequate oxygen delivery during early cooling, and higher HCT (30%) achieved with blood prime results in improved cerebral recovery after circulatory arrest [22]. Pediatrics Less than 18 kg is small enough to warrant a blood prime [23]. Regularly using of blood is essential in extracorporeal procedures like extracorporeal membrane oxygenation (ECMO) [24] and basically in cardiac surgery as prime for CPB unit [25,26]. Blood transfusion; the RBC transfusions which are given to maintain the level of HCT are believed to be most desirable for each neonate’s clinical condition, as management of unexplained growth failure and anemia for some neonatologists. Few years ago, 80% of premature neonates required multiple RBC transfusions and many infants are given cumulative transfusion volumes in excess of their total blood volumes at birth [27,28]. The amount of blood transfused should be applied with flexibility to individual infants facing surgical procedures of varying complexity, however the transfusion of more than 50% of patient blood is considered as massive blood transfusion [29]. Blood transfusion is important in the treatment of many medical problems such as blood disorders, major surgical problems and hemodialysis for small pediatrics (when it is unable to reduce the extracorporeal circuit priming volume, the blood prime may be necessary) [30]. Also for anemic and profound hypotension pediatrics, the blood prime should be employed [31]. In MAPCAs unifocalization it is an off-pump procedure, until de-saturation approached uncompromising level the CPB should be instituted at moderate hypothermia with the heart beating, at this time a calcium-supplemented blood prime is used to maintain normal cardiac function [32,33]. There is no clear threshold beyond which blood use is futile [34]. In congenital corrective surgery the first choice for perioperative blood transfusion is autologous blood donation (ABD) and erythropoietin therapy, but unfortunately pediatric patients with cyanotic disease constitutes absolute contraindications to ABD, and erythropoietin therapy can be used only in older children, where it is generally performed in children older than 5 years and weights > 20 kg and does not decrease blood loss [35]. Types of blood transfusion; in spite of the presence of many indications for FWB transfusion, they are now well managed exclusively with blood component therapy. The guidelines for RBCs transfusion to neonate are controversial and practices vary widely among institutaions [36], and the risk-benefit ratio of transfusion as well as the disease being treated and the clinical condition of the patient must be considered in determining whether to give, blood products or FWB. Basically, the conventional transfusion model of packed RBCs, plasma and platelets actually further dilutes the patient in comparison to the blood he has lost, thus it is not the ideal fluid for coagulopathic patients who require this massive transfusion of products [37-39]. Hemostasis; it can be problematic in infants and neonates following cardiac surgery. Stephan et al.’s study hypothesized that the composition and volume of the pump prime would greatly determine the coagulation profile during and following CPB. For all the studied infants and neonates, the average fibrinogen of the CPB refrigerated whole blood Prime (>48 hours old but <7 days old) was 139 mg/dL. The average pre-operative fibrinogen of 252 mg/dL dropped significantly to 169 mg/dL at the onset of CPB and rose significantly during CPB to 190 mg/dL but remained significantly lower than the preoperative average. If these patients are not treated with platelets, they would show significant decrease in platelets count post CPB [38]. The blood hemostasis is a major consideration in health and disease, where the abnormal clotting of blood is the essential complicating factor in all disease states, where the particulate matter sticks to each other and to the endothelial cells. This phenomena decreases or stops blood flow and therefore oxygen and nutrient supply to tissues and prevents the removal of toxic metabolic byproducts [39]. So there is a clinical coagulopathy importance for relatively fresh blood products [40] and unlike the use of stored blood products. FWB can be anticipated to have full platelet activity [40] and the using of old blood is not preferred especially in these elective surgeries which need relatively small blood quantities compared with large trauma and transplantation centers.
The FWB transfusion systemic effects are; improved intravascular volume, decreased or limited third spacing, enhanced oxygen carrying capacity, and replenishment of coagulation factors—all prove the effectiveness of the FWB therapy, the FWB increase both red cell mass and plasma volume, and it contains clotting factors, which may offset the inherent risks of giving the FWB, so the warm FWB is included in blood treatment strategies, to achieve survival of patients like in hemorrhagic shock cases where less anticoagulants and additives are added. 1 unit of PRBCs (335 mL) plus 1 unit of platelets (50 mL) plus 1 unit of fresh-frozen plasma (275 mL) provide 660 mL of fluid with a hematocrit of 0.29, 88 000 platelets, and 65% coagulation factor activity. In contrast, 1 unit of FWB (500 mL) has a hematocrit of 33% to 43%, 130 000 to 350 000 platelets, and 86% coagulation factor activity [41]. Mohr, et al. in their research suggest that the hemostatic effect of 1 unit FWB after cardiopulmonary bypass is at least equal, if not superior, to the effect of 10 units of platelets [42]. Duchesne JC, et al. demonstrated a significant difference in mortality in patients who received more than 10 units of PRBC (26% vs. 87.5%) when FFP: PRBCs ratio was 1:1 versus 1:4 (p = 0.0001) [43], that makes the core point in blood transfusion is the normal healthy equalized blood components as in normal blood, it is not a matter of large quantity of healthy red blood cells, but it should be with the same quantity ratio with the plasma as in normal human blood. Treatment with FWB is a tried and proven concept [41], it can reverse the dilutional coagulopathy associated with transfusing large amounts of preserved red blood cells [44]. By focusing on the using of Whole blood, One of the most pervasive arguments against the use of FWB is that it is logistically too demanding to be practical, even if indicated [45-47]. This argument focuses on the recruiting, interviewing and testing of a poorly-defined civilian donor population rather than the processing of blood units. The processing of whole blood into components is clearly more costly of labour, time, and material [40]. In Egypt, the capability of local collections (patient’s relatives) achievement is clearly and easily exists to collect FWB, but still the strong argument against the use of FWB relates to concerns about inadequate infectious disease testing, particularly for hepatitis C and HIV. But the calculated and published risks for hepatitis C transmission, using current screening and testing procedures, is 1 in 1.4 million units. The risk for HIV is 1 in 1.6 million units in an otherwise unselected donor population [40]. Using modern tests (enzyme immunoassays) and testing protocols, make risk of acquiring an infection is vanishingly small. Total donor exposure can be anticipated to be less with whole blood transfusions than with similar quantities of component therapy, indeed, comparing donor exposure via 1 unit of fresh whole blood to that of 1unit each of RBC, FFP and apheresis platelets (or 6–11 units of conventional platelets), the use of whole blood can decrease donor exposure at least several fold [40]. A variety of infectious agents can be present in donor blood; like bacteria (the spirochete of syphilis), protozoans (the agents of malaria and babesiosis) and prions (e.g., the agent of variant Crueutzfeldt-Jakob disease), and it could be transmitted to recipients. Blood now checked for the presence of the RNA by the nucleic acid-amplification test for infectious viruses beside the enzyme immunoassays which detect antibodies against the infectious agent. With these precautions the risk of acquiring an infection is vanishingly small. Considering the hemodilution of RBCs to the actual plasma, when the type of blood transfused is FWB, there is no need for extra monitoring for coagulation parameters like PT and PTT [48]. When considering the risk-benefit ratios associated with clinically important complications of FWB, the relatively infrequent risks are even less clinically significant when compared with the likelihood of mortality or morbidity without appropriate managements in the critical medical conditions. The type of blood transfused in the perioperative treatment of cyanotic pediatric patients should be appropriate for their clinical status and considering there is no clear threshold beyond which blood usage is futile [34], so the replacement with FWB is advantageous over conventional component therapy even it is available, where risk-benefit ratio of whole blood transfusion favours its use. The replacement procedure safety; the postoperative needs of cyanotic patients for healthy RBCs but not to increase the hematocrit, in agreement with Hardy J who consider the need of cyanotic patients for healthy blood components in postoperative period [49]. The choice of blood replacement in the current research after CPB is superior over blood transfusion in prime and during operation, where the blood replacement after CPB improve the microcirculation postoperatively to avoid the potential adverse effects of the hyper viscosity due to high HCT during CPB, also to avoid the destructive effect of CPB circuit components on the transfused RBCs. This procedure will be a replacement of the actual pediatric patient’s blood in safe place “intraoperative” instead of postoperative blood transfusion managements during ICU period, where the blood replacement is principally an unavailable procedure. On the other hand such timing for replacement process allows us to reveal easily the blood role in the clinical deterioration of those pediatric patients postoperatively. We can represent the replacement procedure complications as following; 1) The significant decrease in free calcium concentration, solved by going to little bit of hypothermia and adding 10 to 20 ml of 10% calcium chloride/ gluconate for the 500 mL of infused blood, calcium decrease may be lead to the cardiac arrhythmias, but it can be managed easily where the transfusion is still intraoperative and can be terminated in any time. 2) The drop of temperature is managed using the operative table mattress or beer hugger (Cairo University), or by using the heater cooler of cardioplegia delivery device (Aswan University). 3) The use of crossmatched and compatible blood type reduces the transfusion complications and reactions, however, in the incidence of clinically strong incompatible reaction, it is easy to manage rapidly the patient in operating room even by returning back to bypass. To minimize this risk, donors are questioned about their possible exposure to the diseases and each unit of blood is tested for a variety of infectious agents. The suitable volunteer donor’s (parents) blood is collected by the blood bank and donation service in hospital. Many precautions are taken to ensure that blood is safe as possible. 4) The negative pressure created by the over flow which is higher than the proper flow fitting the tube’s size, may lead to vacuum which create gas microbubbles. This is the reason of designing the replacement tubes as following; two stopcocks in the input and output and a connecting tubes in between of ¼ inch size in order to compensate 100 mL flow or more in case. The significant increase of whole blood viscosity which associated to the prolonged hypoxia for group II, in agrement with Matthew J and Katayama, et al. [50,51] and the increase in viscosity naturally accompanied with increase in share stress, influence the RBCs and decrease their rheological parameters. This natural viscosity of cyanotic pediatric patients facilitates and accelerates the RBCs malfunction and destruction, which impair the entire circulation. This change in whole blood rheological parameters potentiate the destructive effect of other factors “as mechanical stress and hypothermia” on circulating RBCs [52]. Testing the integrity of overall RBCs cellular membrane by the solubilization of RBCs cellular membrane by non-ionic detergent passes through four stages, denoted as shown in differentiation curves, as three peaks namely A, B and C. In stage I (limited by peak A) and stage II (limited by peak B) certain detergent concentration was needed to distribute the detergent monomers between the lipid bilayer of RBCs cell membrane and then begans to dissolve the cellular protein molecules (extrinsic and intrinsic respectively) then the lipid bilayer, producing membrane dissolve. This phenomenon can be noticed by the decrease of the scattered light intensity i.e. decrease of the whole volume of the scattering cells. Stage III (which started by peak B and limited by peak C) of the solubiliztion curves represents the process of detergent interaction with the membrane macromolecules where detergent molecules incorporate within the bilayer molecules. Stage IV (which started by peak C and limited by optical cleared mixture) in the solubilization curves represents the complete solubilization of cell membrane. The results of solubilization test showed significant shift of all peaks positions towards the lower detergent concentrations in group II compared to group I. The significant decrease of detergent concentrations used in performing stages I and II, indicates either mutation in the protein structure and/or disappear of these proteins. Hence for the biological membrane proteins, there are mono-, bi- and poly-topic integral and peripheral proteins that refer to the extension of protein in the entire width of the membrane bilayer [53]. The mutation of membrane protein under effect of hypoxia caused disruption of normal cellular function. For example, the “human erythrocyte anion exchanger” (band 3) is a membrane protein, an abundant chloride/bicarbonate exchanger is found in erythorcytes membranes, this protein is involved in membrane stability, erythropoiesis and acid-base regulation of the blood [54]. This protein mutation results in the membrane integrity reduction, and the absence of membrane proteins functioning as signal receptors which affect the cell biological characteristics [55] and we may consider the detergent concentration decrease in these stages as markers for the loss of the cellular receptors. Moreover, these disappeared cellular proteins may recombine or be attached to the membranes of other cells, which accelerate the deterioration of the cellular function for all RBCs. The significant decrease of detergent concentrations used in performing stage III, indicates that, the packing properties of the membrane macromolecules had been changed, this change may be related to the depletion of energy due to chronic hypoxia “as physiological condition”, where phospholipids trafficking in eukaryotic plasma membranes is remarkably dynamic which driven by constitutive energy-dependent processes [56]. Changing the packing properties makes the passage for the detergent molecules within the membrane easier [57,58]. We may imagine the arrangements of macromolecules forming the cellular membrane in a way that “Van Der Waals” forces began to be weaker between these molecules and the intermolecular binding forces are deteriorated between these macromolecules, beside machine share stress as a source of oxidants [59], these affect normal cellular function by disturbing the membrane structure [60], that all these factors enhances existence of lipid detergent mixed micelles and enhances the protein, phospholipids solubilization process [61,62]. The significant decrease of detergent concentrations used in performing stage IV indicates the complete loss of the intermolecular forces of the cellular membrane which confirm the disturbance of cell membrane structure hypothesis. In contrast, the insignificant shift of peaks positions towards lower detergent concentrations for group III compared to group I indicates the improving of overall RBCs membrane integrity and bioactivity under effect of replaced healthy RBCs.
The studying of the osmofragility of overall RBCs can give information about the changes in RBCs cellular membrane permeability and elasticity through the values of Cs%, Cp% and Whmax respectively, where the differentiation curves of osmofragility plots, passes through one main peak which starts at a concentration percentage of NaCl (Cs%), peak position (Cp%) and width at half maximum (Whmax). The results for group II compared to group I (Table 4) showed significant increase of Cs% value that indicates the low osmotic pressure needed to pump water/ion molecules inside the cell, under the effect of lipids and proteins of cell membrane deterioration. The cell membrane permeability increasing, disturb the electrical potential of membrane by interfering the normal distribution of ion channels on cell membrane like K+, Cl-, Ca- and Na+, red blood cells carry electrically positive charges upon their surface which result from pump of K+ from the inner to the outer surface of the cell. These positive charges on the surface of the cell membrane form strong repulsive electrostatic coulomb forces between adjacent cells that prevent cells from being in direct contact. The disrupt in the Na+-K+ pump as a main ion channel leads to losing of cell membrane action potential, the loss of cellular membrane surface charge is mainly due to dramatic changes in the membrane permeability and properties that lead to loss of the Na+-K+ pump [63,64]. All that consequently interrupt the membrane physiological properties and cell surface electrostatic charges resulting in losing cells repulsive electrostatic Coulomb forces between them, then become sticking together with a common cellular membrane. The significant increase in Cp% value for group II compared to group I, indicates the increase of ruptured cells due to the fragility of cell membrane. The significant decrease of Whmax for group II compared to group I, indicates the loss of cellular membrane elasticity, which is directly related to the cell membrane biomechanical properties (deformability). We can contribute the change of biomechanical properties to the change in the packing properties (the intramolecular forces of the phospholipids bilayer macromolecules forming the cell membrane) and arrangement of phospholipids and proteins forming the cell membrane [65], that change may be under effect of hypoxia and machine stress. Loosing membrane elasticity resulted in failure of the cell to be folded in order to pass through blood capillaries (since cellular diameter is in the range 12 to 16 µm while blood capillary diameter is about 6 µm) producing capillaries obstructions [65,66] and renal problems. Losing elasticity consequently resulted in failure of the RBCs to reach the tissues for metabolism leading to organs toxicity and toxins production from anaerobic metabolism, which accumulated on RBC’s surface and incorporated within the cell membrane lipid molecules, and on the other hand the metabolism failure will form anemic diseases unmeasured by traditional medical investigations [64,67,68]. As described peroxidation of membrane lipids-caused by gamma irradiation- as interruption of the healthy building bilayer membrane lipid, or can be treated as change for normal arrangement of lipid membreance, causing the decrease in RBCs deformability [64,67]. In contrast, group III showed insignificant increase of Cs% and Cp% values and insignificant decrease in Whmax, which indicate the improvement of overall RBCs membrane permeability and elasticity, due to the effective replacement process. All these changes in the RBCs cellular membrane characteristics associated with the prolonged hypoxia of cyanotic pediatric patients could affect the RBCs membrane activity.
The polycythemia Secondary to the prolonged hypoxia, accelerates the production of premature RBCs, which affect negatively on its structure and morphology, that besides the deteriorating effect of hypoxia and other factors on RBCs were obvious from the significant decrease of morphological parameters values for group II compared to group I. The imaging statistical data (calculated by image analyzer) supported the chemical calculation of blood rheology (as can be noticed from the data before table 3, RBCs rheological parameters decreased significantly in group II as compared to group I). This significant decreases justified the anemic situation that characterize cyanotic pediatric patients appear as significant decrease in weight, height and body surface area for group II as compared to group I. For group III as a reason of blood replacement with healthy RBCs, the decrease in overall morphological parameter was insignificant.
As a confirmation to the results of solubilization and osmofragility tests, the observational investigation for the image of blood film slides from group II, showed the presence of Acanthocytes with the irregularity in the cellular membrane which indicates changes in the packing properties of the cellular membrane’s macromolecules which will affect cell membrane permeability, that leads to the fragile cell membrane that produce the fusion of several neighboring cells to have common cellular membrane. This phenomenon is normally noticed for cyanotic patients. The loss of the mechanical properties of the RBCs cellular membrane may result in cell membrane morphological changes, which is noticed in blood films for group II as compared with group I. we may state here that RBCs for cyanotic pediatric patients suffer from loss of membrane permeability and elasticity which results in loss of the surface positive charges on the cellular membrane. The loss of these charges resulted in loss of the strong Coulomb repulsive forces between adjacent cells and permitted the sticking of these cells together with common cellular membrane. The sticking of these cells will result in the significant increase in blood viscosity for group II compared with group I. In the presence of other factors as mechanical stress and hypothermia (51, 52), the fragile and destructed RBCs turned to clots, the phenomena which is not predominant in Group III and seems as a mixture of normal healthy and cyanotic RBCs. Also the group II film images showed the presence of fragmentation due to destruction of some cells, then sticked as foreign bodies on the rest of RBCs membrane. We may state that cyanotic pediatric patient’s blood not only suffers from the loss of the RBCs characteristics but also from the free protein fractions in the plasma as a result of squeezing the RBCs by roller pump, where these proteins may be attached to the membrane of other cells, which lead to the deterioration of the cellular function. For group III, as a result of partial replacement of cyanotic pediatric patient’s blood with healthy RBCs, there were free mixing with cyanotic RBCs that confirm the improvement of overall RBCs membrane biomechanical characteristics.
The presumption deterioration of RBCs for group II cell membrane mechanical properties has grown up from the results of blood rheological properties, solubilization, osmotic fragility, RBCs morphology and the blood film investigation.
The study of hemoglobin molecular structure was carried out through the dielectric relaxation, absorption spectra and electrophoretic mobility. For the dielectric relaxation test, the change in ∆Ɛr values represent changes in the dipole moment of hemoglobin molecules which may be caused due to changes of the molecular weight and/or changes in the center of mass of the charge over the molecule, the changes in the Cole-Cole parameter (β) represent changes in the shape of the molecules that means deviation from the spherical form to the non-spherical form or vice versa. The insignificant differences in the measured dielectric parameters refer to the similarity of the hemoglobin structure for groups I and II. Also the results of insignificant changes of electrophoretic mobility and absorption spectra refer to the similarity of hemoglobin structure for the two groups. Therefore, we may state here that, the hemoglobin structure of group II have the same structure of group I.
We can assert that, the negative effect of prolonged hypoxia on RBCs is concentrated in changing the biophysical characteristics of the RBCs membrane, which is responsible for the RBCs function in tissue perfusion and transport oxygen through the body tissues.
As compared to group I, PV and CI showed a significant decrease for group II in the postoperative 24 and 48 hours as sign of cardiac performance deterioration, in contrast with the insignificant decrease for group III refers to the cardiac performance improvement due to the replacement process. The increase of CVP and HR indicate the deterioration of cyanotic patient’s circulation [69,70], which was obvious and significant for group II at 24 and 84 postoperatively, where on the opposite side for group III it was insignificant as sign for improvement of circulation performance due to the partial blood replacement. The deterioration of circulation performance for group II pediatric patients, was also presented as the significant decrease in MBP and the significant increase of the intraoperative needs of drug (inotropic) support [71], along with the intravascular hemolysis which all of them leading to the unstable hemodynamics. Severe intravascular RBC’s destruction due to hyper-viscosity and high mechanical stress during CPB procedure [52] was obvious in the form of severe urine hemolysis intraoperatively at the end stage or after CPB for 51 cases of group III. So generally we can say that insignificant decrease of cardiovascular performance for group III refers to the successful partial blood replacement of whole cyanotic pediatric patient’s blood (efficient replacement for plasma and destructed RBCs) in the same time the renal function improvement to eliminate a good quantity of hemoglobin intraoperative post replacement procedure before reaching ICU.
The results showed significant increase in all sequential organ failure assessment parameters for group II compared to group I, significant increase in extubation time and ICU stay, and the occurrence of all postoperative complications which indicate the clinical deterioration of cyanotic pediatric patients postoperatively. This deterioration was obvious with the significant decrease in Pa O2/Fi O2 (mmHg) indication of insufficient oxygen delivery and glasgow coma score refer to the impared cerebral circulation and the Dopamine and Epinephrine doses confirm the significant decrease in MBP, in bilirubin concentration indicate the imared liver function reflects on the production of propere coagulation factors with the significant decrease in PLTs all Leads to disturbance in the coagulation profile, the significant incrase in creatinine values for group II indicating to the increase of the probability of renal impairment [8-10]. In contract to group II, the primary outcome parameters measurements of group III, except PLT’s count, showed insignificant increase compared to group I, and consequently insignificant increase in SOFA score that indicate clearly the improvement of cyanotic pediatric patient’s organs function, which solve their clinical outcome deterioration. Moreover, about 40% of group III (63 cases who received FWB as partial blood replacement) characterized by slight decrease in extubation time compared with group I, that indicates the improvement of hemostatic status which exceed the status of group I. Such hemostasis status is highly correlated with the improving in coagulation profile occurred by the insignificant increase of PLT’s count for group III compared with group I, this clinical situation seems like returning back the dysfunction PLTs due to CPB procedure effect, to it’s functioning state, in addition to the added fresh functioning PLTs of FWB.
That was clear difference touched by the surgeans and intensivists in their manipulation for such complicated cases. There were less bleeding perioperative and obvious smooth managements, completely different from the usual cyanotic pediatric patient’s outcome postoperatively. Postoperatively the data of hemostasis and coagulation profile enhancement for group III appear without any complications which clearly happened as clearly as a result of FWB partial blood replacement, at the same time, group I pediatrics blood experienced the effect of CPB procedure. So the FWB was incorporated as an adjunct into cardiac paediatric patient’s management protocol up to 2010 in Cairo and Aswan universities. After that the Aswan University switched their strategy to packed RBCs.
Study design and limitations
1) In order to offset the effect of CPB procedure on the biophysical characteristics of cyanotic pediatric patient’s blood, the control group (group I) was chosen to be Acyanotic pediatric patients. 2) The same routine of perioperative blood transfusion managements was applied to the cyanotic pediatric patients who were treated with blood replacement process, therefore the perfusionist cannot suppress the other teams to restrict the blood transfusion to the blood replacement process. These manipulations will limit the ability to have precise results about the blood replacement process efficacy. 3) The individual difference in cardiac output affects the mixed drainage blood of healthy and cyanotic pediatric patient’s blood which returned back to the reservoir, so the exact amount of replaced blood was unknown. At the second minute of the replacement procedure, the patients’ blood started to be mixed with the replaced blood and drained back to the reservoir, that cause a wide variations of the actual replaced blood amount, which interrupt the full blood replacement process assessment.
Conclusions
For cyanotic pediatric patients who undergo corrective surgeries, the partial blood replacement with healthy RBCs immediately postbypass may help in resuming overall RBCs biological activity. Where the RBCs perform its essential roles in organs perfusion post corrective surgery during critical period of ICU, especially after the massive cell destruction in the CPB machine. This will improve the cyanotic pediatric patient’s clinical status in order to survive and overcome their problems postoperatively. The FWB usage was predominant in Cairo and Aswan universities perioperative managements of congenital cardiac disease pediatrics who underwent cardiac corrective surgery until 2010, after that Aswan University switched their strategy to packed RBCs transfusion. The replacement with FWB is advantageous even when conventional component therapy is available, where risk-benefit ratio of whole blood transfusion favors its use. Recommendation; it may be recommended from the present work the following: 1) Using FWB in blood replacement process, where there is no need for extra monitoring of coagulation parameters. 2) Adequate hydration should be maintained in combination with extended diuretic therapy.
Research frontiers: The current research introduce the blood replacement process using healthy RBCs solving the postoperative clinical outcome deterioration of cyanotic pediatric patients underwent corrective congenital surgery. Considering the goals of the blood replacement therapy and blood banking practices, those will best provide safe and effective transfusion treatment. The type of blood transfused should be appropriate for the clinical status of cyanotic pediatric patients postoperatively. Additionally, a thorough understanding of available blood components and indications to know which will be critical for making the decision whether to transfuse FWB or PRBCs. Therefore, further studies are needed to support the medical choice of FWB, however considering the type of transfused blood which belong to the policy and the strategy of the surgery center, we should emphasize the superiority of FWB. The postoperative outcome improvement was highly obvious for cyanotic pediatric patients treated with FWB, the intensivist confirmed that they did not find any difference in postoperative managements of Acyanotic and cyanotic pediatric patients treated with blood replacement process. The intensivist first comment for the first case of group III “for sure, you exchange the cyanotic pediatric patient with another, acyanotic one! Did you?” and the answer was “we only exchange part from his blood”. Also another further researches are needed for a method to clean the cyanotic blood plasma from toxins proteins naturally present and formed during CPB procedure, before starting the blood replacement process.
A lot of thanks for the Cairo university academic staff; prof. Reem H. El-Gebaly the head of biophysics department, faculty of sciences and prof. Mohamed Abdel Raouf the former head of cardiothoracic surgery department, faculty of medicine. And many thanks, to the members of Aswan university hospital. Especial thanks go to the vice president of graduate studies and research Prof. Ayman Mohammad Othman, Prof. Atef Ibrahim Saad the faculty of science dean, and Prof. Ayman Ahmad El-Amin Mohammad the head of the physics department for their interest and support for scientific studies in the field of cardiopulmonary bypass management, in order to provide the highest standard of advanced medical services provided for cardiac patients from the children of Aswan governorate. And a lot of thanks to Nasser Helmy the biomedical engineer at Cairo university and the associated prof. Hisham Hosny for his effort in reviewing the research methodology and data presentation arts.
- O’Brien P and Smith PA (1994) chronic hypoxemia in children with cyanotic heart disease. Crit Care Nurs Clin North Am 6: 215-226. Link: https://bit.ly/3gCiJOS
- Dosanjh A (2018) Neonatal Cyanosis: A Clinical Diagnosis. EC Paediatrics 12: 1164-1168. Link: https://bit.ly/2XLSxJ0
- Komarlu R, Morell VO, Kreutzer J, Munoz RA (2010) Dextro-Transposition of the Great Arteries. In: Munoz RA, Morell VO, da Cruz EM, Vetterly CG and da Silva JP, editors. Critical care of children with heart disease: basic medical and surgical concepts. 2nd ed. Switzerland: Springer, 2010. pp. 359-377. Link: https://bit.ly/2ZzdfO0
- Samarasinghe D (2010) Congenital heart disease: When to act and what to do?. Sri Lanka Journal of Child Health 39: 39-43. Link: https://bit.ly/2Woirm8
- Redlin M, Kukucka M, Boettcher W, Schoenfeld H, Huebler M, et al. (2013) Blood transfusion determines postoperative morbidity in pediatric cardiac surgery applying a comprehensive blood-sparing approach. J Thorac Cardiovasc Surg 146(3): 537-542. Link: https://bit.ly/2WoZLmc
- Şahutoğlu C, Yaşar A, Kocabaş S, Zekiye Aşkar F, Fatih Ayık M, et al. (2018) Correlation between serum lactate levels and outcome in pediatric patients undergoing congenital heart surgery. Turk Gogus Kalp Damar Cerrahisi Derg 26: 375-385. Link: https://bit.ly/32ly2qa
- Zhang Y, Zhang X, Wang Y, Shi J, Yuan S, et al. (2019) Efficacy and Safety of Tranexamic Acid in Pediatric Patients Undergoing Cardiac Surgery: A SingleCenter Experience. Front Pediatr. 7;7: 181. Link: https://bit.ly/2ZzdfO0
- Awad H, El-Safty I, Abdel-Gawad M, El-Said S (2003) Glomerular and tubular dysfunction in children with congenital cyanotic heart disease: effect of palliative surgery. Am J Med Sci. 325(3): 110-114. Link: https://bit.ly/30g92xZ
- Morgan C, Al-Aklabi M, Guerra GG (2015) Chronic kidney disease in congenital heart disease patients: a narrative review of evidence. Can J Kidney Health Dis 2: 27. Link: https://bit.ly/3dd42zF
- Dittrich S, Kurschat K, Dähnert I, Vogel M, Müller C, et al. (2000) Renal function after cardiopulmonary bypass surgery in cyanotic congenital heart disease. Int J Cardiol 73: 173-179. Link: https://bit.ly/36DUmLU
- Fogo AB (2014) Pediatric Renal Pathology. Pediatric Nephrology, SpringerVerlag Berlin Heidelberg. Link: https://bit.ly/3dcOzQ5
- Goel M, Shome DK, Singh ZN, Bhattacharjee J, Khalil A (2000) Haemostatic changes in children with cyanotic and acyanotic congenital heart disease. Indian Heart J 52: 559-563. Link: https://bit.ly/2zG4FTF
- Horigome H, Hiramatsu Y, Shigeta O, Nagasawa T, Matsui A (2002) Overproduction of Platelet Microparticles in Cyanotic Congenital Heart Disease With Polycythemia. J Am Coll Cardiol 39: 1072-1077. Link: https://bit.ly/30grqGV
- Murni IK, Djer MM, Yanuarso PB, Putra ST, Advani N, et al. (2019) Outcome of pediatric cardiac surgery and predictors of major complication in a developing country. Ann Pediatr Card. 12(1): 38-44. Link: https://bit.ly/36Eznso
- Penna GL, Salgado DR, Japiassú AM (2011) Microcirculatory assessment: a new weapon in the treatment of sepsis? Rev Bras Ter Intensiva 23(3): 352-357. Link: https://bit.ly/2ZU0fmG
- Trzeciak S, McCoy JV, Dellinger P, Arnold RC, Rizzuto M, et al. (2008) Early Increases in Microcirculatory Perfusion During Protocol-Directed Resuscitation are Associated with Reduced Multi-Organ Failure at 24 hours in Patients with Sepsis. Intensive Care Med 34(12): 2210-2217. Link: https://bit.ly/3ezRxho
- Trivelli LA, Ranney HM, Lai HT (1971) Hemoglobin components in patients with diabetes mellitus. N Engl J Med 284: 353-357. Link: https://bit.ly/2McGNti
- Parpart AR, Lorenz PB, Parpart ER, Gregg JR, Chase AM (1947) The osmotic resistance (fragility) of human red cells. J Clin Invest 26: 636-640. Link: https://bit.ly/3eG24rv
- Kronig R (1926) On the theory of the dispersion of x-rays. J Opt Soc Am 12: 547-557. Link: https://bit.ly/2C6kV1D
- Rabbat C, Treleaven D, Russell J, Ludwin D, Cook D (2003) Prognostic value of myocardial perfusion studies in patients with end-stage renal disease assessed for kidney or kidney-pancreas transplantation: a meta-analysis. J Am Soc Nephrol 14(2):431-439. Link: https://bit.ly/3jajEHd
- Kula B, Zingle N (2009) Optimizing Circuit Design Using a Remotemounted Perfusion System. J Extra Corpor Technol 41(1): 28-31. Link: https://bit.ly/2WmY3BI
- Shin’oka T, Shum-Tim D, Jonas RA, Lidov HG, Laussen PC, et al. (1996) Higher hematocrit improves cerebral outcome after deep hypothermic circulatory arrest. J Thorac Cardiovasc Surg 112: 1610-1621:Link: https://bit.ly/2B5oHHs
- Duncana BW, Meea RB, Mesia CI, Qureshi A, Rosenthal GL, et al. (2003) Results of the double switch operation for congenitally corrected transposition of the great arteries. Eur J Cardiothorac Surg 24: 11-20. Link: https://bit.ly/2XyqDzK
- Chang AC, McKenzie ED (2005) Mechanical cardiopulmonary support in children and young adults: extracorporeal membrane oxygenation, ventricular assist devices, and long-term support devices. Pediatr Cardiol 26: 2-28. Link: https://bit.ly/2ZuVorA
- Durandy YD, Younes M, Mahut B (2008) Pediatric Warm Open Heart Surgery and Prolonged Cross-Clamp Time. Ann Thorac Surg 86: 1941-1947. Link: https://bit.ly/32qjEgq
- Bishnoi AK, Garg P, Patel K, Solanki P, Surti J, et al. (2017) Effect of Prime Blood Storage Duration on Clinical Outcome After Pediatric Cardiac Surgery. World J Pediatr Congenit Heart Surg 8: 166-1673. Link: https://bit.ly/3chnjON
- Mousa SO (2020) Red blood cell transfusion in preterm neonates: a huge debate on tiny patients. Annals of Neonatology Journal 2: 5-13. Link: https://bit.ly/32qjEgq
- Shanmugha Priya RA, Krishnamoorthy R, Panicker VK, NinanB (2018) Transfusion support in preterm neonates <1500 g and/or <32 weeks in a tertiary care center: A descriptive study. Asian J Transfus Sci 12: 34-41. Link: https://bit.ly/2DHHlq9
- Patil V, Shetmahajan M (2014) Massive transfusion and massive transfusion protocol. Indian J Anaesth 58: 590–595. Link: https://bit.ly/3gvEek5
- Goldstein SL (2003) Overview of pediatric renal replacement therpy in acute renal failure. Artif Organs 27: 781-785. Link: https://bit.ly/2TOWXxz
- Luckritz KE, Symons JM (2009) Renal replacement therapy in the ICU. In: Kiessling SG, Goebel J and Somers MJG, editors. Pediatric Nephrology in the ICU. Spain: Springer 115-126.
- Murthy KS, Reddy KP, Nagarajan R, Goutami V, Cherian KM (2010) Management of ventricular septal defect with pulmonary atresia and major aorto pulmonary collateral arteries: Challenges and controversies. Ann Pediatr Cardiol 3(2): 127– 135: Link: https://bit.ly/2CCwe1h
- McElhinney DB, Reddy VM, Hanley FL (1998) Tetralogy of Fallot with Major Aortopulmonary Collaterals: Early Total Repair. Pediatr Cardiol 19(4): 289-296. Link: https://bit.ly/392JmsU
- Como JJ, Dutton RP, Scalea TM, Edelman BB, Hess JR (2004) Blood transfusion rates in the care of acute trauma. Transfusion 44(6): 809813. Link: https://bit.ly/2WkRCz6
- Pouard P (2005) Blood Conservation in Pediatric Cardiac Surgery. TATM 7: 58-62. Link: https://bit.ly/2OtE7ZB
- Strauss RG (2004) Red blood cell transfusion in the neonate, infant, child, and Adolescent. In: Hillyer CD, Strauss RG and Luban NLC. Editors. Handbook of pediatric transfusion medicine. USA: Academic Press, pp. 131-136. Link: https://bit.ly/32o9JYE
- McMullin NR, Holcomb JB, Sondeen J (2006) Hemostatic Resuscitation. In: Vincent JL. (eds) Yearbook of Intensive Care and Emergency Medicine. Yearbook of Intensive Care and Emergency Medicine. Berlin: Springer 265–278.
- Hornykewycz SJ, Odegard KC, Castro R, DiNardo JA (2006) Infant CPB: Effect of Refrigerated Whole Blood Prime on Platelet Count and Fibrinogen. Pediatric Critical Care Medicine 7(6): S28. Link: https://bit.ly/36G2gEw
- Bruley DF (2007) Anticoagulant blood factor deficiencies (protein C). In J. Maguire Duane F. Bruley David K. Harrison (Eds.) Oxygen Transport to Tissue XXVIII David. (Springer Science+Business Media, LLC, 233 Spring Street, New York, NY 10013 USA) 599: 1-6. Link: https://bit.ly/2zGbbKa
- Repine TB, Perkins JG, Kauvar DS, Blackborne L (2006) The use of fresh whole blood in massive transfusion. J Trauma 60(6 Suppl): S59– S69. Link: https://bit.ly/2ZvtQ5x
- Cassella D, Appenzeller G, Stich J (2009) From Donor to Patient in 20 Minutes, Emergency Resuscitation With Whole Blood During Operation Iraqi Freedom. Critical Care Nurse 29(2): 27-32. Link: https://bit.ly/3ezklq0
- Mohr R, Martinowitz U, Lavee J, Amroch D, Ramot B, et al. (1988) The hemostatic effect of transfusing fresh whole blood versus platelet concentrates after cardiac operations. J Thorac Cardiovasc Surg 96(4): 530534. Link: https://bit.ly/2XTSISD
- Duchesne JC, Hunt JP, Wahl G, Marr AB, Wang YZ, et al. (2008) Review of current blood transfusions strategies in a mature level I trauma center: were we wrong for the last 60 years? J Trauma 65(2): 272-278. Link: https://bit.ly/397NJ5N
- Kauvar DS, Holcomb JB, Norris GC, Hess JR (2006) Fresh Whole Blood Transfusion: A Controversial Military Practice. J Trauma 61(1): 181-184. Link: https://bit.ly/38ZSSwI
- Hess JR, Thomas MJ (2003) Blood use in war and disaster: lessons from the past century. Transfusion 43(11): 1622-1633. Link: https://bit.ly/2CEPLhL
- Maclennan S, Murphy MF (2001) Survey of the use of whole blood in current blood transfusion practice. Clin Lab Haematol 23(6): 391396. Link: https://bit.ly/3jaf0cz
- Mabry RL, Holcomb JB, Baker AM, Cloonan CC, Uhorchak JM, et al. (2000) United States Army rangers in Somalia: an analysis of combat casualties on an urban battlefield. J Trauma 49(3): 515-528. Link: https://bit.ly/2Zyqw9S
- Grosso SM, Keenan JO (2000) Whole Blood Transfusion for Exsanguinating Coagulopathy in a U.S. Field Surgical Hospital in Postwar Kosovo. J Trauma 49(1): 145-148. Link: https://bit.ly/30ffrJJ
- Hardy J, Moerloose P, Samama M (2004) Massive transfusion and coagulopathy: pathophysiology and implications for clinical management. Can J Anesth 51(4): 293–310. Link: https://bit.ly/30jG2W4
- Matthew J (2005) the effect of intermittent hypoxic exposure on haemorheology of Elite meddle distance runners. Mater thesis, school of physiotherapy and exercise science, Griffith University, Australia. Link: https://bit.ly/2Wq9RTU
- Katayama Y, Horigome H, Murakami T, Takahashi-Igari M, Miyata D, et al. (2006) Evaluation of blood rheology in patients with cyanotic congenital heart disease using a microchannel array flow analyzer. Clin Hemorheol Microcirc. 35(4): 499-508. Link: https://bit.ly/36M53ft
- Kameneva M, Ündar A, Antaki J, Watach MJ, Calhoon JH, et al. (1999) Decrease in red blood cell deformability due to effects of hypo-thermia, hemodilution, and mechanical stress: factors related to cardiopulmonary bypass. ASAIO J 45(4): 307–310. Link: https://bit.ly/36E0ZxX
- Tsukihara T, Aoyama H (2000) Membrane protein assemblies - towards atomic resolution analysis. Curr Opin Struct Biol 10(2): 208-212. Link: https://bit.ly/2Wq9sRo
- Peters LL, Shivdasani RA, Liu SC, Hanspal M, John KM, et al. (1996) Anion exchanger 1 (band 3) is required to prevent erythrocyte membrane surface loss but not to form the membrane skeleton. Cell 86(6): 917-927. Link: https://bit.ly/2C6jBvH
- Becker V, Schilling M, Bachmann J, Baumann U, Raue A, et al. (2010) Covering a broad dynamic range: information processing at the erythropoietin receptor. Science (New York, N.Y.) 328: 1404-1408. Link: https://bit.ly/393vKNG
- Martin OC, Pagano RE (1987) Transbilayer movement of fluorescent analogs of phosphatidylserine and phosphatidylethanolamine at the plasma membrane of cultured cells. Evidence for a protein-mediated and ATP-dependent process (es). J Biol Chem. 262(12): 5890-5898. Link: https://bit.ly/3d8TeCq
- Khalid S, Baaden M (2010) Molecular dynamics studies of outer membrane proteins: a story of barrels. In: Sansom M and Biggin P editors. Molecular simulations and biomembranes from biophysics to function. Royal Society of Chemistry 225-247. Link: https://bit.ly/2ZzbGQ8
- Kessel A, Ben-Tal N (2010) introduction to proteins: structure, function and motion. Membrane proteins. 2 nd edition, CRC Press Taylor & Francis Group pp. 415-437.
- Yu G, Bolon M, Laird DW, Tyml K (2010) Hypoxia and reoxygenation-induced oxidant production increase in microvascular endothelial cells depends on connexin40. Free Radic Biol Med 49: 1008-1013. Link: https://bit.ly/38ZSAG8
- Cooper GM (2000) The cell. A Molecular Approach, 2 nd edition. Sunderland (MA):Sinauer Associates. Link: https://bit.ly/3ddO5sF
- Heerklotz H, Seelig J (2007) Leakage and lysis of lipid membranes induced by the lipopeptide surfactin. Eur Biophys J. 36(4-5): 305–314. Link: https://bit.ly/38ZoFha
- Dathe M, Wieprecht T (1999) Structural features of helical antimicrobial peptides: their potential to modulate activity on model membranes and biological cells. Biochim Biophys Acta 1462: 71-87. Link: https://bit.ly/30hLAQW
- Jeong Y. (2011) Introduction to Bioelectricity. In: Yoo HJ., van Hoof C. (eds) Bio-Medical CMOS ICs. Integrated Circuits and Systems. Springer, Boston, MA. Link: https://bit.ly/2ZxMdGY
- Ali FM, Mohamed WS, Mohamed MR (2003) Effect of 50 Hz, 0.2 mT Magnetic Fields on RBC Properties and Heart Functions of Albino Rats. Bioelectromagnetics 24: 535-545. Link: https://bit.ly/3j8zr9B
- Hamilton G, Mathura R, Allsop J, Forton DM, Dhanjal NS, et al. (2003) Changes in brain intracellular pH and membrane phospholipids on oxygen therapy in hypoxic patients with chronic obstructive pulmonary disease. Metab Brain Dis 18(1): 95-109. Link: https://bit.ly/3evpUG5
- Maureen C, Jane M (2007) Calcium-independent phospholipase A2-catalyzed plasmalogen hydrolysis in hypoxic human coronary artery endothelial cells. Am J Physiol Cell Physiol 292: C251-C258. Link: https://bit.ly/3j6OxfO
- Helszer Z, Jozwiak Z, Leyko W (1980) Osmotic fragility and lipid peroxidation of irradiated erythrocytes in the presence of radioprotectors. Experientia 36: 521-524. Link: https://bit.ly/30f6OPt
- Vuong PN, Berry C (2005) The pathology of vessels. Capter I histology of vessels. France: Springer, pp. 1-25. Link: https://bit.ly/2CfUJBB
- Galvis MM, Bhakta R, Mendez MD (2020) Cyanotic heart disease. StatPearls Publishing. Link: https://bit.ly/2M7AvLw
- Kleinman CS, Seri I (2012) Hemodynamics and Cardiology: Neonatology Questions and Controversies. 2nd Edition USA: Elsevier, pp. 3-12.
- Schwartz LI, Ing RJ, Twite MD (2014) Anesthetic Considerations for Children with Congenital Heart Disease Undergoing Non-cardiac Surgery. In: Da Cruz E., Ivy D., Jaggers J. (eds) Pediatric and Congenital Cardiology, Cardiac Surgery and Intensive Care. Springer, London 743-757. Link: https:// bit.ly/2TP2TXo

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



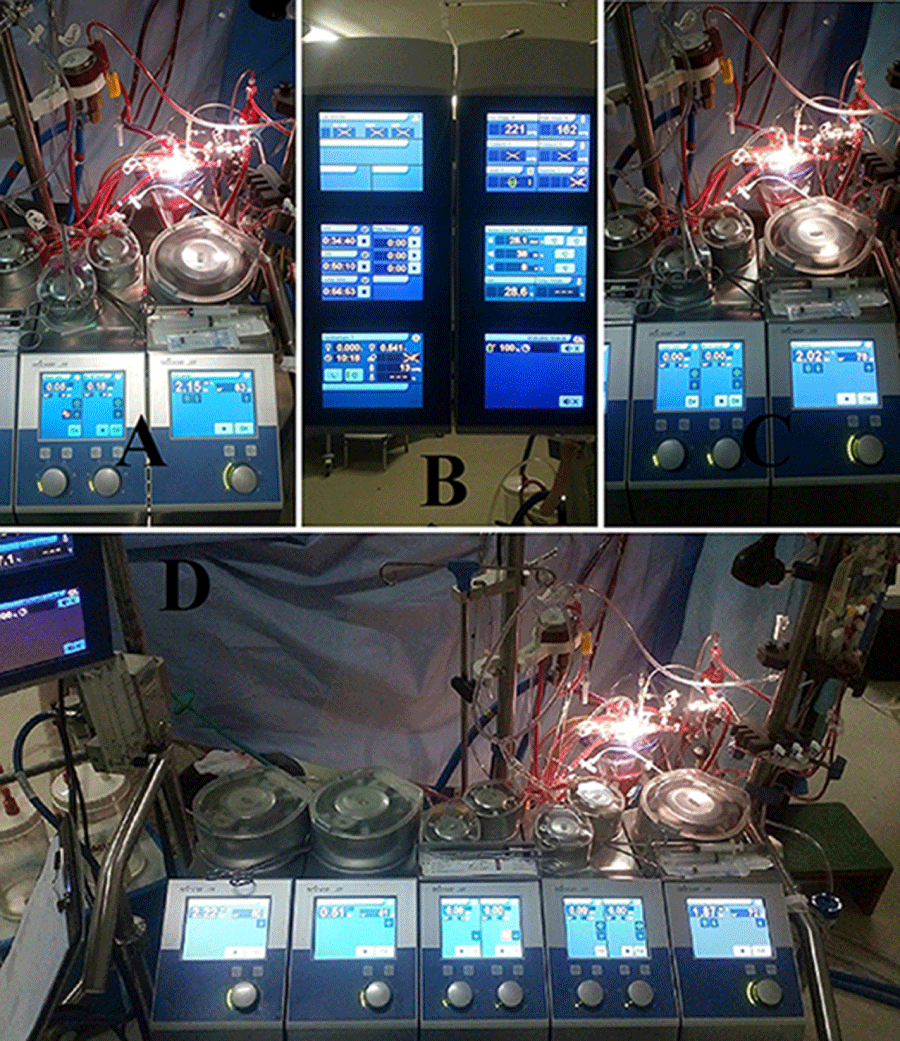


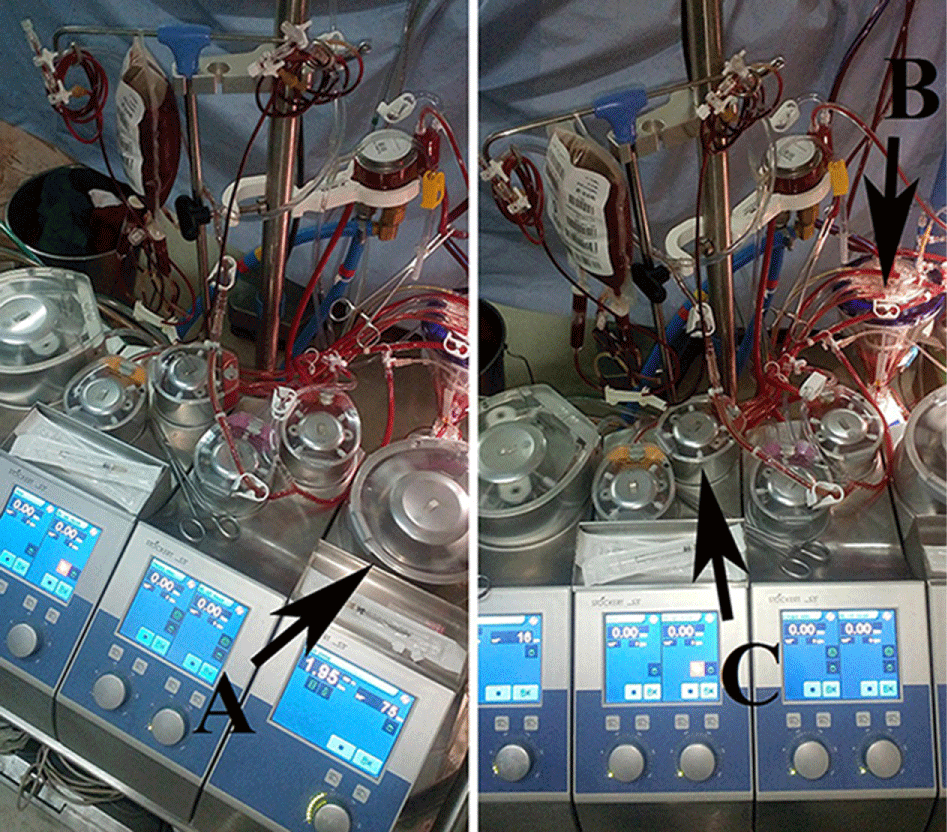




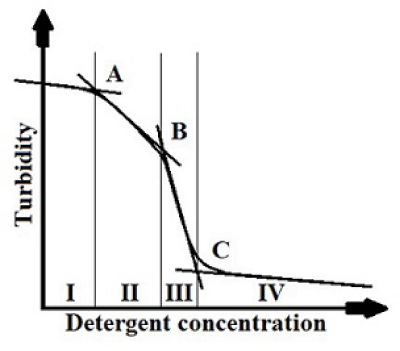
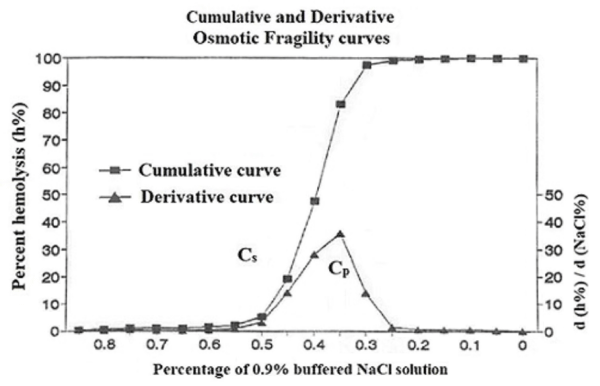
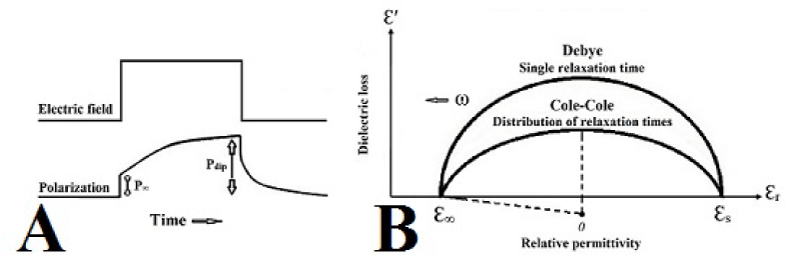
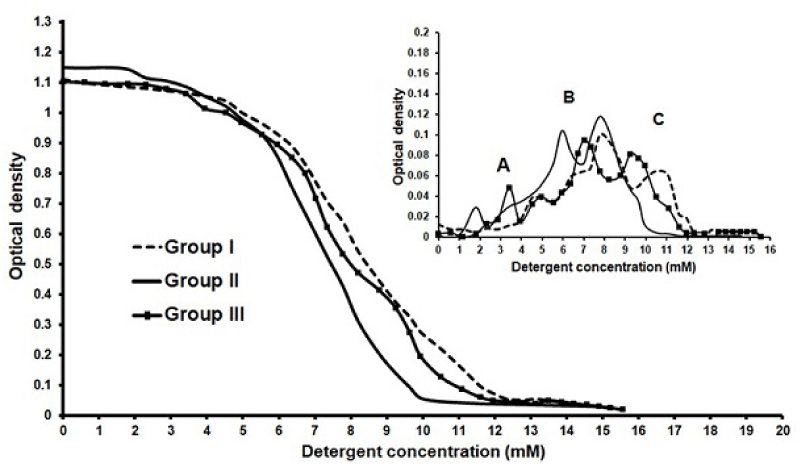
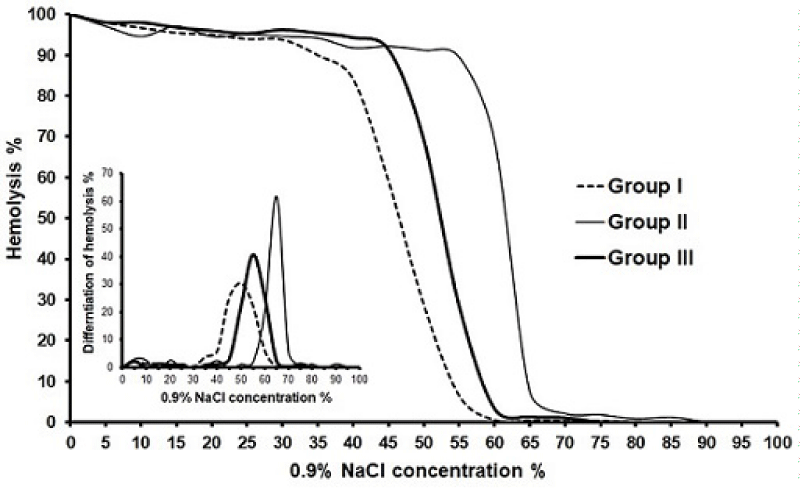
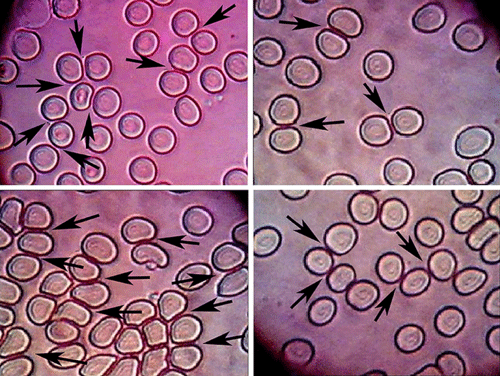
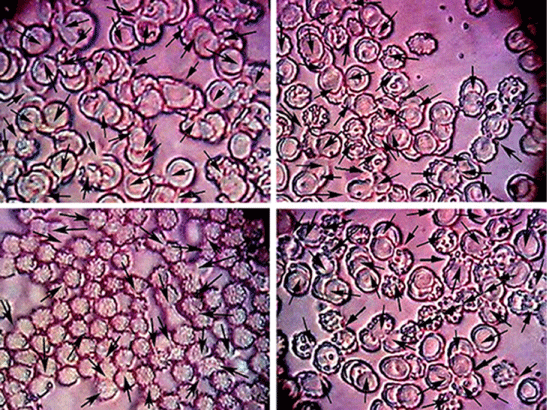

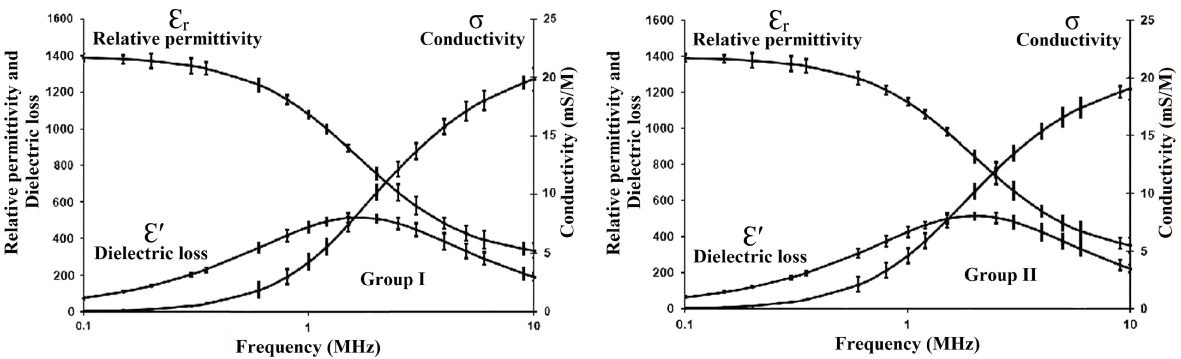

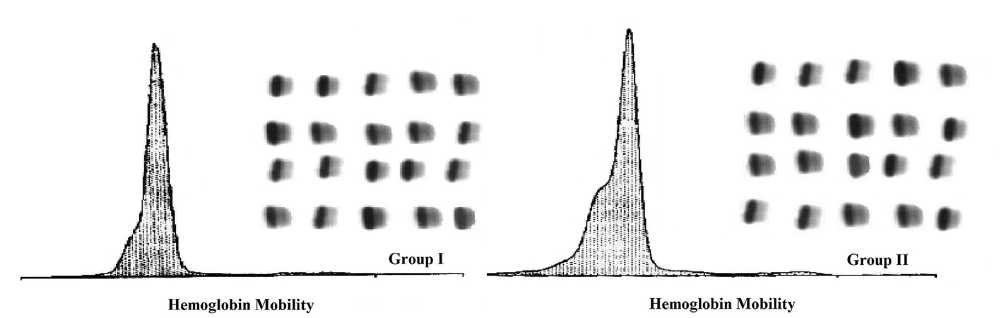

 Save to Mendeley
Save to Mendeley
