Journal of Cardiovascular Medicine and Cardiology
The New Carotid Braided Stent CASPERTM RX. Single center experience in 50 cases
Cabral de Andrade G1,2*, Lesczynski A1,2, Clímaco VM1 and Pereira ER1
2Parana Hospital, Maringá, Brazil
Cite this as
Cabral de Andrade G, Lesczynski A, Clímaco VM, Pereira ER (2020) The New Carotid Braided Stent CASPERTM RX. Single center experience in 50 casesc. J Cardiovasc Med Cardiol 7(2): 129-135. DOI: 10.17352/2455-2976.000127Purpose: Carotid stenosis, as well as dissections and pseudo dissecting aneurysms are etiological factors of Ischemic stroke. A new braided double-layer Nickel titanium Stent CASPERTMRX has super elasticity, shape-memory properties, combined with re-sheathable and repositionable, improving placement accuracy in a closed cell with flow diversion capability. To evaluate in clinical implant behavicor of in many different pathologies.
Materials and methods: It was implanted 53 CASPER-RX stents in 50 lesions (average age of 67 years; 29 men and 21 women) in different pathologies, high-grade symptomatic internal carotid artery stenosis in 43(86%) patients; and dissection in 7(4%) being: 4 dissecting pseudo aneurysms, 2 sub endothelial spontaneous dissections and 1 sub endothelial iatrogenic dissection. In all patients we used dual antiplatelet therapy, before treatment.
Results: Technical success achieved in all patients and complication rate (4%) and a 0% rate of neurological complications at 30 days. No stroke minor or restenosis after 6 months ultrasound or CT Scan examination FU.
Conclusion: CASPER™RX Stent conforms to tortuous anatomy, good wall apposition and a good conformation in tapered ICA-CCA segments in re sheathable delivery system. The very closed cells have a good result as a flow diverter. Technical success was achieved in all patients without clinical complications. However, we need long-term follow up to better assess the efficiency of this new device.
Introduction
The amount of devices aimed to the treatment of the atherosclerotic disease of the carotid artery and other symptomatic lesions as dissections and pseudo aneurysms. This great variety of products makes it difficult to generalize what the best treatment alternative is, because none of them has all the ideal characteristics to treat all kinds of lesions and patients.
All the self-expandable carotid stents are composed by Nickel titanium metal alloy or stainless steel (cobalt). In general, Nickel titanium stents are built with laser cut. Once they are placed in the organism, these stents have thermal memory and adapt themselves to the arterial wall according to its pre-defined form. The only stainless steel available is the Carotid WallStentTM (Boston Scientific, Natick, MA, USA) of closed cells, composed by only one cobalt alloy inside a tubular structure, with 1.08mm of free area from the cells.
During the analysis of the practical factors used on the choice of the stent, Bosiers, et al. [1], conclude that the most important is to minimize the risk of thromboembolic events during its implant. It is directly related to the free cell area, where in the closed cell reduces the risk of thromboembolism during and after its implantation. It happens because of the better retention of the plaque or clot between the mesh and the arterial wall, as well as reduced predisposition of plaque or clot protrusion through the mesh to the interior of the artery, especially in tortuous arteries. However, the analysis of the available data does not define a clear advantage of these two types of devices, of open or closed cells. Nevertheless, there are tendencies that support recommendations of certain stents for specific morphological injury [2]. The ideal stent may cover safely the plate and have good adjustment on the arterial wall. In order to do so, it must have the right balance between flexibility, burst pressure and radial strength [3].
Method
The study was conducted from 2015 to 2018, with prospective data collection, in a singular center, with individual interventional neuroradiologist, in 50 patients (average age of 67 yo; 29 male and 21 female) which included symptomatic atherosclerotic stenosis in 42 patients (average of 80%) and dissection with 4 pseudo aneurysm, 3 dissections (2 spontaneous and 1 iatrogenic) all evaluated by ultrasound, MRI angiography or angio scan. In all cases was used closed cell stent CASPER-RX. A total of 53 stents implanted [7×30(12), 7×40(1), 8×25(4), 8×30(12), 8×40(21), 9×30(3)], using multiple access guiding catheters, all 7F, as well as various kind of cerebral protection filters, micro-guides and angioplasty balloons, compatible with Stent (Table 1).
Casper-RX Stent
CASPER-RX stent (Microinvention, California, Tustin, USA) has a unique design, made with braided Nickel titanium wires with double layer developed to sustain the embolic prevention. It prevents thrombus fragments from being detached and carried by blood flow with fragments trapped between the stent layers. Structure made of closed cell reaching a ~ 375-500 µm cell size with a flexible braid allowing good accommodation in the arterial wall. The free cell size is compatible with about 1/4 of the closed cell stent, which has the lowest free area (WallStent). It can be re-sheathable and repositioned (the implantation of up to 50% is recommended). It has a low profile, which allows a better crossability in case it is necessary to surpass the stent or the use of a second stent. In addition, an expandable stent settles according to the vessel diameter, and can take the conical shape (with a maximum range of 3.5mm). Delivery system: 5F, minimum guiding catheter 7-F, 143 cm of length, fast switch, 0.014” guide. It also has the ability of redirecting the flow.
Data collection
The collected data included different types of lesions in carotid artery, the endovascular treatment performed, immediate results and the clinical follow-up at 30 days and three and six months imaging follow-up. The collected data referring to the patients regarded sex, age, laterality and type of lesion, immediate complications and follow-up. Referring to the treatment, the collection aimed at the technique used, with or without the cerebral protection filter, the pre or post dilatation with the angioplasty balloon and the size and quantity of implanted stents. In addition, complications and clinical follow-up at the 30th day and with the control tests (USG or Angio Scanner) which included ischemic event, morbimortality, relapse or necessity of re-treatment. The treatment included only one type of braided wire stent, closed cells and with the possibility of repositioning, of varied sizes according to the arterial diameter, with numerous other compatible materials like cerebral protection filter, micro-guide and angioplasty balloon.
Treatment indication
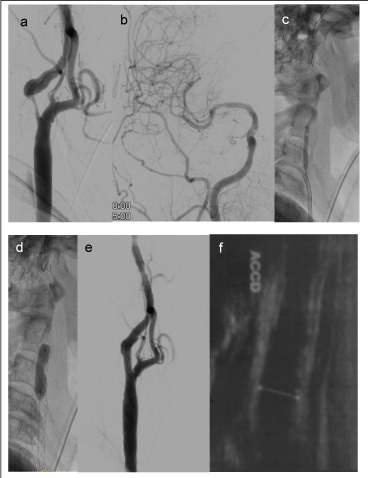
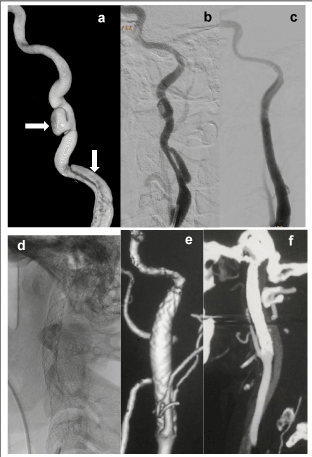
Aiming the evaluation of the CASPER-RX stent use in different pathologies of the extra cranial internal carotid artery, patients with atheromatous disease with stenosis between > 70% and > 90%, and all with ischemic symptoms even the use of antiplatelet drugs, were previously evaluated with ultrasound, angio MRI or angio tomography and lesions confirmed by angiography. As patients with acute and sub-acute disease with spontaneous and sub occlusive iatrogenic dissection and pseudo aneurysms, also with ischemic symptoms. Histories of brief cases and radiographic images in a case of severe and symptomatic stenosis > 90%, symptomatic sub occlusive dissection and sintomatic pseudo aneurysm associated with dissecting shown in Figures 1 (a,b,c,d,e and f), 2(a,b,c,d,e and f) and 3(a,b,c,d and e) respectively.
Endovascular treatment
The procedure was performed under local anesthesia with sedation or general anesthesia. All patients had previous use of dual antiplatelet therapy with clopidogrel 75mg/day and aspirin 200mg/day. In 05 of the cases (in sub occlusive atheromatous disease with stenosis between >80 and ≥90% required pre coronary balloon dilation (cases nº3, nº11, nº12, nº19 and nº29) (Table 1) (Figure 1c). It was used cerebral protection system in all cases of atheromatous disease, in cases of dissection and pseudo aneurysm, only micro-guide 0.014 was used. In one dissection case (case nº7) where there was the presence of free intraluminal clot, it is used a cerebral protection system. All patients received 5,000 units of heparin during the procedure and atropine (0.5 mg) prior to stent implantation. Patients continued with dual antiplatelet therapy after the procedure.
Results
All the stents, a total of 53 and different measures [7×30(12), 7×40(1), 8×25(4), 8×30(12), 8×40(21), 9×30(3)] (Table 1), were satisfactorily implanted, without any difficulties with the navigation and delivery systems, even with the use of telescoped stents. The rate of complications was 4%, a case with a small area dissected of just detachment to the amount of stent, without clinical manifestations and it was opted to put a second telescoped stent to avoid the risk of dissection (case nº9) and femoral hematoma in another case. In two other cases, the use of more than one stent was necessary, in a telescoped way, due to the presence of multiple lesions. Two pseudo aneurysms (case nº8), and a pseudo aneurysm associated with extensive dissection (case nº13) (Figure 3 (a,b,c,d and e)), there were no difficulties to navigate to the implant system through the first implanted stent, as well as the release of a second stent telescoped smoothly. All the patients had a favorable clinical evolution, no thromboembolic event at the clinical and imaging (ultrasound and/or angiography carotid tomography) follow-up that showed patency of the stents in all cases, as well as the absence of re-stenosis, occlusion of pseudo aneurysms and complete recanalization in cases of dissection.
Study limitation
It is a retrospective non-randomized analysis. Even though one could argue that device selection was biased, in all patients there was symptomatic ischemic lesion previously stenting treatment.
Discussion
The nature of most of the neurological events in patients with carotid artery stenosis is not related to the brain hyper perfusion, but have an embolic origin [4].
As demonstrated in the randomized multi-centric prospective trials, carotid endarterectomy (CEA), with the removal of plaque and the source of emboli, it is the gold standard for reducing stroke in symptomatic and asymptomatic patients with significant carotid stenosis (NASCET) [4]. However, carotid artery angioplasty and use of stent has become an alternative to endarcterectomy [5]. In contrast to surgical therapy, 50% to 66% of stroke after CAS occurs within the first 4 weeks, which can be attributed to embolization of thrombus or plaque through the structure of the stent [6].
Arterial dissections can be spontaneous or traumatic/iatrogenic, corresponding to 25% of ischemic strokes in young people up to 45 years old, with favorable outcome in 75% of patients, but with a mortality of about 4%7. Clinical treatment with antiplatelet therapy is still the treatment of choice for these lesions, according to “Cervical Artery Dissection in Stroke Study” (CADISS) [8]. The selection criteria of the candidates for stent therapy include: 1) clinical failure of medical therapy (presence of recurrent TIA, fluctuating neurological signs or neurological deterioration); 2) impending stroke attributable to significant stenosis/occlusion with poor collateral circulation as well as failure of the circle of Willis and decreased perfusion in parenchymal capillary angiographic phase, CT, or MR imaging studies; 3) contraindication for anticoagulation because of intracranial or systemic bleeding; 4) evidence of symptomatic thromboembolic occlusion of cerebral vessels; 5) contralateral CA stenosis/occlusion; 6) need of elective occlusion of the ICA contralateral to another disease; and 7) the need to avoid increasing flow through the anterior communicating artery because of an associated aneurysm [9,10]. In case of sintomatic dissection and arterial subocclusion case stent success rate of 99% and the procedural complication rate was 1.3%, being a safe and effective method in the treatment of these lesions in selected cases, demonstrating viability of this paradigm [11,12].
The pseudo aneurysm of the carotid artery has as the most common causes the atherosclerotic degeneration and congenital connective tissue diseases such as fibromuscular dysplasia or Bechet’s disease or iatrogenic. They can also result from trauma, cerebral vascular dissection or may be iatrogenic as a complication of carotid or cerebral angiography, may be recommended because of relevant thromboembolism risk, and treatment with stenting, especially in distal lesions of pre cerebral internal carotid artery [13,14]. Among the variety of available stents, recent studies have emphasized the superiority of the coated stents for the treatment of large pseudo aneurysms while stent implantation is recommended for small defects [15].
Many features must be taken into consideration in choosing the stent according to the pathology and the patient’s anatomy. The scaffolding properties of the stent (defined as the amount of support given to the vessel wall by stents) are of great importance in order to minimize the risk of embolisms, as well as the free cell area from the results of BIC register [16]. We have learned that stents with this profile are better at retaining material behind the support, which results in significant differences in event rates compared to open cell stents.
The open cell and closed cell stents have been evaluated in several publications with the intention to relate its design with immediate results and long-term treatment of occlusive atherosclerotic disease of the carotid artery. Many conclude that in most cases treated with closed cell design stent there was significantly fewer ischemic stroke and death after 30 days, compared to those treated with stents designed with open cells [17]. In multicenter evaluation, retrospective, nonrandomized, with large group (cohort) of patients and use of multiple closed and open cell stents, was observed a higher rate of ischemic complications after the procedure, within 30 days, with open cell stents, showing a rate of 3.4% against 1.3% in closed cell stents [16].
Another characteristic of the stent is its flexibility, which is reflected in its ability to adapt to vessel tortuosity during its use. In terms of flexibility, closed cell stents, both Nickel titanium and stainless steel do not perform as well as their homologues open cells. Another feature is the ability to adapt to conic anatomy of this region of the carotid artery (vessel wall adaptability). Therefore, the ability of supporting/scaffolding of the current generation of devices is compromised by insufficient flexibility. The improvement in stent design should focus primarily on the combination of scaffolding properties and flexibility [1].
The flexibility and pliability of the stents are related to its configuration, an open cell stent allows greater discovered gaps and is more pliable, making it more suitable for tortuous arteries, where reducing the need for associated handling may reduce the embolic potential and folds (kinking). On the other hand, a larger free cell area allows greater exposure of the plaque, possibly increasing the embolic potential. Closed cell stents provide greater plaque coverage, but are more rigid and require more vigorous manipulation for use, which can also increase the risk of embolic complications [18,19]. A prospective randomized study found no significant difference in the restricted diffusion lesions in MRI when comparing open and closed cell models [20].
The experimental results showed some similarities with the WallStent carotid stent, with relatively low radial force (0,011 N / mm CASPER RX and 0.020 N / mm Wall stent carotid), a very high pressure collapse, bending stiffness almost similar. However, in contrast with the WallStent carotid, adaptation to the arterial wall and curved as in model is better with Casper RX [21].
Another interesting feature of this new device is its flow redirection capability when used in pseudo aneurysms pre cerebral segment of the carotid arteries. Its double braided Nickel titanium layer creates an intraluminal flow bypass, reducing the flow into the aneurysmal sac, and accelerating the formation of thrombus, while maintaining the normal blood flow, in addition, the telescoped stenting coaxial manner causes an increase in the mesh density and better flow redirection with higher embolic protection [22].
In this initial experience, with the medium term follow-up of the patients involved, the use of this new device, the RX CASPER stent, none of them had problems regarding independent navigability of the aortic arch type 1 to 3 or ICA tortuous or not. Other characteristics of the device were observed in this series, as its flexibility, good accommodation according to the vascular anatomy with good navigability. It does not have a high radial strength, however it was sufficient without the need of angioplasty in dissection or pseudo aneurysm and in cases of atheromatous disease, it behaved with good accommodation in the arterial wall after angioplasty. Its crossability also proved to be satisfactory in cases where there was a need of the telescoped stent. Its function as a redirector device flow also proved efficient in cases of pseudo aneurysm occlusion immediately after implantation of the stent, similar to devices diverters of flow used in intracranial aneurysms. Surveillance ultrasound and CT scans documented good wall apposition in all patients and 0% rate of neurological complications at 30 days. In this series we try to observe the behavior of the device with respect to its navigability, risk of intr procedural complications and medium term follow up. There was no kind of early or late thromboembolic complications related to the use of the device.
Conclusion
The CASPER RX stent has the design with double layer of braided Nickel titanium wire with a lower free area of closed cells with re sheathable system. It appears to be safe and effective, with good navigability and enough radial strength with good accommodation in the arterial wall, to treat extra cranial stenosis of the ICA, as well as sub occlusive dissecting symptomatic or high risk lesions, including high lesions in the petrous segment but also in the treatment of the pseudo aneurysm with possibility of immediate occlusion by characteristics for flow redirection. In this series, cases of atheromatous disease it was used cerebral embolic protection device, except in cases of dissection or pseudo aneurysm, however the need for an embolic protection device with this stent should be investigated. We had early or late thromboembolic complications in this series. However, a comparative study with other closed cell devices is necessary evaluate the long term results of this stent.
Ethical standards and patient consent
We declare that all human and animal studies have been approved by the [insert appropriate ethics committee] and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study.
- Bosiers M, Deloose K, Verbist J, Peeters P (2005) Carotid artery stenting: which stent for which lesion? Vascular 13: 205-210. Link: https://bit.ly/2yR9SaR
- Nikas DN, Kompara G, Reimers B (2011) Carotid stents: which is the best option? J Cardiovasc Surg (Torino) 52: 779-793. Link: https://bit.ly/35Tk9iO
- Müller-Hülsbeck S, Schäfer PJ, Charalambous N, Schaffner SR, Heller M, et al. (2009) Comparison of carotid stents: an in-vitro experiment focusing on stent design. J Endovasc Ther 16: 168-177. Link: https://bit.ly/3buaY9A
- North American Symptomatic Carotid Endarterectomy Trial (NASCET) Collaborators. Barnett HJM, Taylor DW, Haynes RB, Sackett DL, et al. (1991) Beneficial effect of carotid endarterec-tomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 325: 445-453.Link: https://bit.ly/2yPSSBG
- Cremonesi A, Castriota F, Secco GG, Macdonald S, Roffi M (2015) Carotid artery stenting: an update. Eur Heart J. 36: 13-21. Link: https://bit.ly/3cES15q
- Piorkowski M, Kläffling C, Botsios S, Zerweck C, Scheinert S, et al. (2015) Postinterventional microembolism signals detected by transcranial Doppler ultrasound after carotid artery stenting. Vasa 44: 49-57. Link: https://bit.ly/2SViHav
- Touzé E, Gauvrit JY, Meder JF, Mas JL (2005) Prognosis of cervical artery dissection. Front Neurol Neurosci 20: 129-139. Link: https://bit.ly/2TgT8B1
- Kennedy F, Lanfranconi S, Hicks C, Reid J, Gompertz P, et al. (2012) Antiplatelets vs anticoagulation for dissection: CADISS nonrandomized arm andmeta-analysis. Neurology 79: 686-689.Link: https://bit.ly/2WsxJ9W
- Donas KP, Mayer D, Guber I, Baumgartner R, Genoni M, et al. (2008) Endovascular repair of extracranial carotid artery dissection: current status and level of evidence. J Vasc Interv Radiol 19: 1693-1698. Link: https://bit.ly/2zw1D3W
- Biondi A, Katz JM, Vallabh J, Segal AZ, Gobin YP (2005) Progressive symptomatic carotid dissection treated with multiple stents. Stroke 36: e80-e82. Link: https://bit.ly/2Ah3wlk
- Ohta H, Natarajan SK, Hauck EF, Khalessi AA, Siddiqui AH, et al. (2011) Endovascular stent therapy for extracranial and intracranial carotid artery dissection: single-center experience. J Neurosurg 115: 91-100. Link: https://bit.ly/3fJotFY
- Pham MH, Rahme RJ, Arnaout O, Hurley MC, Bernstein RA, et al. (2011) Endovascular stenting of extracranial carotid and vertebral artery dissections: a systematic review of the literature. Neurosurgery. 68: 856-866. Link: https://bit.ly/2YXr1KA
- Li Z, Chang G, Yao C, Guo L, Liu Y, et al. (2011) Endovascular stenting of extracranial carotid artery aneurysm: a systematic review. Eur J Vasc Endovasc Surg 42: 419-426. Link: https://bit.ly/2zBrG9L
- Maras D, Lioupis C, Magoufis G, Tsamopoulos N, Moulakakis K, et al. (2006) Covered stent-graft treatment of traumatic internal carotid artery pseudoaneurysms: a review. Cardiovasc Intervent Radiol 29: 958-968. Link: https://bit.ly/3fJmoJY
- Welleweerd JC, Jan de Borst G, de Groot D, van Herwaarden JA, Lo RT, et al. (2015) Bare metal stents for treatment of extracranial internal carotid artery aneurysms: long term results. J Endovasc Ther 22: 130-134. Link: https://bit.ly/3dDBEq0
- Bosiers M, De Donato G, Deloose K, Verbist J, Peeters P, et al. (2007) Does free cell area influence the outcome in carotid artery stenting? Eur J Vasc Endovasc Surg 33: 135-141.Link: https://bit.ly/2T0FCBe
- Hart JP, Peeters P, Verbist J, Deloose K, Bosiers M (2006) Do device characteristics impact outcome in carotid artery stenting? J Vasc Surg 44: 725-730. Link: https://bit.ly/3dMq641
- Tadros RO, Spyris CT, Vouyouka AG, Chung C, Krishnan P, et al. (2012) Comparing the embolic potential of open and closed cell stents during carotid angioplasty and stenting. J Vasc Surg 56: 89-95. Link: https://bit.ly/2WQSGtV
- Hopf-Jensen S, Marques L, Preiß M, Müller-Hülsbeck S (2015) Initial Clinical Experience With the Micromesh Roadsaver Carotid Artery Stent for the Treatment of Patients With Symptomatic Carotid Artery Disease. J Endovasc Ther 22: 220-225. Link: https://bit.ly/2T041GV
- Timaran CH, Rosero EB, Higuera A, Ilarraza A, Modrall JG, et al. (2011) Randomized clinical trial of open-cell vs closed-cell stents for carotid stenting and effects of stent design on cerebral embolization. J Vasc Surg 54: 1310-1316. Link: https://bit.ly/3bnz9GJ
- Wissgott C, Schmidt W, Brandt C, Behrens P, AndresenR (2015) Preliminary Clinical Results and Mechanical Behavior of a New Double-Layer Carotid Stent. J Endovasc Ther 22: 634-639. Link: https://bit.ly/2SZzjxS
- Kabbasch C, Bangard C, Liebig T, Majd P, Mpotsaris A, et al. (2016) The Dual Layer Casper Micromesh Stent: Taking Advantage of Flow-Diverting Capabilities for the Treatment of Extracranial Aneurysms and Pseudoaneurysms. Cardiovasc Intervent Radiol 39: 472-476. Link: https://bit.ly/2T0xqRD

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
