Journal of Cardiovascular Medicine and Cardiology
Targeting metalloproteinases in cardiac remodeling
Anestakis Doxakis1*, Konstantinidou Polyanthi1, Tsepa Androniki1, Petanidis Savvas1, Zagelidou Eleni2, Leontari Roubini3 and Raikos Nikolaos1
2Forensic Medical Service of Thessaloniki, Ministry of Justice, Transparency, and Human Rights, Greece
3Forensic Medical Service of Larisa, Ministry of Justice, Transparency, and Human Rights, Greece
Cite this as
Doxakis A, Polyanthi K, Androniki T, Savvas P, Eleni Z, et al. (2019) Targeting metalloproteinases in cardiac remodeling. J Cardiovasc Med Cardiol 6(3): 051-060. DOI: 10.17352/2455-2976.000092During the last years metalloproteinases as a family of proteases have been implicated in various human diseases and catalytic mechanisms of pathological disorders. Metalloproteinases can be divided into two subgroups, matrix metalloproteinases (MMPs) and adamalysins (ADAMs). The MMPs consist of 6 basic subgroups which are inhibited by the tissue inhibitors of metalloproteinase (TIMP), while ADAMs consists of approximately 40 members. It is confirmed that metalloproteinases are an important factor for the cardiac remodeling process, interfering with the alteration of the cardiac structure induced by cardiac injury or increased haemodynamic load. The aim of this review is the analysis of the impact of metalloproteinases on cardiac remodeling and the factors affecting metalloproteinase activity. Metalloproteinases induce the remodeling process of the cardiac tissue in both beneficial and detrimental ways. TIMPs and various administered pharmacological agents may limit the effectiveness of metalloproteinases, therefore interfering in cardiac remodeling.
Ventricular remodeling (or cardiac remodeling)
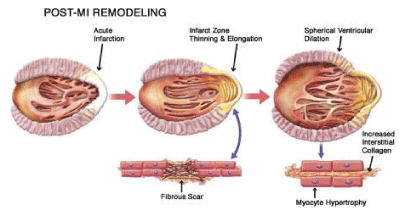
Cardiac remodeling is defined as alterations of the size, shape and function of the cardiac tissue, induced mainly by cardiac lesion. These alterations affect the molecular, cellular and interstitial cardiac structure, as well as the genome expression[1]. Cardiac remodeling is manifested through modifications of the shape of the ventricle chamber. This process can be induced by consecutive trauma of the cardiac tissue or through a direct lesion of the ventricle. Cardiac remodeling is associated with cardiac hypertrophy, dilatation and heart failure [2]. Left ventricular (LV) dilatation during the remodeling process deteriorates LV dysfunction, but the exact mechanisms remain unclear [3]. Myocyte is the cardiac cell that primarily aids the remodeling process [1]. During cardiac remodeling, alterations of the architecture and alignment of the cardiac myocytes are induced, as well as modifications in the structure and activity of the extracellular matrix (ECM) [4]. Relevant mechanisms are ischemia, cell death and apoptosis [1]. Even though many clinical trials and efforts have been conducted for the interpretation of the structural, cellular and molecular procedures and mechanisms that take place during cardiac remodeling, they remain partially unclear [2]. Cardiac remodeling might occur due to: 1) Myocardial infarction (MI), 2) Pressure overload (aortic stenosis and hypertension), 3) Inflammation of the myocardium (Myocarditis), 4) Idiopathic dilated cardiomyopathy or volume overload (valvular regurgitation), 4) Compensatory physiological modifications in cardiac proportions and function on athletes [1]. Even though the origins of these diseases are distinct, they share similar molecular, biochemical and mechanical pathways [1]. The mechanism of pathological cardiac remodeling is established on three basic patterns; concentric, eccentric and post-infarction remodeling. Concentric remodeling is induced by cardiomyocyte’s thickness growth due to pressure overload. Eccentric remodeling is activated by cardiac volume load leading to cardiomyocyte elongation. Post-infarction remodeling process includes pressure and volume load in the non-infarcted area along with cellular-matrix interplay of the cardiac scar [5] (Figure 1).
Clinical procedure of LV remodeling
LV remodeling causes ventricular dilatation and fibrosis and worsens the cardiac function. The severity of the remodeling is considered an indicator of morbidity and mortality for post infarction patients [6]. The process of LV post-infraction remodeling progresses rapidly, most usually within some hours after an episode of myocardial infarction. The time course is affected by the severity of the underlying disease, secondary events, genotype, treatment and other factors [1]. Animal studies have shown that it is possible that infract expansion, regional dilatation and thinning of the infract zone may occur in one day after an MI episode [1]. The exact procedures and cells that take place in cardiac remodeling are not completely clear. It’s proposed that, on a molecular level, the stretched myocytes increase the local norepinephrine activity and the releasement of angiotensin and endothelin. Other factors that are thought to be stimulated are being studied [1]. The alterations caused by cardiac remodeling stimulate the expression of modified proteins and myocyte hypertrophy, leading to deterioration of cardiac function and increased neurohormonal activity. Furthermore, the increased aldosterone and cytokine secretion might stimulate collagen synthesis, causing fibrosis and ECM remodeling [1].
Alterations associated with cardiac remodeling
Alterations of the cell structure and function result to eccentric hypertrophy which increases LV chamber volume, muscle mass and fibrous tissue. The normal elliptical shape of the left ventricle is becoming more spherical (Walsh, 2005). MI causes asymmetric remodeling that is associated with infarct extension, an acute dilatation of the infarction region after a premature ventricular remodeling followed by coronary occlusion. Generally, infarct extension is caused by myocyte death and slippage, dominates the apical region of the left ventricle and induces dramatic modifications in ventricular volume and geometry [7]. Different causative agents, such as arterial hypertension or valvular disease,yet the ventricular hypertrophy remains symmetric. Decompensated cardiac hypertrophy is concentric and is characterized by a thick ventricular wall and interventricular septum, normal internal volume and wall tension as well as high mass to volume ratio. Heart failure is progressive and followed by ventricular cavity enlargement, while the mass to volume ratio is normalized [7]. The initial phase of cardiac remodeling, which leads to restoration of the necrotic area and scar formation, can be considered beneficial at some point. This cellular reorganization of the ventricular wall is associated with cardiac output maintenance or improvement, accompanied with increased LV volume [1]. The magnitude of the alterations induced by cardiac remodeling are related to the infarct size. Large infarcts cause greater dilatation and increases in systolic and diastolic pressure than small ones. In progressive post-infarction dilatation, the end-systolic volume index increases progressively while ejection fraction decreases [1]. Alterations on cellular mass that follow cardiac remodeling include myocyte hypertrophy, necrosis, apoptosis, fibrosis, increased fibrillar collagen and fibroblast proliferation. Circulating or locally secreted angiotensin II is considered a modifying agent in altering gene expression through stimulation of second messenger systems [1].
Cardiac myocytes in cardiac remodeling
Myocytes and other types of cardiac cells are considered to have a fundamental effect in the process of cardiac remodeling. These specific cells have received considerable attention due to their contractile activity and their great amount on the cardiac mass [1]. In case of cardiac injury the number of myocytes is decreased and the functional myocytes are either elongated or hypertrophied due to an initial compensatory process initiated for the maintenance of the systolic volume, after the loss of contractile tissue. In addition, there is an increase of the thickness of the ventricular wall [1]. Alterations in loading conditions lead to stretching of the cell membrane that might initiate the expression of genes related to hypertrophy. If the cardiac myocytes are affected, they may initiate the synthesis of contractile proteins and the formation of sarcomeres [1]. The increased stress of the ventricular wall might worsen the energy imbalance and ischemia, two major factors of the demand for myocardial oxygen. It is believed that this fact might lead to an endless cycle of elevated wall stress and thickness as well as deterioration of the disturbed energy balance and ischemia [1].
Fibroblasts in cardiac remodeling
Fibroblasts are the basal cardiac cells, since they constitute the 70% of the overall rate of cells found in the normal left ventricular. Respectively, the myofibroblasts are the main source of ECM proteins on the cardiac tissue, while they modulate the local immune system [8]. However, fibroblasts can be found within all human tissues and their functions and role is differentiated among them. Atrial fibroblasts display different responses to pathological attacks compared to the fibroblasts of the ventricle. This can be explained from the fact that atrial fibroblasts origin form different cells, depending on the heart development stage and cellular substance [8]. Cardiac injury induces the activation of cardiac fibroblasts and they are differentiated into myofibroblasts via mechanical alterations and inflammatory mediators [8]. During cardiac remodeling, cardiac fibroblasts participate in the process of fibrosis [9]. In the post-MI process, the myofibroblasts stimulate the activation of ECM proteins in order to replace the cells of the infarcted myocardium, induce infarct contraction and produce factors that control the inflammatory and fibrotic responses. Myofibroblasts can communicate directly with the protein components of ECM via stress fibers at their cell-integrin-matrix junction sites and with other cells with the use of N-cadherin-type adherens junctions. These junctions serve the myofibroblasts by increasing their contractility, a basal factor for the structure, as well as the newly formed ECM by supporting it on the infarct scar [8]. Myofibroblasts tend to migrate into the infarcted tissue in post-MI and restore the lost ECM. Stimulating factors include pro-inflammatory cytokines and hormones, derived by inflammatory and preexisting cells and alterations in the mechanical microenvironment. Also, cardiac fibroblasts may obtain and use a partially differentiated phenotype called proto-myofibroblast, as a response to the unbalanced ECM and increased mechanical stress and platelet-derived growth factor (PDGF) levels. As a result, myofibroblasts are able to increase their proliferation and migration degree, produce matrix metalloproteinases (MMPs) and control the expression of cytokines and growth factors, like tumor necrosis factor-α (TNF-a), interleukin (IL)-1, IL-6 and transforming growth factor beta (TGF-β) [8].
Role of collagens
The human myocardium consists of myocytes bound to support tissue network. This network is mainly structured by fibrillar collagen, which is synthesized and degraded by fibroblasts [1]. Myocardial collagenase is considered as an important proenzyme which can be found in the cardiac ventricle, in its inactive form. The activation of this proenzyme after the myocardial injury contributes to the increase of the dimension of the cavity as a response to the dilated pressure. This process is accused for myocyte slippage, a theoretical factor of cavity remodeling [1]. Circulating collagen peptides can be used often as biomarkers of myocardial fibrosis and a predictor of the cardiac function [8].
Apoptosis in cardiac hypertrophy
Apoptosis is a type of cell death known as type II, a well-characterized process. The molecular events that occur and contribute to apoptosis are analyzed and cleared [10]. It is speculated that cardiac remodeling is caused partially from continuous myocyte death. The importance of apoptosis in cardiac remodeling in humans has yet to be fully established, but it has been noted that it occurs in an increased rate after ischemia, reperfusion and myocardial infarction [1]. Apoptosis might be an important regulatory mechanism that contributes to the response mechanism to pressure overload, which is connected to apoptosis with cardiac hypertrophy, for the initial phase. Some factors that might also lead to apoptosis are the function of cytokines (mainly TNF-α and interleukins), the oxidative stress and impairments conducted to mitochondria. It is believed that myocytes might proliferate in an increased rate in the wounded heart [1].
Metalloproteinases
Many pathophysiological and normal procedures that take place in the human body such as embryogenesis, healing and carcinogenicity can be instigated with the contribution of many different types of proteolytic enzymes. These proteolytic enzymes can remodel the extracellular matrix and modify the cell-matrix interactions [11]. Human proteinases consist of four groups, and each of them has a different amino acid molecule connected to their active site [11]. Metalloproteinases are one of the four human proteinases and their key trait is that their active site is enclosed by a Zn2+ ion or less frequently by a Cu2+ ion [11]. The first enzyme of the metalloproteinases family that was recognized is the matrix metalloproteinase 1 or MMP-1 [12]. The metalloproteinases group is classified into many sub-groups, with different primary structure and specialization [11]. The basic sub-groups are the matrix metalloproteinases or MMPs and adamalysins or ADAMs.
Matrix metalloproteinases & classification
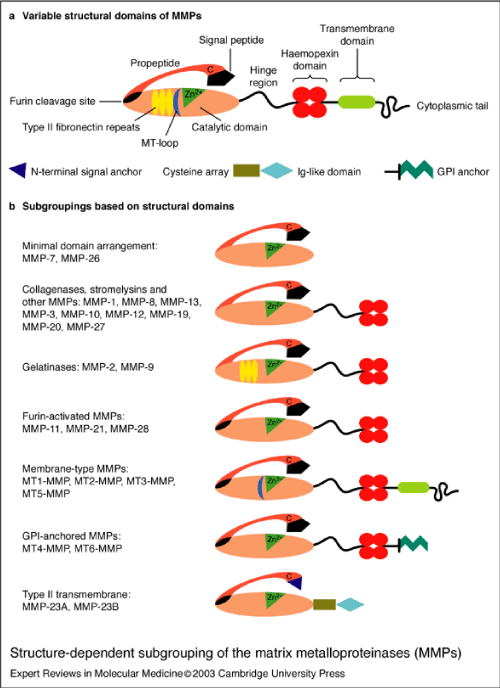
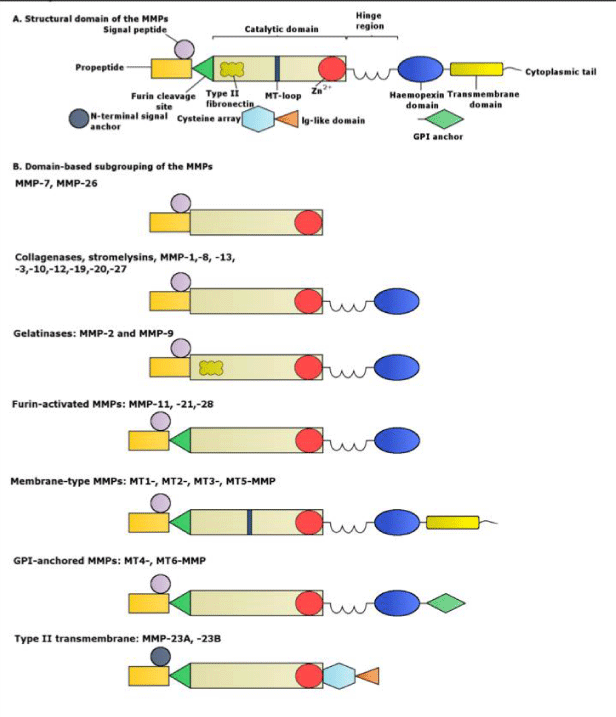
Matrix metalloproteinases or MMPs are proteolytic enzymes [8], or zinc endopeptidases that play a basic role in the ECM degradation and participate in tumor expansion, angiogenesis and cardiac remodeling [2]. MMPs are released mainly in their zymogen form, with the exception of MMP-11 and MMP-28 [8]. The initial form of the MMPs is known as pro-MMPs and their activation can be intracellular or extracellular, through proteolytic cleavage [13]. The MMPs can control the process of cell migration, proliferation and apoptosis, since they regulate the cellular components that take part in these procedures. Specifically, the MMPs are able to digest the ECM and basement membrane proteins, while they are cleaving other MMPs, proteases and inhibitors of proteases, as well as growth factors, growth factor binding proteins (GFBP), chemokines, cytokines, cell surface receptors and cell adhesion molecules [2]. MMP activity is regulated by mechanisms such as oxidative stress and protein kinase B (PKB/AKT) and extracellular-signal-regulated kinase (ERK) pathways [9]. Their effect on ECM proteins is coordinated most of the time but interactions between them or redundancy is a possible observation [14]. MMPs are responsible for various tissues remodeling during physiological processes such as morphogenesis, trophoblast migration and mammary development [4]. MMPs are categorized into five basic sub-groups that have different substrate specificity and localization. These groups are the collagenases, gelatinases, stromelysins, matrilysins, and membrane type MMPs [8].
Role of collagenases
The collagenases group consists of the matrix metalloproteinases MMP-1, MMP-8 and MMP-13. MMP-1 or fibroblast collagenase or collagenase-1 was the first member of the MMPs family that was ever discovered and was named in 1966. Expression of MMP-1 can be activated by myocytes, macrophages and fibroblasts. In addition, MMP-1 is the responsible proteinase for the cleavage of collagen, gelatin, laminin and non-ECM components like complement C1q, interleukin IL-1β and tumor necrosis factor alpha (TNF-α) [8]. MMP-8 or collagenase-2 was known as neutrophil collagenase; however it can be secreted from neutrophils and macrophages. They function by disintegrating the fibrillar collagens, via the binding of collagen type I α1 and α2 chains, a helping process for the cell migration [8]. MMP-13 or collagenase-3 expression is induced by macrophages and fibroblasts. The functional ways of MMP-13 and their effect on cardiac remodeling remains partially unclear. However, it is known that MMP-13 stimulates the expression of MMP-9 [8]. The collagen group participates in the cardiac remodeling process by their contribution to the degradation of ECM. Their function lies in the cleavage of all the alpha chains of native types I, II and III collagens in a specific location and into ¾ and ¼ segments. Those segments can denature into nonhelical gelatin derivatives, without any pre-planning and under physiological temperatures [14].
Role of gelatinases
The gelatinases consist of the MMP-2 and MMP-9. MMP-2 or gelatinase A substrate may consist of both extracellular and intracellular proteins, like aggrecan, elastin, fibronectin, citrate synthase, fusion protein, interleukin IL-1β, prolysyl oxidase, as well as other MMPs like MMP-1, MMP-9, and MMP-13. Intracellular substrates of the MMP-2 are the myosin chain and troponin I. Expressers of MMP-2 are the myocytes, fibroblasts, myofibroblasts, vascular smooth muscle cells and endothelial cells [8]. MMP-2 is known for their ability to disintegrate ECM proteins throughout the human body [13]. MMP-9 or gelatinase B can cleave proteins, such as collagen, elastin, fibronectin, laminin, aggrecan, galectin-3, interleukin IL-1β and osteonectin (ON), also known as secreted protein acidic and rich in cysteine (SPARC). However, MMP-9 might also activate the transforming growth factor beta 1 (TGF-β1), through the activation of the latent TGF-β binding protein. Increased MMP-9 levels are associated with deterioration of LV dysfunction [8], while some of the MMP-9 substrates participate in the cardiac remodeling process [15]. Fundamental function of the gelatinases sub-group is the further cleavage of the collaganase degradation products into smaller peptides. Later, these peptides are cleaved by other proteases [14].
Role of stromelysins
The stromelysins sub-group consists of MMP-3, MMP-10 and MMP-11. MMP-3 and MMP-10 share similar activity, but MMP-3 is prevalent [16]. MMP-3 or stromelysin-1 is expressed by myocytes, fibroblasts and macrophages. It cleaves casein and proteoglycan and activates the MMPs MMP-1, MMP-3, MMP-7, MMP-8, MMP-9 and MMP-13 [8]. MMP-10 or stromelysin-2 shares similar structure and substrate specificity with MMP-3 and can cleave ECM components and take place in the proMMP activation [17]. The differences between MMP-11 or stromelysin-3 and the other stromelysins members is that it is released as an active enzyme. This enzyme can be activated in the intracellular space by furin, a trans -Golgi-associated protein [16]. The substrates of the stromelysins consist of proteoglycan core proteins, laminin, fibronectin, elastin, as well as nonhelical regions of collagens. However, MMP-11 differs from the other stromelysins, since its substrate has serine proteinase inhibitors (serpins). Because of this, MMP-11 has a small to zero impact to the ECM components [16]. Another main difference between the sub-group of stromelysins and the other MMP sub-groups is that they are more drastic to the other ECM components, other than the serpins [16].
Role of matrilysins
Matrilysins are comprised of a sole member, the MMP-7, which was originally detected in a human rectal carcinoma cell line [16]. MMP-7’s function is the cleavage of some ECM proteins and the activation of the MMP-1, MMP-2, MMP-8, and MMP-9 [8]. MMP-7 effect against versican, a chondroitin sulfate proteoglycan, is considered more drastic than the effect of the other MMPs [16]. Furthermore, the MMP-7 is considered the most resistant to the inhibitory activity of the tissue inhibitors of MMPs (TIMP), compared to the other members of the MMP family [16].
Membrane type MMPs
In the human body six members of the membrane type MMPs (MT-MMPs) have been found. They are the MT1-MMP, MT2-MMP, MT3-MMP, MT4-MMP, MT5-MMP and MT6-MMP. However, those MMPs are also known as MMP 14, MMP 15, MMP 16, MMP 17, MMP 24 and MMP 25 respectively [2]. MT-MMPs are divided into two groups based on their mechanism of attachment to the plasma membrane, the transmembrane-type MT-MMPs and the glycosylphosphatidyl-inositol (GPI)-type MT-MMPs. MT1-MMP, MT2-MMP, MT3-MMP and MT5-MMP are transmembrane proteins type I and contain a short cytoplasmic tail that assists the control mechanisms of intracellular trafficking and activity of those MT-MMPs. MT4-MMP and MT6-MMP are GPI type proteins, which are bound to the cell surface [2]. MMP-14 or MT1-MMP has a latent form (65 kDa) and an active form (54/45/40 kDa). This protease can degrade collagen, fibronectin and gelatin and activates MMP-2 and MMP-13. According to clinical studies, an increase of MMP-14 levels in post infarcted patients was followed by cardiac fibrosis, worse LV function and lower survival [8].
The MT-MMPs function is adjusted by the proenzyme activation and by the process of inhibition. In addition, their activity can be regulated by shedding and endocytosis, by other factors such as cytokines (interleukin. TNF-α or TNF-β), by mechanical stretch and interactions with ECM proteins, such as collagen and fibronectin or fibrinogen [2]. MT-MMPs can cleave many ECM components, such as fibronectin, tenascin, nidogen, aggrecan, perlecan, laminin and type-I and type-III collagens. They can degrade membrane or pericellular proteins, such as cell adhesion molecules (CD44 and pro-αv integrin) and cytokines (pro-TNF-α), as well [2].
Special types of MMPs
There are some other MMPs that are not included in the stated sub-types. These are the MMP-12, MMP-19, MMP-20, MMP-21, MMP-23, MMP-27 and MMP-28. MMP-12 or metalloelastase has an adversely influence to elastin and cleaves ECM components, like fibronectin, laminin, vitronectin, proteoglycans, type IV collagen, and heparin sulfate [16]. It is a necessary MMP for the macrophage migration [17]. MMP-19 degrades many ECM components, such as the structural basement membrane components. It is expressed by many human tissues [17]. MMP-20 or enamelysin is expressed on the newly formed tooth enamel and cleaves amelogenin [17]. MMP-21 is expressed in adult and fetal organisms, as well as in basal and squamous cell carcinomas [17]. MMP-23 has a unique structure; it contains a C-terminal cysteine-rich immunoglobulin-like domain, instead of a hemopexin domain [17]. MMP-27 can cleave gelatin and casein and cause enzymatic autolysis. However, its effects on the human tissue remain unknown [17]. MMP-28 or epilysin is one of the newest members of the MMP family and was cloned in 2001. In normal conditions, MMP-28 is expressed mainly in the cardiac tissue. Targeted deletion of MMP-28 might worsen LV dysfunction and increases mortality [8].
Biochemical structure
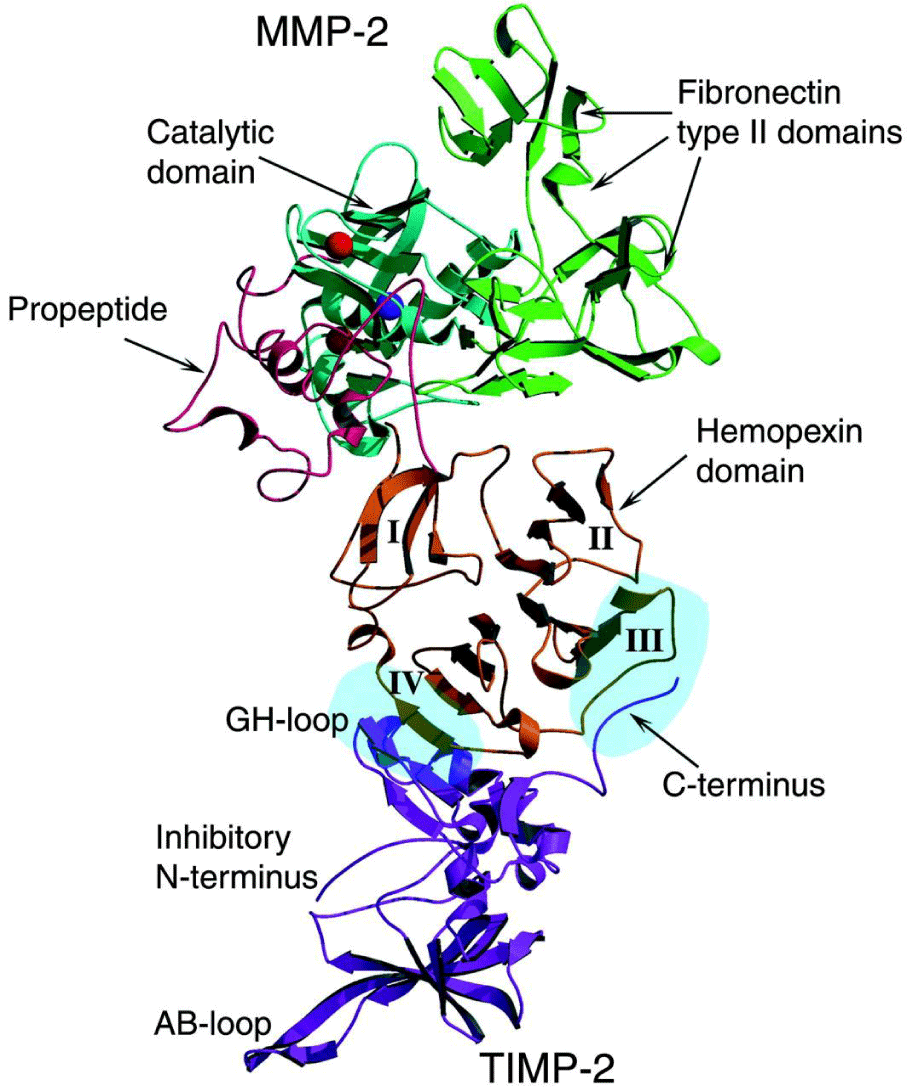
MMPs are initially synthesized in an inactive zymogen form, which has an autoinhibitory propeptide domain [13]. The basic structure of the MMPs consists of four domains, the pro-domain, the catalytic domain, the hinge region and the hemopexin domain [8]. Another key element is the signal peptide that guides the MMPs towards the endoplasmic reticulum [2]. The pro-domain is a propeptide with a zinc-interacting thiol group that maintains the MMPs in its latent form, such as a zymogen. The catalytic domain contains a highly conserved Zn2+ binding site. The hemopexin domain can be found in all the MMPs, with the exception of MMP-23, MMP-7 and MMP-26 and affects the substrate specificity and the MMP binding on their inhibitors. The catalytic domain and the hemopexin domain are connected via a proline-rich hinge region [2]. Hinge region and haemopexin domain bind various proteins with the ability to alter MMP activity [13]. Metalloproteinases MMP-2 and MMP-9 show an additional domain, the fibronectin type II-like domain, which is inserted into the catalytic domain and contributes to the collagen degradation [8]. The membrane type MMPs contain all of the previously mentioned protein domains. However, the main difference between them and the other MMPs lies in the membrane-anchoring domain, which is present only in the MT-MMPs, allows those MMPs to perform a unique cellular localization and provides a number of substrates for regulation. In addition, they allow distinct interactions with the tissue inhibitors of MMPs, while they create a non-conventional regulatory mechanism of these MMPs, which includes enzyme internalization and processing as well as ectodomain shedding [2]. The structure of the matrix metalloproteinases are shown in Figures 2.1-3 [1].
Activation signaling
Activation of the MMPs can be executed either in the intracellular space by other MT-MMPs at the cell surface or in the extracellular space from the activity of other proteases. However, MMP activation can also occur by previously activated MMPs through a process known as stepwise activation [16]. All the MMPs except MMP-23 can be activated with the intervention of a cysteine switch mechanism. During the process of this mechanism, the catalytic MMP domain is exposed because of the separation of the cystein-73 residue from the Zn2+ atom [8]. An important regulatory factor of the MMP activation and activity are their tissue inhibitors (TIMPs). Additionally, a factor that reduces the activity of specific members of the MMP family is the tissue factor pathway inhibitor 2 (TFPI-2), which effects MMP-2 and MMP-9 function and the ability of MMP-1 and MMP-13 to cleave collagen triple helix [16].
Tissue inhibitors of metalloproteinases (TIMPs)
TIMPs are endogenous inhibitors of active MMPs and consist of four sub-types, TIMP-1, TIMP-2, TIMP-3 and TIMP-4 [18]. Generally, they are cysteine-rich proteins of low molecular mass [19]. TIMP-1 is a glycoprotein with a molecular weight of 29 kDa and is synthesized in myocytes and fibroblasts [8]. TIMP-2 has 28 kDa of molecular mass, is secreted mainly in fibroblasts and its expression takes place mainly in the normal myocardium. TIMP-2 is the only member of the TIMP family that is necessary for the cell surface activation of pro-MMP2, maybe due to TIMP-2’s inhibitory function on MMP-14 [8]. TIMP-3 (24 kDa) is ECM bound. The main TIMP-3 expressers are the cardiac fibroblasts. It is considered as the most powerful inhibitor from the TIMP family [8]. TIMP-4 has a 24 kDa form and a 28 kDa glycosylated form, expressed by cardiomyocytes [8]. The TIMP biochemical structure consists of 184–194 amino acids, a N-terminal and a C-terminal subdomain. Each of these two domains includes three conserved disulfide bonds [17]. In the N-terminal domain the inhibition of the MMPs takes place [19]. The secretion of TIMPs is performed by different cells, such as smooth muscle cells and macrophages. TIMP activity is altered by various interleukins and increased by platelet-derived growth factor (PDGF) and TGF-β [16]. Even though all of the TIMPs can inhibit endogenous MMPs, they display differences in their activity. TIMP-1 is a poor MT1-MMP, MT3-MMP, MT5-MMP and MMP-19 inhibitor. TIMP-3 inhibits ADAMs (ADAM-10, -12 and -17) and ADAMTSs (ADAMTS-1, -4 and -5) [17]. TIMPs inhibit the MMP by binding to their active site in a 1:1 stoichiometrie, which prevents them from accessing the collagen substrate. Furthermore, binding of latent MMPs at the aminoterminus leads to the prevention of MMP activation [18]. TIMP-2 and TIMP-3 are effective inhibitors for the MT-MMPs. Furthermore, TIMP-3 is the only member of the TIMP family with the ability to inhibit TNF-α converting enzyme [18]. Studies in mice deficient in the TIMP-1 gene has shown increased LV mass and end diastolic volume. These results lead to the assumption that TIMP-1 has an important role in the prevented activation of the protease cascade and the preservation of ECM balance. At this point, the TIMPs can be affected by inflammatory mediators, since interleukin IL-1 and TNF-α might reduce the expression of TIMP-1 [18].
Adamalysins (ADAMs)
Adamalysins are transmembrane proteases known as ADAMs that contain a disintegrin and metalloprotease domain [14]. Like MMPs, ADAMs belongs to the metalloproteinases family [2]. ADAMs biochemical structure consists of a disintegrin domain, which is relevant to integrin-binding ligands in snake venom, as well as a metalloprotease catalytic domain, which might display an activity similar to MMPs [20]. The difference between them and MMPs is the integrin binding receptor, which is present in the ADAMs domain [2]. ADAMs 10, 11, 12, 15, 17 and 19 are expressed in muscles, but 14 ADAMs members can be expressed by various human tissues. Studies on patients with atrial fibrillation have shown increased mRNA and protein levels of the ADAMs 10 and 15, compared to non patients. These results suggest that these ADAMs contribute to the atrial remodeling process, which follows atrial fibrillation. The expression of ADAM 11 was thought to occur in the central nervous system; however it was detected in the fetal and perinatal heart as well [2]. Basic functions of the ADAMs is the degradation of ECM molecules, regulation of the cell–cell and cell–matrix interactions with the integrin receptor and activation key biopeptides, cytokines and growth factors in the interstitiu [14]. A protein group related to ADAMs is the ADAM proteins with a thrombospondin motif (ADAMTS). While ADAMs is transmembrane proteins with substrates consisting of other transmembrane proteins, ADAMTS are soluble ECM proteases and their substrate consists of other ECM proteins [14]. However, ADAMTs bind to ECM, like ADAMs [2]. It is possible that both ADAMs and ADAMTS have a basic role in the process of ventrivular remodeling [14].
Metalloproteinases in cardiac remodeling
MMPs in cardiac tissue: The presence of MMPs in the normal myocardium was detected using zymographic techniques and myocardial specimens obtained during cardiac transplantation. MMP-2 and MMP-9 were identified in the myocardial tissue by using a gelatin-impregnated electrophoretic platform [21]. Further immunochemical and gene expression clinical trials showed the presence of all main MMP sub-groups in the normal human myocardial tissue. Some of the localized MMPs were MMP-1, -13 and -8 [21]. The presence of MMP-1 in the myocardial tissue was anticipated, since this MMP can be expressed by all human tissues. However, MMP-13 was considered to be expressed in high levels exclusively in rodents and malignant carcinomas. Thus, the trial results indicated the presence of many MMP sub-groups in the normal human myocardium [21]. The expression of MMP-8 was considered to be initiated only by peripheral inflammatory cells, until clinical trials focused on the human myocardium have shown that endogenous cells, like mast cells, are responsible for MMP-8 expression as well [21]. Clinical results have also showed the presence of stromelysins and membrane-bound MMPs in the myocardial tissue, which proves the existence of a localized proteolytic pathway for the purpose of activation of the pro-MMPs [21]. Other clinical trials, with an immunohistochemical as well as a quantitative immunochemical approach, showed that MMP distribution in the myocardial tissue might differ between the cardiac chambers. For example, MMP-1 levels in the left ventricle are mainly higher than the MMP-1 levels of the right ventricle. A confirmed theory for this fact hasn’t been provided yet, but these differences of the MMP levels between the chambers are considered to be caused by factors such as alterations in loading conditions, the embryological origin and the cellular response to various stimuli [21]. The four members of TIMP family were also present in the normal myocardial tissue. More specifically, TIMP-4, which is mainly expressed in the cardiovascular tissues, was detected in all of the chambers [21]. Alterations in the normal MMP and TIMP levels in the myocardium are likely to cause regulation of the structure and function of the myocardial ECM. These regulations of the myocardium have been proven to occur in humans.
MMP activity in cardiac remodeling: MMPs and TIMPs are recognized for their important role in cardiac remodeling [21]. Studies in animals and patients with heart failure have shown that inhibition of MMP expression and activity after LV dilatation with the use of pharmacological agents prevented the progression of cardiac remodeling [22]. After ischaemia/reperfusion injury, MMPs stimulate the cardiac fibroblasts, being an important factor in cardiac remodeling and fibrosis [9]. It is known that any imbalance of the collagenase family and their inhibitors alters the ECM structure, which assists the remodeling process [23]. TIMPs can disrupt the MMP/TIMP balance if their levels are decreased due to limited expression or increased consumption. Imbalance in MMP and TIMP levels subsequently affects cardiac remodeling [24]. MMP and TIMP effect is characterized as temporal and spatial [6]. MMPs degrade the pre-existing ECM, by disrupting the fibrillar collagen network, which releases inflammatory cells. The inflammatory cells have the ability to release MMPs, cytokines, growth factors and angiogenic factors [6]. After cardiac myocyte necrosis in post-MI cardiac tissue, MMPs degrade ECM and coronary vasculature (a process that declines by the end of the first week post-MI), while TIMP expression levels increase. MMP activity facilitates the migration of neutrophils and macrophages in the infarct area, contributing to the proteolytic digestion and phagocytosis of the infarct [25] Also, Sakata, et al. [22], demonstrated that the remodeling process is stimulated mainly by the simultaneous activation of few MMPs, such as MMP-2 and MMP-9. Some of these MMPs are the collagenases, the gelatinases sub-groups and the stromelysins MMP-3 and MMP-10 [26]. MMP effect on the cardiac remodeling process can be beneficial and detrimental as well. Myocyte slippage might cause cardiac rupture, LV dilatation and dysfunction [6]. Increased MMP-9 levels in the early post-MI stage are related to an adverse myocardial remodeling expressed as LV dilatation during the later post-MI stage [27]. The presence of high MMP-2 levels in cardiac tissue contributes to the remodeling process via causing systolic dysfunction, fibrosis, and cardiomyocyte dropout. Targeted deletion of MMP-2 led to attenuation of the left ventricular rupture and post-infarction remodeling [28]. However, MMP-2 activation without the simultaneous activation and activity of MMP-9 does not appear to cause LV dilatation, while genetic deletion of MMP-9 limits dilatation, even with elevated MMP-2 levels [22]. In human tissues, within the first 24 hours of the cardiac remodeling, expression of MMP-9 is induced mainly by infiltrating neutrophils and macrophages. MMP-9 levels are further increased on the third day of cardiac remodeling and the main cells responsible for MMP-9 expression are neutrophils. Once the MMP-9 levels reach their peak they gradually decrease. On the other hand, MMP-2 activity is drastically increased on the fourth day of the remodeling, reaches the highest levels on the seventh day and remains increased for the remodeling process. In the initial phase of the remodeling, the main MMP-2 sources are the activated macrophages, fibroblasts and myocytes [6]. MMP-3 levels are increased on the second day of remodeling and reach a peak on the fourth. Animal studies suggest that MMP-3 levels remain increased during the whole process of cardiac remodeling [6]. In human cardiac tissues, MMP-3 levels reach the highest levels at 3 months post-MI [29]. In addition, MMP-3 contributes to the remodeling process by affecting many ECM components, while it is believed that MMP-3 regulates the activity of other MMPs. The relevancy between MMP-3 and post-MI cardiac remodeling was assessed with echocardiography [29]. MMP-13 has been found in the human myocardium, but its association with cardiac remodeling remains unclear [27]. The inflammatory process which is stimulated post-MI leads to the elevation of MMP-8 levels, secreted by inflammatory cells. Three days post-MI, the MMP-8 levels are increased due to the activity of the macrophages during the MI healing progress [27]. The mRNA levels of MMP-1 are increased only on the third day of remodeling and rebound until the seventh day. These changes on the MMP-1 levels are representative of their activity, since they are highly increased on the third day of remodeling and reach a peak on the seventh. There are no other alterations in MMP-1 levels for the rest of the remodeling process. Expression is induced mainly by fibroblast-like cells [6]. MMP-10 and its effect on the remodeling process remain vague, but MMP-10 affects ECM by degrading many components. Furthermore, MMP-10 is associated with LV dilatation in patients with heart failure [30]. During cardiac fibrosis processes, various pathological factors like ischemia-reperfusion injury, inflammation and induction of TGF-β might increase MMP activity [9]. In mice MMP-2 and MMP-9 levels are increased within seven days, while in rabbit models, the MMP-9 activity returned to their normal level in four days and MMP-2 showed minimal increase. In rats, MMP-2 levels were increased from the first 24 hours of remodeling and MMP-9 showed increased activity for the first 16 weeks [6].
Specific MMP expression: High levels of the gelatinases sub-group (MMP-2 and MMP-9) may contribute to the degradation of the ECM basement membrane in the myocardial tissue [31]. Metalloproteinases MMP-2 and MMP-9 proteolyse ECM proteins and have an important role in cardiac remodeling [32]. The activity of the MMPs MMP-9, MMP-2, MMP-1 and the TIMPs TIMP-1 and TIMP-3, along with their importance in the process of cardiac remodeling has been explained through clinical trials conducted on knockout and transgenic mice [6]. Targeted deletion of MMP-9 changes the LV remodeling process [4]. Animal studies in MMP-9 deficient rats showed reduction of the remodeling process post-MI [31] and limitation of LV dilatation. It is possible that MMP-9 is a mediator of the molecular organization of the collagen network. However, in knockout mice these results didn’t indicate that MMP-9 inhibition was always associated with improved cardiac remodeling. MMP-9 deficient mice showed high levels of MMP-3 and MMP-13 compared to normal mice [6]. Targeted deletion of MMP-9 appears to limit the LV remodeling process, post-MI [32]. The deficiency of MMP-9 expression in mice with post-infarction cardiac remodeling caused the deceleration of the wound healing process of the infarcted area, characterized by reduced leukocyte inflow in the infarct and by larger residual necrotic areas. However, lack of MMP-9 activity almost prevented infarct rupture [6]. Other animal studies on post-MI, MMP-9 deficiency models have shown that this protease limited LV cavity enlargement and collagen accumulation [15]. Animal studies with MMP-2 deficient mice have shown higher survival rates post-MI than with wild mice. This result is attributed to the inhibition of early cardiac rupture and the development of subsequent LV dysfunction during the first 28 days after MI. Targeted deletion of MMP-2 contributed to the restriction of post-MI LV dilatation in the late phase, without altering the infarct. Results have also shown decreased levels of phagocytic removal of necrotic cardiomyocytes, while degradation of ECM components such as laminin and fibronectin was restrained [6]. Knockout mice, with MMP-3 and MMP-12 deficiency haven’t shown alterations in leukocyte infiltration, collagen deposition and incidence of cardiac rupture, for the first 14 days of remodeling [6]. In a clinical trial for the ascertainment of MMP-1 function, transgenic mice that expressed human MMP-1 in the cardiac tissue were used, since adult normal mice don’t express MMP-1. At 6 months of age, compensatory myocyte hypertrophy and increased cardiac collagen were detected. They were probably caused by a corresponding increase in collagen type III transcript levels. At 12 months of age, the mice developed ventricular dysfunction, induced by reduction in interstitial collagen. The results suggest that overexpression, even of a single member, of the MMP family might lead to cardiac dilatation and dysfunction [6].
Pharmacological agents that affect MMP activity: Pharmacological treatment for coronary disease might affect MMP expression, such as nitroglycerin, heparin, rosiglitazone, amlodipine and diltiazem, losartan, statins and estrogen [16]. Nitroglycerin is used in the symptomatic relief of stable and unstable angina. It affects MMP activity by increasing the expression and activity of MMP-2, MMP-7, and MMP-9 and decreasing TIMP-1 levels [16].
Heparin affects mRNA and protein levels of TIMP-1 and TIMP-2 similar to nitroglycerin [16]. Rosiglitazone may decrease mMP-9 levels through the peroxisome proliferator-activated receptor gamma (PPARγ) [16]. In cultured vascular endothelial cells, the calcium channel blockers, amlodipine and diltiazem, caused increased activity of MMP-1 and MMP-2. However, all calcium channel blockers sub-groups are known to increase IL-6 expressions, which induces TIMP-1 expression. In addition, amlodipine decreases MMP-1 levels in human endothelial cells that induce IL-1β [16]. Angiotensin-converting-enzyme (ACE) inhibitors effects on MMP expression remain unclear [16]. Animal studies including models after heart failure have shown that ACE inhibitors limit the remodeling process, by reducing LV dilatation. Clinical trials on animals which had been treated with ACE inhibitors, MMP inhibitors or combined treatment have shown no difference in MMP activity of the left ventricular tissue [15]. However, angiotensin II appears to be related with decreased MMP-1 activity, indicating that ACE inhibitors might increase MMP-1 activity [16]. Losartan appears to increase MMP-2 activity in vascular smooth cells [16]. Clinical trials have shown that statins or HMG-CoA reductase inhibitors participate in MMP expression. Fluvastatin and lovastatin have inhibitory effect on MMP-1. Simvastatin and fluvastatin decrease the secretion of MMP-9 from macrophages. Pravastatin reduces MMP-2 levels, while it increases TIMP-1 levels [16]. Estradiol or 17β-estradiol has a stimulative effect on MMP levels and their activity in mesangial cells. More specifically, raloxifene, a selective estrogen receptor modulator, increases MMP-1 expression in monocytes. However, increased estradiol levels limit MMP-1 activity. The exception to this rule is progesterone, which reduces MMP activity [16]. A different pharmacological approach against MMP activity included MMP inhibition on animal models with LV dysfunction.
TIMPs effect on MMP activity: TIMPs have an important role in the cardiac remodeling process by inhibiting the MMP members and regulating the remodeling processes [8]. In post-MI ventricular remodeling, there is a loss of TIMP-mediated control. In mice and rats the TIMP-1 levels have a rapid increase within 3 days and reach a peak until the seventh day, only in the infarcted myocardial tissue. However, protein levels of TIMP-1 are increased later, within the first 2 to 16 weeks of the remodeling [6]. The TIMP-1 deficient mice have shown amplified hypertrophic response and adverse LV remodeling. As a conclusion, MMP-1 regulation of the endogenous MMP activity in the early phase of cardiac remodeling is of great importance [6].
TIMP-3 proteins have shown decreased expression in animal and human tissues of MI patients. It appears that lack of MMP-3 expression leads to increased mortality, since it is responsible for increased cardiac rupture, infarct expansion and worse LV dilatation and dysfunction. Mice with TIMP-3 deficiency had limited collagen synthesis and disorganized deposition as well as decreased myofibroblast levels and MMP activity. On the other hand, MMP inhibition reverses these adverse effects. Conclusively, proteolytic activity in the early phase in post-MI patients induces adverse LV remodeling and dysfunction, while the timing of intervention is determinant for the improvement of the ventricular response [8]. Loss of TIMP-4 expression might lead to increased LV rupture and mortality, but MMP inhibition might prevent it. The plasma TIMP-4 levels, in contrast to the corresponding TIMP-1 and TIMP-2 levels, can predict cardiac remodeling in post-MI patients [8]. The TIMP-1 and TIMP-2 mRNA levels are dramatically increased in the early phase of remodeling, but TIMP-2 proteins remain unchanged until the 2nd, 5th and 16th week of remodeling process in rats. This indicates the post-translation processing of TIMP-2. TIMP-4 mRNA levels remain stable, while the protein levels of TIMP-4 are decreased during the first week of remodeling and return to its normal levels [6].
ADAMs in cardiac remodeling: ADAMs might affect the cardiac structure due to their cell-cell and cell-matrix interactions. Specifically, adamalysins can affect the remodeling process, which alters cardiac tissue structure, thanks to cleavage-secretion of surface-bound proteins [33]. In addition, ADAMs and ADAMTSs might contribute to the growth factor release from the extracellular matrix. These enzymes seem to increase protein levels, such as Heparin-binding EGF-like growth factor (HB-EGF) and transforming growth factor alpha (TGF-α). TGF-α is cleaved and activated by ADAM-17 and seems to affect cardiac ECM homeostasis by pressure overload [34-37].
- Cohn J, Ferrari R, Sharpe N (2000) Cardiac remodeling—concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. JACC 35: 569-582. Link: https://bit.ly/2Mw1ult
- Manso AM, Elsherif L, Min Kang S, Ross RS (2005) Integrins, membrane-type matrix metalloproteinases and ADAMs: Potential implications for cardiac remodeling. Cardiovasc Res 69: 574-584. Link: https://bit.ly/2YwtnMP
- You Li Y, McTiernan C, Feldman A (2000) Interplay of matrix metalloproteinases, tissue inhibitors of metalloproteinases and their regulators in cardiac matrix remodeling. Cardiovasc Res 46: 214-224. Link: https://bit.ly/2Yh60eC
- Bradham WS, Bozkurt B, Gunasinghe H, Mann D, Spinale FG (2002) Tumor necrosis factor-alpha and myocardial remodeling in progression of heart failure: a current perspective. Cardiovasc Res 53: 822-830. Link: https://bit.ly/2SSwtt8
- Bujak M, Frangogiannis NG (2007) The role of TGF-β signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res 74: 184-195. Link: https://bit.ly/2Yu36yp
- Vanhoutte D, Schellings M, Pinto Y, Heymans S (2006) Relevance of matrix metalloproteinases and their inhibitors after myocardial infarction: A temporal and spatial window. Cardiovasc Res 69: 604-613. Link: https://bit.ly/2KeOiyO
- Swynghedauw B (1999) Molecular Mechanisms of Myocardial Remodeling. APS 79: 215-262. Link: https://bit.ly/2YcFL9a
- Ma Y, Castro Brás LE, Toba H, Padmanabhan R, Hall ME, et al. (2014) Myofibroblasts and the extracellular matrix network in post-MIcardiac remodeling. European Journal of Physiology. Link: https://bit.ly/316hnTS
- Jun JH, Cho JE, Shim YH, Shim JK, Kwak YK (2011) Effects of propofol on the expression of matric metalloproteinases in rat cardiac fibroblasts after hypoxia and reoxygenation. Br J Anaesth 106: 650-658. Link: https://bit.ly/2Mw3iLh
- Tian XF, Cui MX, Yang SW, Zhou YJ, Hu DY (2013) Cell death, dysglycemia and myocardial infarction. Biomedical Reports 1: 341-346. Link: https://bit.ly/2K8yj6F
- Cuvelier A, Kuntz C, Sesboüé R, Muir JF, Martin JP (1997) Les métalloprotéinases de la matrice extracellulaire (MMP): structure et activité. Revue des Maladies Respiratoires 14: 1-10.
- Newby AC, Pauschinger M, Spinale FG (2006) From tadpole tails to transgenic mice: Metalloproteinases have brought about a metamorphosis in our understanding of cardiovascular disease. Cardiovasc Res 69: 559-561. Link: https://bit.ly/2OqJ20a
- Kandasamy AD, Chow AK, Ali M, Schulz R (2010) Matrix metalloproteinase-2 and myocardial oxidative stress injury: beyond the matrix.Cardiovasc Res 85: 413-423. Link: https://bit.ly/2GCgWJd
- Janicki JS, Brower GL, Gardner JD, Forman MF, Stewart JA, et al. (2006) Cardiac mast cell regulation of matrix metalloproteinase-related ventricular remodeling in chronic pressure or volume overload. Cardiovasc Res 69: 657-665. Link: https://bit.ly/2Ow4Szr
- Reinhardt D, Sigusch HH, Henβe J, Tyagi SC, Körfer R, et al. (2002) Cardiac remodelling in end stage heart failure: upregulation of matrix metalloproteinase (MMP) irrespective of the underlying disease, and evidence for a direct inhibitory effect of ACE inhibitors on MMP. Heart 88: 525-530. Link: https://bit.ly/2KieqZq
- Jones CB, Sane DC, Herrington DM (2003) Matrix metalloproteinases: A review of their structure and role in acute coronary syndrome. Cardiovasc Res 59: 812-823. Link: https://bit.ly/2Yfrnxc
- Nagase H, Visse R, Murphy G (2006) Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res 69: 562-573. Link: https://bit.ly/2YBd993
- Rutschow S, Li J, Schultheiss HP, Pauschinger M (2006) Myocardial proteases and matrix remodeling in inflammatory heart disease. Cardiovasc Res 69: 646-656. Link: https://bit.ly/2K3o7w2
- Chow AK, Cena J, Schulz R (2007) Acute actions and novel targets of matrix metalloproteinases in the heart and vasculature. Br J Pharmacol 152: 189–205. Link: https://bit.ly/2yoqKSD
- Brauer PR (2006) MMPs - Role in Cardiovascular Development and Disease. Frontiers in Bioscience 11: 447-478. Link: https://bit.ly/2K4io94
- Spinale FG (2007) Myocardial Matrix Remodeling and the Matrix Metalloproteinases: Influence on Cardiac Form and Function. APS. 87: 1285-1342. Link: https://bit.ly/2K5KfWM
- Sakata Y, Yamamoto K, Mano T, Nishikawa N, Yoshida J, et al. (2004) Activation of matrix metalloproteinases precedes left ventricular remodeling in hypertensive heart failure rats: its inhibition as a primary effect of Angiotensin-converting enzyme inhibitor. Circulation 109: 2143-2149. Link: https://bit.ly/2GFmMt9
- Li MJ, Huang CX, Okello E, Yanhong T, Mohamed S (2009) Treatment with spironolactone for 24 weeks decreases the level of matrix metalloproteinases and improves cardiac function in patients with chronic heart failure of ischemic etiology. Can J Cardiol 25: 523–526. Link: https://bit.ly/2Ysbdjh
- Nilsson L, Hallén J, Atar D, Jonasson L, Swahn E (2012) Early measurements of plasma matrix metalloproteinase-2 predict infarct size and ventricular dysfunction in ST-elevation myocardial infarction. Heart 98: 31-36. Link: https://bit.ly/2KeO22O
- Sun Y (2009) Myocardial repair/remodelling following infarction: roles of local factors. Cardiovasc Res 81: 482-490. Link: https://bit.ly/2OvmXhc
- Chancey AL, Brower GL, Peterson JT, Janicki JS (2002) Effects of matrix metalloproteinase inhibition on ventricular remodeling due to volume overload. Circulation 105: 1983-1988. Link: https://bit.ly/2YBbCjj
- Webb Cs, Bonnema DD, Ahmed SH, Leonardi AH, McClure CD, et al. (2006) Specific Temporal Profile of Matrix Metalloproteinase Release Occurs in Patients After Myocardial Infarction: Relation to Left Ventricular Remodeling. Circulation 114: 1020-1027. Link: https://bit.ly/2YcpdON
- Bergman MR, Teerlink JR, Mahimkar R, Li L, Zhu BQ, et al. (2007) Cardiac matrix metalloproteinase-2 expression independently induces marked ventricular remodeling and systolic dysfunction. Am J Physiol Heart Circ Physiol 292: 1847-1860. Link: https://bit.ly/2yrg5q4
- Kelly D, Hkan S, Cockerill G, Ng LL, Thompson M, et al. (2008) Circulating Stromelysin-1 (MMP-3): A novel predictor of LV dysfunction, remodelling and all-cause mortality after acute myocardial infarction. Eur J Heart Fail 10: 133-139. Link: https://bit.ly/2MnV7Rj
- Wei Y, Cui C, Lainscak M, Zhang X, Li J, et al. (2011) Type-specific dysregulation of matrix metalloproteinases and their tissue inhibitors in end-stage heart failure patients: relationship between MMP-10 and LV remodelling. Journal of Cellular and Molecular Medicine 15: 773–782. Link: https://bit.ly/316fB4Y
- Peterson JT, Hallak H, Johnson L, Li H, O' Brien PM, et al. (2001) Matrix Metalloproteinase Inhibition Attenuates Left Ventricular Remodeling and Dysfunction in a Rat Model of Progressive Heart Failure. Circulation 103: 2303-2309. Link: https://bit.ly/2ytNIra
- Lalu MM, Pasini E, Schulze CJ, Ferrari-Vivaldi M, Ferrari-Vivaldi G, et al. (2005) Ischaemia–reperfusion injury activates matrix metalloproteinases in the human heart. Eur Heart J 26: 27-35. Link: https://bit.ly/2YxF75C
- Arndt Μ, Lendeckel U, Röcken C, Nepple K, Wolke C, et al. ((2002) Altered Expression of ADAMs (A Disintegrin And Metalloproteinase) in Fibrillating Human Atria. Circulation 105: 720-725. Link: https://bit.ly/2yrzya6
- Berk BC, Fujiwara K, Lehoux S (2007) ECM remodeling in hypertensive heart disease. J Clin Invest 117: 568–575. Link: https://bit.ly/2LTbtSq
- Briest W, Hölzl A, Raßler B, Deten A, Baba HA, et al. (2003) Significance of matrix metalloproteinases in norepinephrine-induced remodelling of rat hearts. Cardiovasc Res 57: 379-387. Link: https://bit.ly/2YlSsPn
- Gupta SP (2012) Matrix Metalloproteinase Inhibitors: Specificity of Binding and Structure-Activity Relationships. London: Springer 9. Link: https://bit.ly/2KfStdG
- Li H, Simon H, Bocan TMA, Peterson JT (2000) MMP/TIMP expression in spontaneously hypertensive heart failure rats: the effect of ACE- and MMP-inhibition. Cardiovasc Res 46: 298-306. Link: https://bit.ly/2K7lObs

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
