Journal of Cardiovascular Medicine and Cardiology
Efforts to visually match 5- and 60-minute post-stress images following a single injected dose of sestamibi clearly demonstrate changes in sestamibi distribution: Demonstrating once and for all clinical recognition that sestamibi redistributes
Richard M Fleming1*, Matthew R Fleming1 and Tapan K Chaudhuri2
2Eastern Virginia Medical School, Norfolk, Virginia, USA
Cite this as
Fleming RM, Fleming MR, Chaudhuri TK (2019) Efforts to visually match 5- and 60-minute post-stress images following a single injected dose of sestamibi clearly demonstrate changes in sestamibi distribution: Demonstrating once and for all clinical recognition that sestamibi redistributes. J Cardiovasc Med Cardiol 6(2): 030-035. DOI: 10.17352/2455-2976.000087Importance: Sestamibi imaging has been available for approximately three decades. During that time there has been considerable evidence published in the medical literature demonstrating that Sestamibi redistributes. Despite these publications, Lantheus has continued to insist that Sestamibi does not redistribute and that two doses of radioactive isotope are needed to conduct myocardial perfusion imaging. Isotope package inserts have maintained that Sestamibi is initially taken up by the heart within minutes without redistribution making it not only possible but acceptable to wait until 60-minutes to begin imaging. This approach then requires a second dose of Sestamibi to acquire comparative images for clinical decision-making according to the Sestamibi package insert.
Objective: This study not only considers those publications validating Sestamibi redistribution, but looked at whether experienced professionals were able to match 10-patients initial 5-minute images with sequential 60-minute images, which would be identical if in fact there was no Sestamibi redistribution.
Design: Nineteen trained and certified nuclear medicine professionals were provided with two sets of images from 10-different patients, each of whom had different areas of ischemia on their myocardial perfusion imaging studies, making every patient different from the others. Professionals were then asked to match initial and sequential images of these 10-patients, basing their matching of images purely upon the premise that Sestamibi does NOT redistribute making the initial and sequential images identical.
Setting: The images were presented at a Nuclear Medicine Conference, where professionals were receiving continuing medical education and credit. Participants were volunteers. Images were projected onto conference Screens and participants were allowed to examine the studies from any position in the conference hall.
Results: Of the 19 participants, only one matched three of the patients, with one of these being the patient with a dilated cardiomyopathy. Five matched two of the patients while 31.6% matched only one patient and 36.8% matched none of the patients. When the patient with a dilated cardiomyopathy was removed from the data set, there were only 15 of 180 correct matches.
Conclusions: The failure of these professionals to match the two sets of images demonstrates that the sequential images are different from the initial images and that SESTAMIBI DOES IN FACT REDISTRIBUTE. By failing to image at 5-minutes post-stress and sequentially imaging at 60-minutes, there is a failure to find up to 40% of actionable ischemic heart disease.
Introduction
The introduction of technetium-99m (Tc-99m) isotopes in the late 1980s offered the promise of better qualitative imaging using higher doses of radioactive isotope than that made possible by thallium-201 (Tl-201). This was possible because the longer 73.01-hour half-life of Tl-201 limited the amount of Tl-201, which could be safely given to a patient to 2-3 mCi.
The shorter Tc-99m half-life of 6.01-hours, meant larger doses of such isotopes could be given; doses up to 30 mCi, thus increasing the amount of isotope available for emission imaging and presumptively providing better “appearing” qualitative imaging.
To utilize nuclear isotopes for diagnostic imaging requires several fundamental steps: (1) You must have a patient; human or otherwise. (2) You must have an isotope, which will be delivered to the area within the body you are interested in determining if there is a problem. (3) You need to have a way to introduce that isotope into the body so it can arrive at the target organ. (4) You must have a nuclear camera, which can detect the emissions resulting from the isotope and that camera (SPECT/Planar or PET) is determined by the actual photon energy the isotope “emits.” (5) One must actually point the camera at the right area of the body to detect the emissions, and finally (6) You must actually point the camera at the right area of the body at the right time to detect these emissions.
This later point is easy to understand using a simple example. Imagine, you are asked to describe an accident that just occurred. If you were looking in another direction at the time the accident happened and you were asked to describe what happened, you really cannot. You may have heard the crash and turned around, but the fact that you were not looking at the accident site at the right time means you cannot describe what you did not see.
Since the introduction of nuclear imaging with Blumgart in 1926, two very important principles of nuclear imaging have been known; First, any isotope introduced into the human body moves around (redistributes) and Secondly, the best method for seeing this redistribution is to “measure” (quantify) it and not depend upon your eyes (qualitative imaging) to see it, because odds are, you won’t see changes with the naked eye.
When Tl-201 was introduced, it was well recognized that detection of its redistribution pattern required imaging the heart at 1-hour and 4-hours after the “single” injected dose of isotope was given. The importance of understanding this “redistribution pattern” made it possible to determine if there was either ischemic or infarcted tissue present.
Similarly, understanding the “redistribution patterns” of Tc-99m isotopes is important to fully appreciate if ischemia or infarcted tissue is present. There are multiple publications, by our group and others including papers from Harvard, Brigham Women’s, UCLA, Cedars Sinai, et cetera [1-38], showing sestamibi redistribution can bee seen when early images (approximately 5-minutes post injection) are compared with sequential images at 60-minutes post isotope injection. Lantheus has continued to deny Sestamibi redistribution, which we have addressed elsewhere [37,38]. Unfortunately the most critically ill individuals displaying “wash-in” may have coronary artery disease (CAD), CAD which can be missed by waiting for 1-hour following isotope injection and comparing that result with images taken after a second-injection of isotope is given hours later or on another day [39-40]. Just as the details of the traffic accident are missed by not looking in the right direction at the right time, so too are the redistribution patterns of Tc-99m isotopes (Sestamibi, Teboroxime, Tetrafosmin) missed by not looking early enough to see them; resulting in the potential misdiagnosis of individuals while increasing their radiation exposure caused by the addition of a second injection of the isotope.
To further demonstrate that Sestambi redistributes, redistribution which is readily observable when comparing 5-minute and 60-minute acquisitions [37,38], following “stress”, we provided an opportunity for attendees at a recent Nuclear Medicine Conference to participate in a study, asking professionals to match 10-patients [38], initial 5-minute images with their sequential images projected onto conference screens.
Methods
Nineteen experienced attendees at the 2019, 48th Annual Florida Nuclear Medicine Conference volunteered to undergo a matching exercise comparing initial and sequential images of 10-patients that had undergone “stress” followed by a single-injected dose of sestamibi and serial imaging. This conference is recognized by the State of Florida as meeting all the necessary requirements for continuing education of the attendees to maintain their licenses.
All participants are certified in Nuclear Medicine and are actively involved in Nuclear Imaging studies. Participants were told patients had undergone only one injection of sestamibi and to presume the isotope did not redistribute. Given the premise that sestamibi does not redistribute, the initial (5-minute) and sequential (60-minute) images would then be identical.
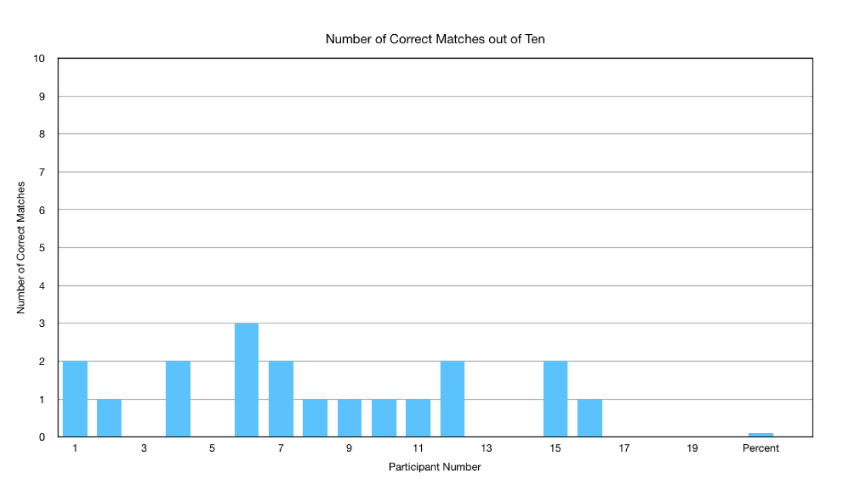
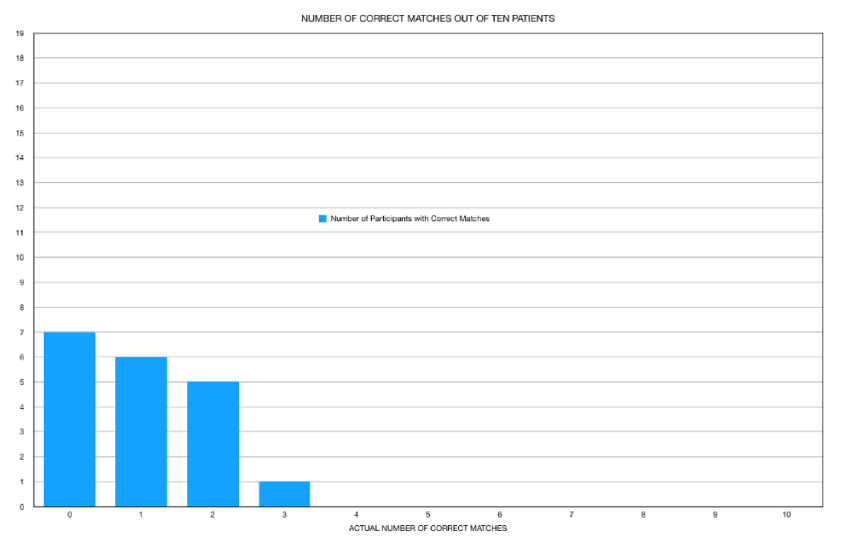
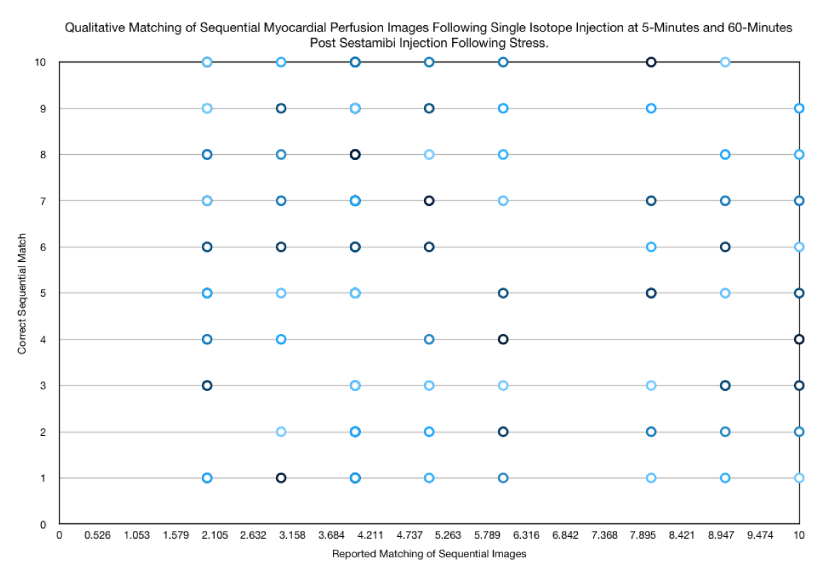
The patient acquired images were displayed with the initial images projected onto one conference screen and the sequential images displayed on another conference screen. Participants were allowed to freely move around and analyze the images anywhere in the conference room, including going up to the screens based upon each individual’s preference for reading the images. The results were then recorded, analyzed and graphed as shown in figures 1-3.
Results
The actual clinical myocardial perfusion imaging (MPI) findings, which were “measured/quantified” [37-40] during the initial 5-minute and sequential 60-minute MPIs are shown in table 1.
As shown in table 2 and figure 1, of the 19 participants, 7 (36.8%) incorrectly matched all 10-patient studies. Six (31.6%) individuals were able to match 1 of the 10 sets of images with 5 participants correctly matching 2 of the 10 patient results. Only 1 participant correctly matched a third study. No participants were able to match 4, 5, 6, 7, 8, 9 or 10 of the studies. The cumulative results are shown in figure 2.
Further analysis of the results reported by participants (Figure 3) revealed absolutely no pattern in the answers provided by participants absent the one patient who had a dilated heart (Table 1). On further questioning of participants, the cumulative correct matches (19 of 190) revealed that 4 of the 19 (21% of correct matches) was the direct result of participants identifying the one heart as dilated and not based upon their ability to match perfusion results. Excluding this patient with a dilated heart; participants correctly matched images only 8% of the time (15 of 180).
Discussion
Based upon the premise that sestamibi does not redistribute and that the isotope is immediately (within 5-minutes) taken up by myocardial tissue and retained, any defects seen at 60-minutes would be identical to that, which would be seen at 5-minutes. In each of these 10-patient studies the ischemia present in the sequential 60-minute images was different from the 5-minute images and they were different for each individual patient as detailed in table 1 [41]. The actual quantitatively measured redistribution varied depending upon the extent of ischemia determining wash-in and washout as already described [42] in this journal. Consequently, the corresponding initial 5-minute images would also be different from each other if there were no redistribution and sestamibi uptake was immediate as stipulated by Lantheus Medical Imaging.
If the images are consequently identical, then the matching rate should be nearly 100% for individuals experienced in MPI. The participants in this study were in fact such experienced professionals. All individuals are licensed in the state of Florida and received continuing education credit by the State of Florida approved through Health and Human Services for attendance and participation. The Florida Board of Pharmacy has also submitted for approval. Many of these individuals had a decade or more of clinical experience with one having 49-years.
As can be seen from the results (Table 2, Figures 1-3), more than two-thirds of the trained, experienced professionals matched one or none of the studies. If sestamibi does not redistribute, then it would have been relatively easy for these professionals to match the initial images with the corresponding identical sequential images. The only logical explanation for the inability of these professionals to be able to match the initial and sequential images is that the results of the cardiac images changed between the initial and sequential images. The only way these images could have changed is if the results of the perfusion imaging had changed. The only way the perfusion imaging could have changed is for redistribution to have occurred.
The only alternative to this logical conclusion is that these trained professionals are the cause of the failure to correctly match images from 10-patients. If this is the case then we have a much more serious problem in our medical system than anyone can account for or consider acceptable considering the critical significance of coronary artery disease and the potential loss of life from diagnostic errors. It would mean that the entire training, certification and continued education of professionals within the nuclear imaging component of medicine is flawed and would require correction. No, this is not the problem!
The misrepresentation that sestamibi does not redistribute, has resulted in each patient undergoing myocardial perfusion imaging (MPI) receiving a second injection of radioactive isotope since the introduction of the Tc-99m isotopes in the late 1980’s. Using a conservative estimate [43] there are approximately 10 million MPI studies performed each year in the United States. Using 10 mCi as an estimate for these second injected doses of radioactive isotope, this means there has been an additional 3 Billion millicuries or an extra 3 Million curies radiation being given to patients; radiation which hospital and clinic personnel have been exposed to, which was not medically needed. Placed in perspective, the Fukushima Daiichi 2011 event released 10 Million curies.
These same conservative estimates would place the sale of these 300 million additional second injections around $12 billion. Diagnostically, by failing to image at 5-minutes post-stress and sequentially initiating imaging at 60-minutes, there is a failure to find up to 40% of actionable ischemic heart disease (redistribution “wash-in”). Hence, there is a conservative [43] death rate of 100 thousand Americans each year equaling approximately 3 million potential deaths due to misdiagnosis by failing to look for ischemic heart disease at the right time. This is a problem much more critical than simply looking the wrong direction to witness a car accident.
Conclusion
The evidence from multiple clinical studies have clearly demonstrated that based upon ischemia and consequential cellular injury, sestamibi is taken up by cells of the heart beginning almost immediately, with sequential changes occurring based upon regional blood flow changes and cellular metabolism/viability [1-34]. The extent of this disease is reflected by the “redistribution pattern” (wash-in or washout) seen when image acquisition occurs at the correct time [37-42] to see the redistribution. While quantification of these differences enhances the diagnostic capabilities of MPI, it is clear that Sestamibi redistributes, just like Tl-201 redistributes and every other drug introduced into the body redistributes.
This information has been publicly available through peer reviewed medical journals since the introduction of sestamibi. Despite multiple published papers [1-34], Lantheus has continued to deny sestamibi Redistribution; denial of which has resulted in (1) a significant increase in isotope sales, (2) increased radiation exposure of patients and clinical imaging and ancillary personnel and (3) the failure to detect critical heart disease by the insistence of a timing sequence too late to discover many critically ill patients.
Compliance with Ethical Standards
Possible COI: “The Fleming Method for Tissue and Vascular Differentiation and Metabolism (FMTVDM) using same state single or sequential quantification comparisons” patent issued to first author. However, the MPIs used in this study were qualitatively compared by the experts and did not use the quantitative results obtained using FMTVDM, therefore we believe there is no actual COI.
The study did not require experimentation on human subjects or animals. All MPIs were from prior studies performed in diagnostic clinical laboratories where informed consent was obtained for MPI imaging. No other informed consent was required. The nuclear medicine professionals all volunteered to participate in this study.
We would like to thank Mr. Brian Wheeler, AGS, NRP, Senior Lecturer, UCLA Paramedic Program for his thoughts, insights and experience into pediatric intubation.
- Sheikine Y, Berman DS, Di Carli MF (2010) Technetium-99m-Sestamibi Redistribution after Exercise Stress Test Identified by a Novel Cardiac Gamma Camera: Two Case Reports. Clinical Cardiology 33: E39-E45. Link: https://bit.ly/2WSj6yh
- Fallahi B, Haghighatafshar M, Farhoudi F, Salehi Y, Aghahosseini F (2014) Comparative evaluation of the diagnostic accuracy of 99mTc-sestamibi gated SPECT using five different sets of image acquisitions at stress and rest phases for the diagnosis of coronary artery disease. Am J Nucl Med Mol Imaging 4:10-16. Link: https://bit.ly/2Kvv2zy
- Beiki D, Fallahi B, Mohseni Z, Khalaj A, Fard-Esfahani A, et al. (2010) Initial and delayed stress phase imaging in a single-injection double-acquisition SPECT. The potential value of early 99mTc-MIBI redistribution in assessment of myocardial perfusion reversibility in patients with coronary artery disease. Nuklearmedizin 49: 19-27. Link: https://bit.ly/31RgcIS
- Crane P, Laliberté R, Heminway S, Thoolen M, Orlandi C (1993) Effect of mitochondrial viability and metabolism on technetium-99m-sestamibi myocardial retention. Eur J Nucl Med 20: 20-25. Link: https://bit.ly/2Y4zdph
- Holman BL, Jones AG, Lister-James J, Davison A, Abrams MJ, et al. (1984) A new Tc-99m-labeled myocardial imaging agent, Hexakis(t-butylisonitrile)-Technetium(I) [Tc-99m TBI]: Initial experience in the human. Journal of Nuclear Medicine 25: 1350-1355. Link: https://bit.ly/2WXFnpw
- Li QS, Solot G, Frank TL, Wagner HN Jr, Becker LC (1990) Myocardial redistribution of Technetium-99m-Methoxyisobutyl Isonitrile (SESTAMIBI). Journal of Nuclear Medicine 31: 1069-1976. Link: https://bit.ly/2Fkf5bh
- Maublant JC, Gachon P, Moins N (1988) Hexakis (2-methoxy isobutylisonitrile) technetium-99m and thallium-201 chloride: uptake and release in cultured myocardial cells. Journal of Nuclear Medicine 29: 48-54. Link: https://bit.ly/2KvFSFL
- Pace L, Catalano L, Del Vecchio S, De Renzo A, Fonti R, et al. (2005) Washout of [99mTc] sestamibi in predicting response to chemotherapy in patients with multiple myeloma. The Quarterly Journal of Nuclear Medicine and Molecular Imaging 49: 281-285. Link: https://bit.ly/31L4qA5
- Hurwitz GA, Ghali SK, Husni M, Slomka PJ, Mattar AG, et al. (1998) Pulmonary uptake of Technetium-99m- Sestamibi induced by dipyridamole-based stress or exercise. Journal of Nuclear Medicine 39: 339-345. Link: https://bit.ly/2X1dFNB
- Hurwitz GA, Fox SP, Driedger AA, Willems C, Powe JE (1993) Pulmonary uptake of sestamibi on early post-stress images: angiographic relationships, incidence and kinetics. Nucl Med Commun 14: 15-22. Link: https://bit.ly/2XzrQJu
- Saha M, Farrand TF, Brown KA (1994) Lung uptake of technetium-99m-sestamibi: relation to clinical, exercise, hemodynamic, and left ventricular function variables. J Nucl Cardiol 1: 52-56. Link: https://bit.ly/2Ydb0x7
- Giubbini R, Campini R, Milan E, Zoccarato O, Orlandi C, et al. (1995) Evaluation of technetium-99m-sestamibi lung uptake: correlation with left ventricular function. J Nucl Med 36: 58-63. Link: https://bit.ly/2RuRKIU
- Sugiura T, Takase H, Toriyama T, Goto T, Ueda R, et al. (2006) Usefulness of Tc-99m methoxyisobutylisonitrile scintigraphy for evaluating congestive heart failure. J Nucl Cardiol 13: 64-68. Link: https://bit.ly/2IxJHb9
- Kumita S, SeinoY, Cho K, Nakaio H, Toba M, et al. (2002) Assessment of myocardial washout of Tc-99msestamibi in patients with chronic heart failure: comparison with normal control. Annals of Nuclear Medicine 16: 237-242. Link: https://bit.ly/2LdP1SG
- Matsuo S, Nakae I, Tsutamoto T, Okamoto N, Horie M (2007) A novel clinical indicator using Tc-99m sestamibi for evaluating cardiac mitochondrial function in patients with cardiomyopathies. J Nucl Cardiol 14: 215-220. Link: https://bit.ly/2x89Tmj
- Ikawa M, Kawai Y, Arakawa K, Tsuchida T, Miyamori I, et al. (2007) Evaluation of respiratory chain failure in mitochondrial cardiomyopathy by assessments of 99mTc- MIBI washout and 123I-BMIPP/99mTc-MIBI mismatch. Mitochondrion 7: 164-170. Link: https://bit.ly/2xbirc9
- Sperker B, Karsten C, Meyer Zu Schwabedissen H, Seeland U, et al. (2002) Expression and localization of P-glycoprotein in Human Heart: Effects of Cardiomyopathy. J Histochem Cytochem 50: 1351-1356. Link: https://bit.ly/2Ydc589
- Ono S, Yamaguchi H, Takayama S, Kurabe A, Heito T (2002) Rest delayed images on 99mTc-MIBI myocardial SPECT as a noninvasive screen for the diagnosis of vasospastic angina pectoris. Kaku Igaku 39: 117-124. Link: https://bit.ly/2XnsNV7
- Ono S, Takeishi Y, Yamaguchi H, Abe S, Tachibana H, et al. (2003) Enhanced regional washout of technetium-99msestamibi in patients with coronary spastic angina. Annals of Nuclear Medicine 17: 393-398. Link: https://bit.ly/2RxbAD9
- Fukushima K, Momose M, Kondo C, Kusakabe K, Kasanuki H (2007) Myocardial kinetics of (201) Thallium, (99m) Tc-tetrofosmin, and (99m) Tc-sestamibi in an acute ischemia-reperfusion model using isolated rat heart. Ann Nucl Med 21: 267-273. Link: https://bit.ly/2N4gcSw
- VanBrocklin HF, Hanrahan SM, Enas JD, Nandanan E, O’Neil JP (2007) Mitochondrial avid radioprobes. Preparation and evaluation of 7’(Z)-[125I] iodorotenone and 7’(Z)-[125I] iodorotenol. Nucl Med Biol 34: 109-116. Link: https://bit.ly/2Y5TDOn
- Tanaka R, Nakamura T, Chiba S, Ono T, Yoshitani T, et al. (2006) Clinical implication of reverse redistribution on 99mTc-sestamibi images for evaluating ischemic heart disease. Ann Nucl Med 20: 349-356. Link: https://bit.ly/2L5SliV
- Liu Z, Johnson G, Beju D, Okada RD (2001) Detection of myocardial viability in ischemicreperfused rat hearts by Tc-99m sestamibi kinetics. Journal of Nuclear Cardiology 8: 677-686. Link: https://bit.ly/2RrZNpH
- Shin WJ, Miller K, Stipp V, Magour S, Mazour S (1995) Reverse redistribution on dynamic exercise and dipyridamole stress technetium-99m-MIBI myocardial SPECT. J Nucl Med 36: 2053-2055. Link: https://bit.ly/31L5IuV
- Takeishi Y, Sukekawa H, Fujiwara S, Ikeno E, Sasaki Y, et al. (1996) Reverse redistribution of technetium- 99m-sestamibi following direct PTCA in acute myocardial infarction. J Nucl Med 37: 1289-1294. Link: https://bit.ly/2FstqCm
- Fujiwara S, Takeishi Y, Atsumi H, Yamaki M, Takahashi N, et al. (1998) Prediction of functional recovery in acute myocardial infarction: comparison between sestamibi reverse redistribution and sestamibi/BMIPP mismatch. J Nucl Cardiol 5: 119-127. Link: https://bit.ly/2KwpUuW
- Ayalew A, Marie PY, Menu P, Mertes PM, Hassan N, et al. (2000) A comparison of the overall first-pass kinetics of thallium-201 and technetium-99m MIBI in normoxic and low-flow ischaemic myocardium. Eur J Nucl Med 27: 1632-1640. Link: https://bit.ly/2J1syWn
- Richter WS, Cordes M, Calder D, Eichstaedt H, Felix R (1995) Washout and redistribution between immediate and two-hour myocardial images using technetium-99m sestamibi. European The Journal of Nuclear Medicine 22: 49-55. Link: https://bit.ly/2Y6D6K4
- Meerdink DJ, Leppo JA (1990) Myocardial transport of hexakis(2-methosyisobutyl isonitrile) and thallium before and after coronary reperfusion. Circulation Research 66: 1738-1746.
- Ayalew A, Marie PY, Menu P, Mertes PM, Audonnet S, et al. (2002) 201 Tl and 99m Tc-MIBI retention in an isolated heart model of low-flow ischemia and stunning: Evidence of negligible impact of myocyte metabolism on tracer kinetics. J Nucl Med 43: 566-574. Link: https://bit.ly/2J4haJt
- Takahashi N, Reinhardt CP, Marcel R, Leppo JA (1996) Myocardial uptake of 99m Tc-tetrofosmin, Sestamibi, and 201 Tl in a model of acute coronary reperfusion. Circulation 94: 2605-2613. Link: https://bit.ly/2Y4w74x
- Tanaka R, Nakamura T, Chiba S, Ono T, Yoshitani T, et al. (2006) Clinical implication of reverse redistribution of 99mTc-sestamibi images for evaluating ischemic heart disease. Annals of Nuclear Medicine 20: 349-356. Link: https://bit.ly/2L5SliV
- Sinusas AJ, Bergin JD, Edwards NC, Watson DD, Ruiz M, et al. (1994) Redistribution of 99mTc-Sestamibi and 201-Tl in the Presence of a Severe Coronary Artery Stenosis. Circulation 89: 2332-2341. Link: https://bit.ly/2IASZ6x
- Taillefer R, Primeau M, Costi P, Lambert R, Léveillé J, et al. (1991) Technetium-99m-Sestamibi Myocardial Perfusion Imaging in Detection of Coronary Artery Disease: Comparison Between Initial (1-Hour) and Delayed (3-Hour) Postexercise Images. J Nucl Med 32: 1961-1965. Link: https://bit.ly/2IYs4QQ
- Blumgart HL, Yens OC (1926) Studies on the velocity of blood flow: I. The method utilized. J Clin Invest 4: 1-13. Link: https://bit.ly/2xaTyNO
- Drew T, Vo MLH, Wolfe JM (2013) The invisible gorilla strikes again: Sustained inattention blindness in expert observers. Psych Sci 9: 1848-1853. Link: https://bit.ly/2xmKfZM
- Fleming RM, Chaudhuri TK, McKusick A (2019) The FDA, HHS, Sestamibi Redistribution and Quantification. Acta Scientific Pharmaceutical Sciences 3: 47-69. Link: https://bit.ly/2L4u4db
- Fleming RM, Fleming MR, Chaudhuri TK, McKusick A (2019) Definitive Human Studies Showing Indisputable Proof of Clinically Significant Sestamibi Redistribution. J Clin Med Imag and Short Reports 1: 1-7. Link: https://bit.ly/2WZeim6
- Fleming RM, Fleming MR, McKusick A, Chaudhuri TK (2018) FMTVDM©℗ Nuclear Imaging Artificial (AI) Intelligence but fires we need to clarify the use of (1) Stress, (2) Rest, (3) Redistribution and (4) Quantification. Biomed J Sci & Tech Res 7: 1-4. Link: https://bit.ly/2Net494
- Fleming RM, Fleming MR, McKusick A, Chaudhuri T (2018) FMTVDMTFM©℗: True Quantification requires Standardization of the tool being used to Measure, with a Known, Unchanging Standard to produce accurate, consistent and reproducible Quantified Measurements. J Nucl Card 1-4. Link: https://bit.ly/2N6bQup
- Fleming RM, Fleming MR, Chaudhuri TK, Mckusick A (2019) Definitive Human Studies Demonstrating Clinically Important Sestamibi Redistribution. ACTA Sci Med Sci 3: 66-77.
- Fleming RM, Fleming MR, Chaudhuri TK (2019) FMTVDM provides first patented Quantitative Method to accurately Measure both Heart Disease and Breast Cancer on the “Health-Spectrum. J Cardiovasc Med Cardiol 6: 019-020.
- (2011) Nuclear Medicine Communications 1997 (vol 18) and Canadian Medical Association Journal.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
