Journal of Cardiovascular Medicine and Cardiology
Myocardial bridges and competitive sports fitness between past and future
Assisi Elio1*, E Libener2, S Grossgasteiger1, Ottavio Marine1 and S Resnyak1
2School of Specialisation in Sports Medicine, University of Verona, Italy
Cite this as
Elio A, Libener E, Grossgasteiger S, Marine O, Resnyak S (2023) Myocardial bridges and competitive sports fitness between past and future. J Cardiovasc Med Cardiol 10(1): 012-016. DOI: 10.17352/2455-2976.000193Copyright License
© 2023 Elio A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.The myocardial bridge is an intramural course of a coronary artery in which a more or less long section of a coronary branch, instead of running normally on the epicardial surface of the heart, deepens early in the myocardium, coming to be surrounded by a ring or sleeve of muscle fiber cells that, contracting in systole, can cause an ab-extrinsic “throttling” of the artery. The guidelines for granting eligibility for competitive sports have evolved over time: from 2009, when any case of myocardial bridging indicated exclusion of eligibility, to today, when only significant bridges, i.e. ‘long’ bridges > 1 cm and > 3 mm deep, place a restriction on competitive sports activity.
Introduction
The myocardial bridge is defined as an anatomical coronary variant where a segment of an epicardial coronary artery has an intramural course through the myocardium, defining the so-called “tunneled coronary artery”.
It was first described during an autopsy in 1737 by Reyman and the first angiographic relief was in 1960 by Portmann and Iwing.
The affected segment is usually the middle/distal tract of the anterior descending (DA) and angiographic images show a systolic compression of the epicardial coronary artery although studies with Doppler and intracoronary ultrasound have also documented alterations in flow in the diastolic phase. The degree of coronary obstruction depends on certain factors such as the thickness and length of the bridge and the degree of myocardial contractility.
That a section of the DA decorated below the muscle fibers cannot be considered per se as a congenital affection of the coronary arteries, most often as an anatomical variant of their course, without significant pathophysiological and clinical consequences.
In a small number of cases (0.5%-2.5%), however, AD tunneling may take on the characteristics of a congenital coronary disease.
In fact, in systole, the tunneled tract may be compressed by the muscular bands interfering with the flow of the coronary artery; this interference with the flow inevitably leads to the potential for ischaemia with its associated consequences.
The two forms are distinguished from each other by the anatomical and functional characteristics of the muscle fibers that make up the tunnel.
If the anterior descendant is covered by the superficial oblique fibers which cross the two ventricles obliquely, it appears covered by thin and relatively short fibers which are not capable of exerting a systolic compression able to interfere with the coronary reserve.
This variant does not show up distinctly on angiography, is not usually associated with clinical symptoms, and does not change the patient’s prognosis.
If, on the other hand, the anterior descending is tunneled by deep, thicker, and longer fibers, with a right ventricle-ventricular septum apex course, the DA in the tunneled tract will appear displaced towards the right and in-depth, and above all, it can be compressed in systole to a different degree; this variant will be visible on angiography (milking effect) and has the potential to modify the coronary reserve to be modified.
The association of vasospasm or endoluminal thrombosis may contribute to the hemodynamic significance and clinical symptomatology of these conditions. In fact, abnormal coronary vasoreactivity has been reported after administration of acetylcholine at the level of the myocardial bridge; this explains both the permanent asymptomaticity of the majority of subjects carrying the anomaly and the variability in the time of onset of symptoms, as well as the broad spectrum of manifestations ascribable to the anomaly in published clinical case histories (stress angina, myocardial infarction, unstable angina, episodes of acute pulmonary edema, ventricular tachycardia, sudden death) [1-5].
In addition, atheromatous stenosis of other coronary districts, particularly proximal to the bridge, may also coexist.
The diagnosis is made by means of coronary CT angiography or coronarography when patients generally present with ‘classic’ angina symptoms and with positive ischaemia provocative tests. It is also important, for the purposes of prognosis and therapeutic options, to establish the haemodynamic efficiency of the obstruction and to exclude the coexistence of atherosclerotic coronary stenoses that may contribute to the symptoms experienced by the patient.
The aim of our study is to examine and evaluate the evolution over time of the inclusion criteria for competitive sports fitness in subjects who underwent a sports medicine examination from 2014 to 2021 at the Sports Medicine Center of the Provincial Service of the South Tyrolean Health Authority and were found to have at least one myocardial bridge after undergoing a third-level cardiological examination such as coronary angiography.
Can subjects who have a superficial (< 3 mm deep) but > 1 cm long, or > 3 mm deep and short course myocardial bridges (< 1 cm) be considered fully eligible?
Materials and methods
A total of 18972 users over 35 years of age were analyzed for competitive sports activity eligibility at the Sports Medicine Center of the Provincial Service of the South Tyrolean Health Authority. The 0,9% of them, which corresponds to 183 patients (126 men and 57 women) were tested for our study because they presented a strongly doubtful maximal ergometric test of stress-inducible myocardial ischaemia.
The inclusion criteria on which the investigation was based were the symptoms (angina pectoris, dyspnoea, extrasystolic tachycardic heartbeat) and above all the presence of significant horizontal/descending underdevelopment equal to or greater than 2 mm during the maximum exercise test.
On the basis of the diagnostic suspicion of reduced coronary reserve, the 183 users were subjected to an in-depth level third cardiological diagnosis by means of coronary angiography.
A memorandum of understanding was introduced between the Company Service of Sports Medicine and the Radiology Service of the South Tyrol Health Authority on which users were referred with the diagnostic question of ischaemic heart disease and, in the specific case of our study, myocardial bridging with the request for, in the event of the latter being found, characterization in terms of length and depth in the light of the importance of this in terms of prognostic suitability.
Results
The coronary CT angiography response of the 183 users was positive for myocardial bridging in 15% of cases, which means 27 subjects (Table 1).
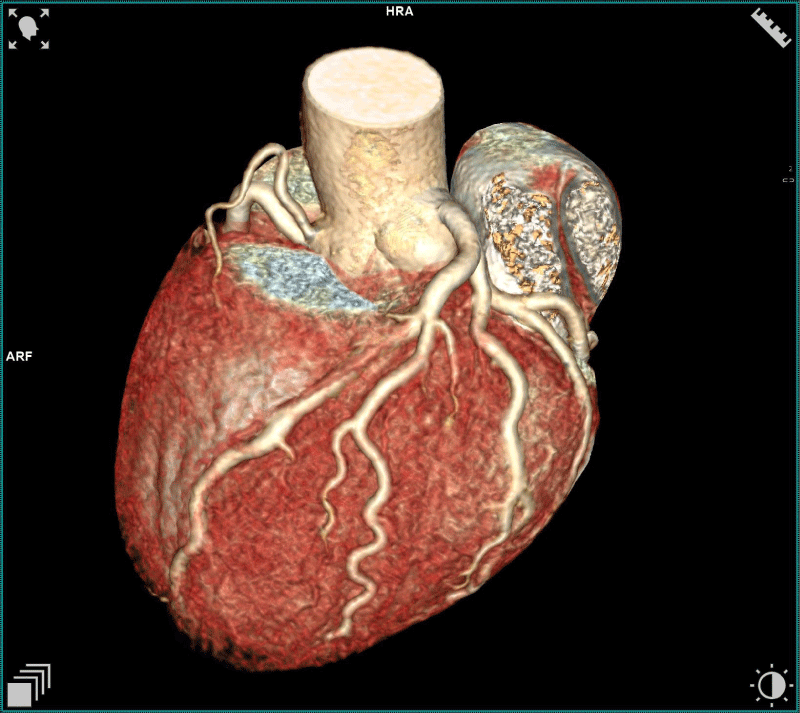
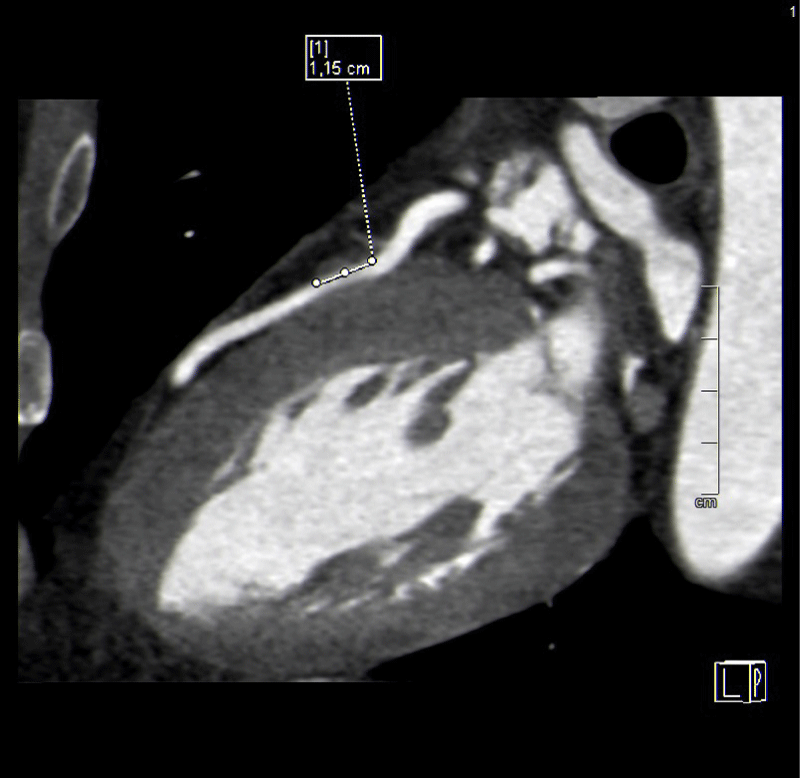
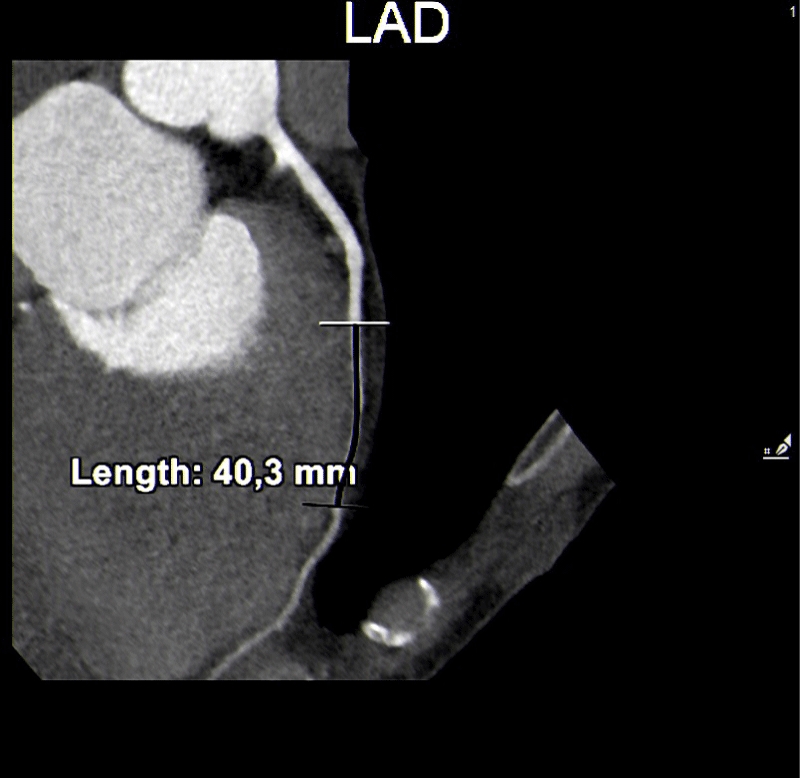
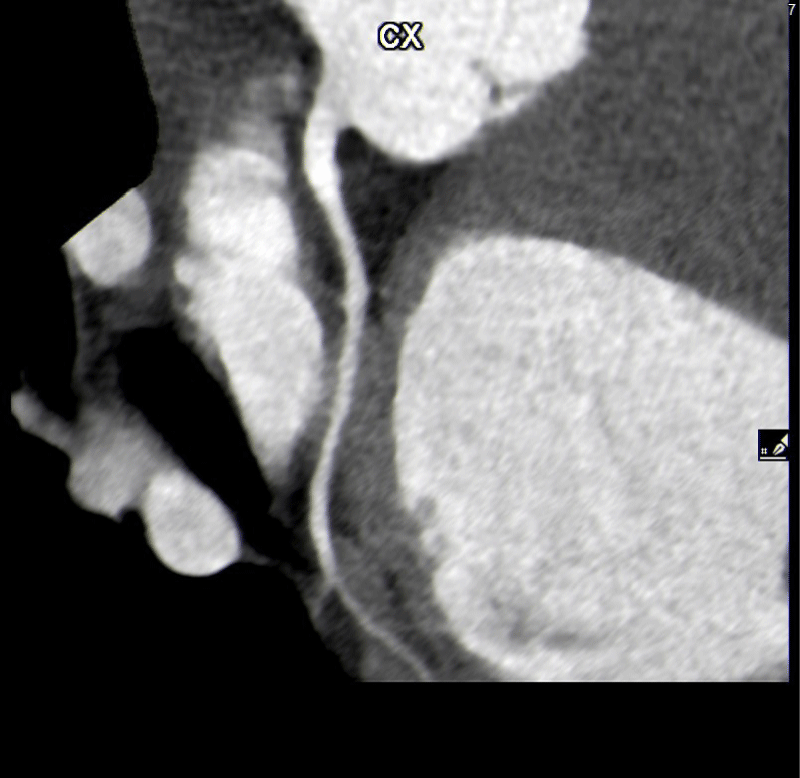
Of the 27 users with positive coronary CT angiograms, 13 (48%) had a short and superficial course of the myocardial bridges in the anterior descending. In one case, which turned out to be the most complex from a prognostic point of view, there were two myocardial bridges: the bridge on the anterior descending was >1 cm in length and superficial < 3 mm, while in the circumflex artery, the bridge was < 1 cm and < 3 mm long. (Figures 1-3).
We also found 3 (11%) cases of isolated myocardial bridging in the right coronary artery, also non-significant, i.e. short and superficial.
There were five cases (18,5%) of intramyocardial bridges of the right coronary artery and the anterior descending artery at the same time, which would have given us prognostic doubts if it was not for the fact that the patients, asymptomatic as all those tested, had short and superficial intramural segments.
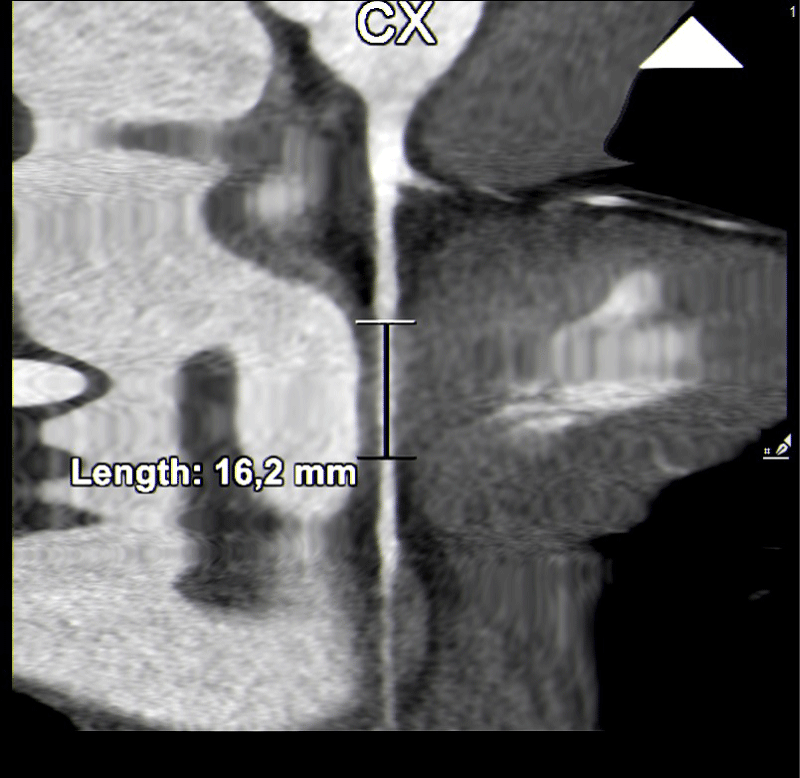
Finally, five cases (18,5%) of myocardial bridging were found over a circumflex coronary artery, only one of which was more than 3 mm deep (Figures 4,5).
This means that out of the 183 patients with a positive stress test, none of the subjects examined presented significant bridges, according to the 2017 COCIS guidelines, with the coexistence of both conditions of length (> 1 cm) and depth (> 3 mm).
Discussion
A myocardial bridge is frequently found at autopsy (30% of cases) but these are, in the majority of cases, thin muscular shoots causing insignificant compression of the vessel in systole, whereas the most significant bridges are visualized in 5% of coronarographies.
Myocardial bridges with a long deeply tunneled segment (most frequently the anterior descending artery) can cause exertional ischaemia, be symptomatic for exertional angina, and be related to some cases of sudden death during sporting activity.
So much has been done over time in terms of the criteria for judging suitability for competitive sport in Italy, fundamental criteria to be followed when formulating eligibility for competitive sport, the certification of which has been compulsory by law since the ministerial decree of 1982 [6-10].
In the past, according to the 2009 COCIS eligibility protocols, athletes with a myocardial bridge, even without evidence of exertional ischaemia, could be at risk of sudden death, which is why they were excluded from competitive sports eligibility apart from sports with a ‘neurogenerative’ type of cardiovascular commitment, i.e. bowls, golf, sport hunting, billiards, bridge, draughts, chess.
Today, however, (COCIS 2017 to the present day), the assessment in this regard is much more defined as myocardial bridges have been classified into significant long and deep ones, with both conditions of length ( > 1 cm) and depth ( > 3 mm) having to coexist as the only abnormal autopsy finding in some cases of sudden death occurring during physical activity.
From a forensic point of view, a number of issues arise: in terms of criminal liability, it may be necessary to establish whether the autopsy finding of a myocardial bridge can be correlated with the death of a patient and, if so, whether it was possible to reach such a diagnosis while the patient was alive, i.e., whether the abnormality had been diagnosed and the appropriate treatment had been taken [11-20].
In the absence of anamnestic findings of symptoms and/or angina equivalents and transient ischaemia on instrumental examinations, the autopsy finding of myocardial bridging is to be considered occasional.
The problem arises in cases of acute myocardial infarction or sudden death. In fact, if myocardial necrosis affects the area supplied by the coronary artery with a myocardial bridge, it is possible to attribute the infarction itself to an acute obstruction at the level of the bridge. In the case of sudden death, in the absence of documentable acute myocardial necrosis, the dilemma arises as to whether the myocardial bridge may have triggered a malignant ventricular arrhythmia (sustained ventricular tachycardia or ventricular fibrillation). In this case, the presence or absence of anamnestic and instrumental findings of ischaemia (resting ECG, ergometric tests, Holter ECG), the absence of other pathologies that could justify the triggering of a malignant ventricular arrhythmia (long QT syndromes, Brugada syndrome, Wolff Parkinson White syndrome, the absence of significant atheromatous lesions in the remaining coronary tree) may help to settle the question [21-29].
Conclusion
In our study, of the 183 users with a doubtful ergometric test for exertion ischaemia, none presented significant bridges with the coexistence of both conditions of length (> 1 cm) and depth (> 3 mm). In view of this finding, since these sportsmen are fit for competitive sport according to the 2017 COCIS guidelines, which replace the previous 2009 guidelines, more restrictive in this aspect, we wonder whether it is legitimate to count these ergometries as false positives and thus exclude a causal link between the ST sub slope of 2 mm at the various sites and the angiographic finding of myocardial bridging. Another hypothesis could be a possible ischaemic effect in the systolic phase such as to produce such a marked graphic ECG sign: in this case, however, there could be a reduction in the coronary lumen that does not produce symptoms. Further studies, as well as a long-term clinical follow-up of these patients, are needed to elaborate a standard approach for the diagnosis and management of these asymptomatic sports patients.
Clearly the picture changes in the case of symptoms such as angina, heart palpitation or tachycardia in which, beyond any classification, the patient is a candidate for medical therapy or stent implantation in the trapped segment or even for surgical treatment.
- Piseri M., Halasz G., Biasini V., Capelli B., Nardecchia S., Fallavolita L., Petroni R., Penco M., Romano S.. Electrocardiographic characteristics of a population of athletes aged 8-16 years: a comparison with the young adult athlete. Sports Medicine. 2019 Sep;72(3):385-94
- Landry CH, Allan KS, Connelly KA, Cunningham K, Morrison LJ, Dorian P; Rescu Investigators. Sudden Cardiac Arrest during Participation in Competitive Sports. N Engl J Med. 2017 Nov 16;377(20):1943-1953. doi: 10.1056/NEJMoa1615710. PMID: 29141175; PMCID: PMC5726886.
- Karam N, Marijon E, Jouven X. Cardiac Arrest during Competitive Sports. N Engl J Med. 2018 Apr 12;378(15):1463. doi: 10.1056/NEJMc1802593. PMID: 29648426.
- Pelliccia A, Fagard R, Bjørnstad HH, Anastassakis A, Arbustini E, Assanelli D, Biffi A, Borjesson M, Carrè F, Corrado D, Delise P, Dorwarth U, Hirth A, Heidbuchel H, Hoffmann E, Mellwig KP, Panhuyzen-Goedkoop N, Pisani A, Solberg EE, van-Buuren F, Vanhees L, Blomstrom-Lundqvist C, Deligiannis A, Dugmore D, Glikson M, Hoff PI, Hoffmann A, Hoffmann E, Horstkotte D, Nordrehaug JE, Oudhof J, McKenna WJ, Penco M, Priori S, Reybrouck T, Senden J, Spataro A, Thiene G; Study Group of Sports Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology; Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Recommendations for competitive sports participation in athletes with cardiovascular disease: a consensus document from the Study Group of Sports Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2005 Jul;26(14):1422-45. doi: 10.1093/eurheartj/ehi325. Epub 2005 May 27. PMID: 15923204.
- Corrado D, Basso C, Rizzoli G, Schiavon M, Thiene G. Does sports activity enhance the risk of sudden death in adolescents and young adults? J Am Coll Cardiol. 2003 Dec 3;42(11):1959-63. doi: 10.1016/j.jacc.2003.03.002. PMID: 14662259.
- Angelini P, Velasco JA, Flamm S. Coronary anomalies: incidence, pathophysiology, and clinical relevance. Circulation. 2002 May 21;105(20):2449-54. doi: 10.1161/01.cir.0000016175.49835.57. PMID: 12021235.
- Angelini P, Trivellato M, Donis J, Leachman RD. Myocardial bridges: a review. Prog Cardiovasc Dis. 1983 Jul-Aug;26(1):75-88. doi: 10.1016/0033-0620(83)90019-1. PMID: 6346395.
- Reyman HC. Diss. de vasis cordis propriis. Bibl Anat. 1737;2:359-379.
- PORSTMANN W, IWIG J. [Intramural coronary vessels in the angiogram]. Fortschr Geb Rontgenstr Nuklearmed. 1960 Feb;92:129-33. German. PMID: 14434245.
- Faruqui AM, Maloy WC, Felner JM, Schlant RC, Logan WD, Symbas P. Symptomatic myocardial bridging of coronary artery. Am J Cardiol. 1978 Jun;41(7):1305-10. doi: 10.1016/0002-9149(78)90890-1. PMID: 307341.
- Channer KS, Bukis E, Hartnell G, Rees JR. Myocardial bridging of the coronary arteries. Clin Radiol. 1989 Jul;40(4):355-9. doi: 10.1016/s0009-9260(89)80118-7. PMID: 2527105.
- Irvin RG. The angiographic prevalence of myocardial bridging in man. Chest. 1982 Feb;81(2):198-202. doi: 10.1378/chest.81.2.198. PMID: 7056084.
- Schwarz ER, Klues HG, vom Dahl J, Klein I, Krebs W, Hanrath P. Functional, angiographic and intracoronary Doppler flow characteristics in symptomatic patients with myocardial bridging: effect of short-term intravenous beta-blocker medication. J Am Coll Cardiol. 1996 Jun;27(7):1637-45. doi: 10.1016/0735-1097(96)00062-9. PMID: 8636548.
- Ge J, Erbel R, Rupprecht HJ, Koch L, Kearney P, Görge G, Haude M, Meyer J. Comparison of intravascular ultrasound and angiography in the assessment of myocardial bridging. Circulation. 1994 Apr;89(4):1725-32. doi: 10.1161/01.cir.89.4.1725. PMID: 8149538.
- Klues HG, Schwarz ER, vom Dahl J, Reffelmann T, Reul H, Potthast K, Schmitz C, Minartz J, Krebs W, Hanrath P. Disturbed intracoronary hemodynamics in myocardial bridging: early normalization by intracoronary stent placement. Circulation. 1997 Nov 4;96(9):2905-13. doi: 10.1161/01.cir.96.9.2905. PMID: 9386156.
- Sánchez V, Zamorano J. New approach to the diagnosis of myocardial bridging by intracoronary ultrasound and Doppler. Eur Heart J. 1999 Dec;20(23):1687-8. doi: 10.1053/euhj.1999.1776. PMID: 10562473.
- Kneale BJ, Stewart AJ, Coltart DJ. A case of myocardial bridging: evaluation using intracoronary ultrasound, Doppler flow measurement, and quantitative coronary angiography. Heart. 1996 Oct;76(4):374-6. doi: 10.1136/hrt.76.4.374. PMID: 8983690; PMCID: PMC484555.
- Rossi L, Dander B, Nidasio GP, Arbustini E, Paris B, Vassanelli C, Buonanno C, Poppi A. Myocardial bridges and ischemic heart disease. Eur Heart J. 1980 Aug;1(4):239-45. doi: 10.1093/oxfordjournals.eurheartj.a061125. PMID: 7274234.
- GEIRINGER E. The mural coronary. Am Heart J. 1951 Mar;41(3):359-68. doi: 10.1016/0002-8703(51)90036-1. PMID: 14818948.
- Tauth J, Sullebarger T. Myocardial infarction associated with myocardial bridging: case history and review of the literature. Cathet Cardiovasc Diagn. 1997 Apr;40(4):364-7. doi: 10.1002/(sici)1097-0304(199704)40:4<364::aid-ccd9>3.0.co;2-7. PMID: 9096936.
- Arjomand H, AlSalman J, Azain J, Amin D. Myocardial bridging of left circumflex coronary artery associated with acute myocardial infarction. J Invasive Cardiol. 2000 Aug;12(8):431-4. PMID: 10953110.
- Amitani K, Yamaguchi T, Takahashi N, Uchida T, Kushikata Y, Munakata K. Two cases of myocardial bridging associated with myocardial ischemia [Japanese]. J Nippon Med Sch. 2000;67:206-209.
- Schar B. Myocardial bridging: symptoms of a coronary artery disease that sometimes is not [German]. Schweiz Rundsch Med Prax. 2001; 90:1923-1928.
- Akdemir R, Gunduz H, Emiroglu Y, Uyan C. Myocardial bridging as a cause of acute myocardial infarction: a case report. BMC Cardiovasc Disord. 2002 Sep 21;2:15. doi: 10.1186/1471-2261-2-15. PMID: 12243650; PMCID: PMC128836.
- Yetman AT, McCrindle BW, MacDonald C, Freedom RM, Gow R. Myocardial bridging in children with hypertrophic cardiomyopathy--a risk factor for sudden death. N Engl J Med. 1998 Oct 22;339(17):1201-9. doi: 10.1056/NEJM199810223391704. PMID: 9780340.
- Murtaza G, Mukherjee D, Gharacholou SM, Nanjundappa A, Lavie CJ, Khan AA, Shanmugasundaram M, Paul TK. An Updated Review on Myocardial Bridging. Cardiovasc Revasc Med. 2020 Sep;21(9):1169-1179. doi: 10.1016/j.carrev.2020.02.014. Epub 2020 Feb 18. PMID: 32173330.
- Tauth J, Sullebarger T. Myocardial infarction associated with myocardial bridging: case history and review of the literature. Cathet Cardiovasc Diagn. 1997 Apr;40(4):364-7. doi: 10.1002/(sici)1097-0304(199704)40:4<364::aid-ccd9>3.0.co;2-7. PMID: 9096936.
- Sternheim D, Power DA, Samtani R, Kini A, Fuster V, Sharma S. Myocardial Bridging: Diagnosis, Functional Assessment, and Management: JACC State-of-the-Art Review. J Am Coll Cardiol. 2021 Nov 30;78(22):2196-2212. doi: 10.1016/j.jacc.2021.09.859. PMID: 34823663.
- Aden D, Phulware RH, Mittal S, Ahuja A. Myocardial bridging - Sudden unexpected death of a young girl: A case report. Indian J Pathol Microbiol. 2022 Jan-Mar;65(1):157-159. doi: 10.4103/IJPM.IJPM_1177_20. PMID: 35074984.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
