Journal of Cardiovascular Medicine and Cardiology
A possible easy way to predict response to cardiac resynchronization therapy: The role of QRS Index
Giuseppe Coppola, Cristina Madaudo*, Amedeo Prezioso, Enrico Bonnì, Giuseppina Novo, Vincenzo Sucato, Gianfranco Ciaramitaro, Alfredo Ruggero Galassi and Egle Corrado
Cite this as
Coppola G, Madaudo C, Prezioso A, Bonnì E, Novo G, et al. (2023) A possible easy way to predict response to cardiac resynchronization therapy: The role of QRS Index. J Cardiovasc Med Cardiol 10(1): 001-006. DOI: 10.17352/2455-2976.000191Copyright License
© 2023 Coppola G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Background: Some studies have evaluated the role of QRS duration (QRSd) as predictor of response to Cardiac Resynchronization Therapy (CRT). However, their results are still not entirely clear. The goal of our study was to determine the correlation between the relative change in QRS narrowing index (QI) compared to clinical outcome and prognosis in patients who underwent CRT implantation.
Methods: We collected clinical and echocardiographic data of 115 patients in whome a CRT device was implanted in accordance with current guidelines. QRS duration before and after CRT implantation and QI were measured.
Results: After 6 months, a significant improvement in all echocardiographic parameters was detected. QI was correlated to reverse remodelling (r = +0.19; 95% CI: 0.006 to 0.35, p = 0,049). The value of QI that predicted best LV reverse remodelling after 6 months of CRT was 12.25% (sensitivity = 65,5%, specificity = 75%, area under the curve = 0.737, p = 0,001). Independent predictors of QI are sex, serum creatinine and eGFR measured at baseline and LVEF pre-CRT performed by echocardiography.
We observed an betterment in their HF clinical composite score and NYHA class at 12 months.
We have also investigated the clinical outcomes and the possible sex differences related to QI.
Conclusions: Patients with a larger QI after CRT initiation showed greater echocardiographic reverse remodelling and better outcome from death or cardiovascular hospitalization. QI seems to be an easy-to- measure variable that could be used or evaluated to predict CRT response but further studies are needed.
Introduction
Cardiac Resynchronization Therapy (CRT) is a successful strategy for Heart Failure (HF) patients with severe reduction of Left Ventricular Ejection Fraction (LVEF). The rationale of CRT is based on the possibility of inducing left ventricular reverse remodelling while the pre-requisite for the response is the evidence of electrical dyssynchrony on the surface electrocardiogram usually as Left Bundle Branch Block (LBBB); in HF patients underwent to CRT, left ventricular “reverse remodelling” (changes in LV end-systolic volume relative to baseline ≥10%) has been shown to be able to predict long-term outcome with higher reproducibility and predictive power than changes in LVEF. Then, reverse remodelling is currently considered the strongest predictor of mortality and HF-hospitalization [1-3]. However, since the preliminary studies providing benefits of CRT, the major concerns have been targeted to the problem of responders’ identification considering that a significant proportion of implanted patients fails to respond sufficiently or in a predictable manner. A lot of studies have reported a renewed interest on the role of simple, low cost and easily available electrocardiographic parameters; in particular narrowing of QRS duration has been shown to correlate with favorable structural changes (ventricular volumes, LVEF and even functional mitral regurgitation) tailored by biventricular pacing and outcomes [4-7]. The “early” identification of potential non responder (before six months as recommended) could help physicians in planning the timing for clinical follow-up and device optimization [8].
Materials and methods
Aim and study design
Our study is a retrospective, single-centre cohort study designed to identify a parameter for a simple and precocious identification of responders to CRT. We identified and observed QRS Index (QI), as an aspect of electrical remodelling after CRT, and its connection with anatomic reverse remodelling, ecocardiographically assessed with a six-month follow-up [6-8]. The design of the study has been published previously [8]. The population of our study is 115 patients implanted in our center from 2014 to 2019, who received a device like Pacemaker (PM) or an Implantable Cardioverter Defibrillator (ICD) for cardiac resynchronization therapy administration following the guidelines available at the time of implantation. The study complied with the declaration of Helsinki guidelines.
A transvenous approach through the coronary sinus into the lateral or posterolateral cardiac vein was chosen to insert the Left Ventricular (LV) pacing lead. Clinical and demographic data were collected at the time of the first visit and included: age, sex, etiology of cardiomyopathy, risk factors, other comorbidities, therapy, creatinine level, history of atrial fibrillation, New York Heart Association (NYHA) functional class and electrocardiographic and echocardiography evaluations. Glomerular Filtration Rate (GFR) was calculated using the MDRD equation [9].
All devices were set to the dual chamber pacemaker (DDD) or dual chamber rate adaptive pacemaker (DDDR) mode with standard pacing rates (50 – 60 beats/min – 120 – 130 beats/min). Before patients were discharged, devices were programmed to achieve atrial-synchronous biventricular pacing (for patient in sinus rhythm) and highest percentage of biventricular pacing. Interventricular programmed delay was set automatically, depending by device’s technical characteristics. Specific defibrillation, tachycardia and bradycardia programming is set according to the clinical practice of our center.
12-lead ECGs (25 mm/s, 0.05 mV/mm) were collected at the time of implantation according to international recommendations for the standardization and interpretation of the electrocardiogram [10]. QRS duration was measured during spontaneous conduction and during biventricular pacing. ECG measurements were automatically recorded and subsequently reviewed centrally by two different expert cardiologists, blinded to lead position and echocardiographic and clinical response. The QRS duration was the maximum value in any lead. As described in previous studies, we defined the QI as: [(pre implant QRS duration — QRS duration during CRT)/pre implant QRS duration × 100] with positive values corresponding to QRS narrowing on biventricular pacing [7,8].
End points
Our primary endpoint was the anatomical reverse remodelling at the 6-month follow-up visit. A positive echocardiographic response was defined as a ≥10% decrease in LV end-Systolic Volume (LVESV) from the baseline to the 6-month follow-up visit (2). During follow-up, patients were classified according to a Clinical Composite as follows [8,11]:
− worsened: Death; hospitalization due to or in association with worsening heart failure; worsening of NYHA class; permanent discontinuation of CRT due to or in association with worsening heart failure.
− improved: The patient’s condition has not worsened (as defined above); NYHA class improvement.
− unchanged: The condition of patient has neither improved nor worsened.
The following adverse events were used to assess clinical outcome: death from any cause and hospitalization for non-fatal heart failure exacerbations over a 12-month period after CRT implantation.
Statistical analysis
Continuous variables were expressed with means and standard deviations or medians with interquartile range. Categorical variables were evaluated by percentages on the total population and compared using the χ2 test and Fisher’s exact test. To compare two groups, the Student’s T-test or, when necessary, the Mann-Whitney test was used. ROC analysis was performed to determine the value that with the best sensitivity and specificity of QI predicts left ventricular reverse remodeling after CRT. A multivariate linear analysis was performed using ANOVA type III. A Kaplan-Meier survival analysis was done to measure the number of subjects free from events or survived after that intervention over a period of time.
A two-tailed p - value <0.05 was considered statistically significant. Bonferroni’s correction was used to correct for significance of multiple tests. All statistical analyzes were performed using R studio software (version 1.4.1103 2009 - 2021 RStudio).
Results
We have evaluated 115 patients. Table 1 highlights the basic clinical and echocardiographic parameters and drug therapies.
Patients were mostly male (n = 81, 71%) with a mean age of 66,3 ± 10,4 years; 45 (40%) were in NYHA class III/IV at the time of implantation. The mean value of LVEF was 27 ± 7,3%, with a LVESV of 136,9 ± 59 ml and a LVEDV of 184,2 ± 67,5 ml. The RV lead was positioned in the RV apex in 77 patients (67,5%) and in the RV septum in the remaining patients (32,5%). The LV lead was deployed in a target zone from anterolateral to posterior in 114 patients (99%). The overall mean QRS duration (QRSd) decreased on CRT (from 157,4 ± 18,5 ms at baseline to 132,8 ± 18,8 ms post implantation, p < 0,0001). The median [25th–75th] QI was 15,5% [14–18,1].
After 6 months, a significant improvement in all echocardiographic parameters was observed (LVESV from 136,9 ± 59 ml to 111,3 ± 57,7 ml, p 0,0001; LVEDV from 184,2 ±67,5 ml to 166,2 ± 70 ml, p 0,0001 and LVEF from 27 ± 7,3 % to 33 ± 10%, p 0,0001).
In 73,5% of our population LVESV decreased by 10% or more. The absolute difference between pre implant QRSd and QRSd during CRT (ΔQRS) was found to be statistically associated with CRT response (ΔQRS = 26.5 ms in responders vs. 19,7 ms in non- responders, p = 0,0349).
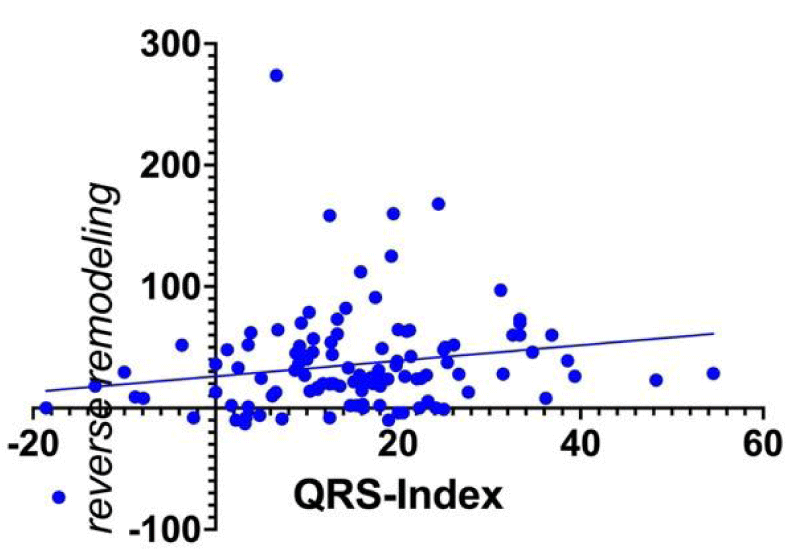
QI was related, with a borderline statistical significance, to reverse remodelling (r = + 0.19; 95% CI: 0.006 to 0.35, p = 0,049, Figure 1). The Figure shows a low correlation and a wide dispersion around the regression line and even if it has been considered such as a limitation in this population, it could be interesting understand the way and/or how the relation between QI and reverse remodeling will change its behavior in a larger one.
Twenty-two (19%) patients were in NYHA class III/IV after the implantation, 23 patients (21%) displayed an improvement in their HF clinical composite response at 12 months.
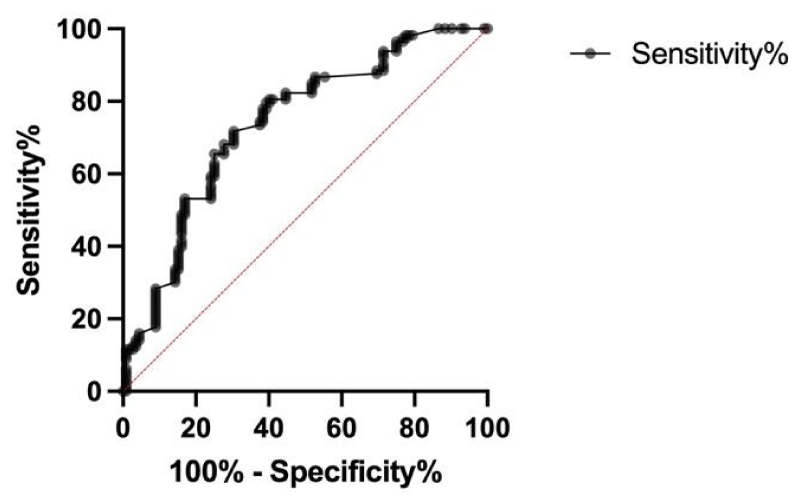
On the basis of the ROC analysis, the cut-off value of QI that best predicted LV reverse remodelling after 6 months of CRT was a value of QI > 12.25% and < 12.55% (sensitivity 65,49%, specificity 75%, area under the curve 0.737, p = 0,001) (Figure 2).
A multivariate linear analysis was performed analysis to evaluate an independent predictor of QI for reverse remodeling. Female sex, serum creatinine and eGFR and echocardiographic parameter like LVEF are independent predictors of QI with statistical significance (Table 2).
After CRT initiation, 73 (64%) of 115 patients had a QI value >12.25%. At 6 months follow up, a decrease in LVESV ≥10% was observed in 69% (n = 50) of these patients, while in patients with QI ≤ 12.25% responders was 60% (n = 25) (p = 0,3).
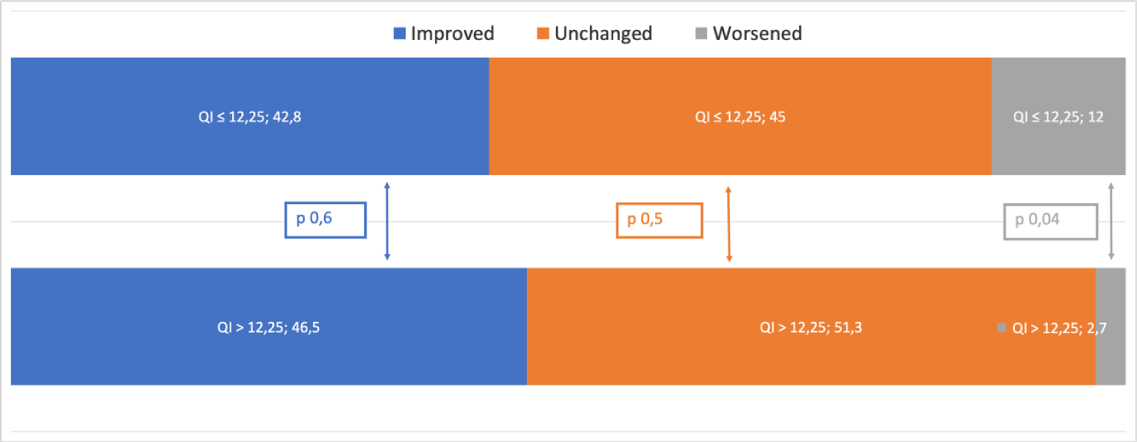
Considering the improvement in NYHA class, of the 73 patients with QI > 12.25%, 34 (46,5%) had a reduced NYHA class at 12 months post implantation. Of the remaining 39 patients, 37 (51,3 %) had the same NYHA class at control and only 2 (2,7%) worsened. Regarding 42 patients with QI ≤ 12.25%, 18 (42,8%) had a reduced NYHA class at 12 months post implantation. Of the remaining 24 patients, 19 (45%) had the same NYHA class at control while 5 patients (12%) worsened (Figure 3A).
A statistically significant difference was found between patients with a QI < 12.25% and those with a QI > 12.25%, in terms of NYHA class worsening (p 0,04).
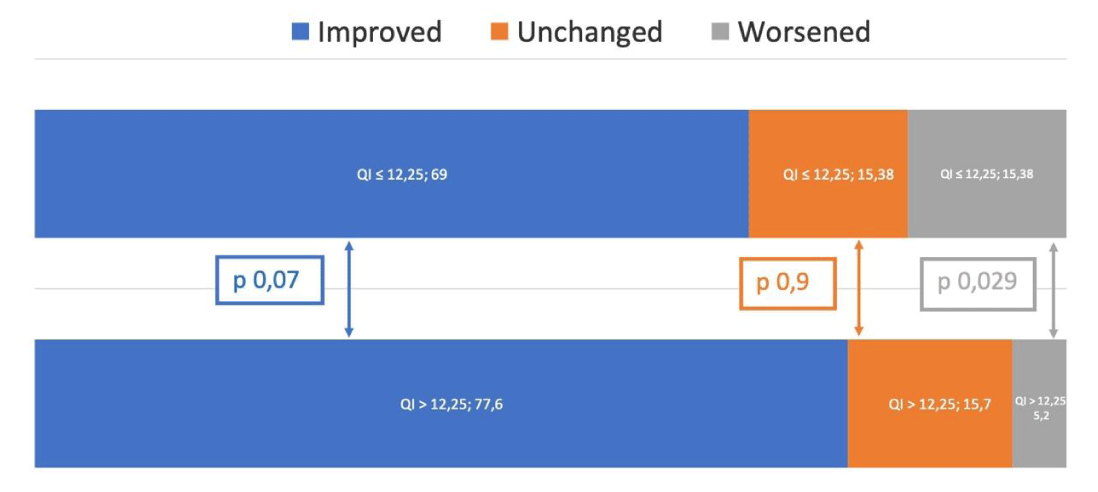
Analysis of the clinical composite score (CCS) at 12 months shows that 73 patients with a QI above the cut-off has shown an improvement in 59 (77,6%), compared with 39 patients with QI ≤ 12.25% (p = 0,07). On the contrary, the clinical status worsened in 15,4% of patients with QI ≤ 12.25% and in 5,2% of patients with QI > 12.25%, p = 0,029 (Figure 3B).
During follow up at 12 months, 6 patients (5,2%) died, 3 (2,6%) of these patients died for cardiovascular causes. The average of the QI values in patients who died from cardiovascular causes was 3,03 while the average of the QI values in patients who died of other causes was 20,39 with statistical significance (p 0,0074).
Seven patients (6,1%) had been hospitalized for cardiovascular events. The mean of the QI in patients hospitalized for heart failure exacerbation was 10,65 while the mean of the QI in non-hospitalized patients was 17,79 and this difference was statistically significant (p 0,049).
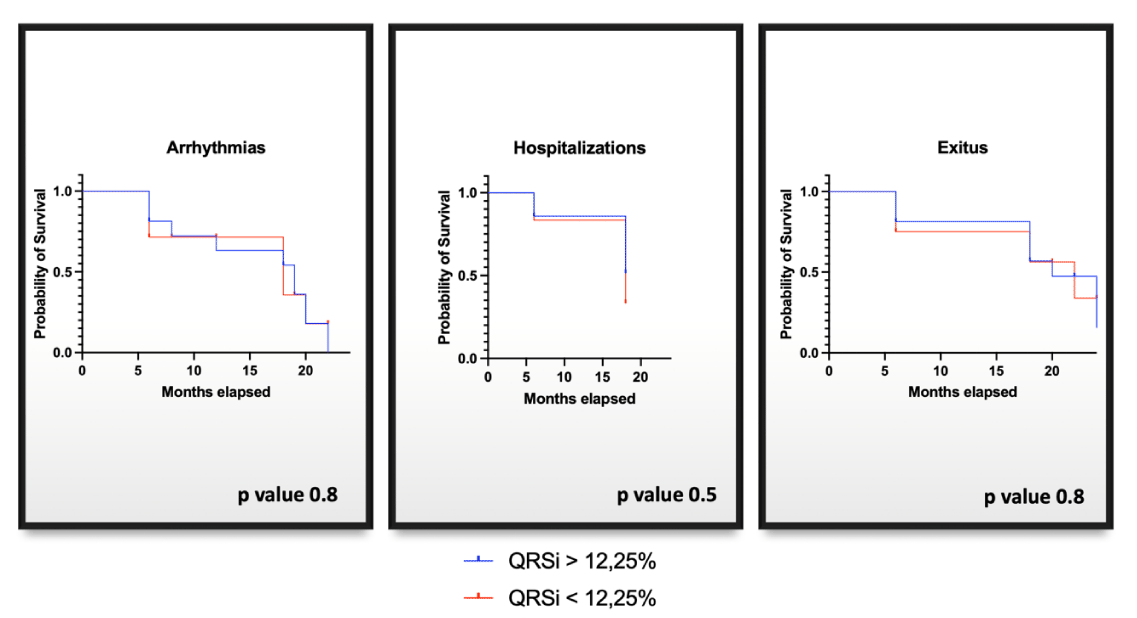
A Kaplan-Meier survival analysis was performed to evaluate clinical outcomes after two years between patients with QI > 12.25% and those with QI ≤ 12.25%. There were no significant differences between the two groups although the group with QI > 12.25% was more numerous (Figure 4).
Gender differences
As was already observed in the multivariate analysis (Table 2), female sex appears to be an independent factor of CRT-response. Furthermore, looking for possible sex differences related to QI, we have classified our population into 4 subgroups based on gender and the QI cut off of 12.25% (Table 3). We have found that women with QI > 12.25% had a statistically significant greater improvement in LVEF at follow up than men with QI > 12.25% (p 0,03).
Discussion
The main problem of CRT, which still remains unclear, is the possibility of easily and quickly identifying possible non-responder patients in order to intervene early with all the measures to obtain the best result. QI is an available, reproducible and easily measured electrocardiographic parameter that can be used to predict CRT response at the time of implantation. This study demonstrates that patients with a greater early reduction in QRS duration after CRT compared to baseline have greater reverse echocardiographic remodeling and better survival from cardiovascular death or hospitalization [7,8]. After its first adoption by Rickard et al, it was found that QI was associated with reverse remodeling, as measured by a reduction of LVESV ≥ 10% [6]. Furthermore, QI was correlated with the echocardiographic index of reverse left ventricular remodeling proposed by Yu et al., suggesting a correlation between electrical and anatomic remodeling and confirming that a greater acute reduction in QRS duration leads to a better response to CRT [7,8]. Identifying a QI cut-off value that is capable of predicting worsening of clinical status and NYHA class assessed by CCS could explain how QI appears to be able to predict reverse remodeling and be independently associated with a favorable outcome of CRT supporting the hypothesis that correction of electrical dyssynchrony is an important measure of the effect of CRT. Yang et al. have also argued in their work that QRS shrinkage is strongly related to regression of the fragmented QRS complex, a marker of reverse electrical remodeling associated with CRT response in support of the hypothesis that QRS shrinkage reflects early resynchronization achieved by CRT, while regression of fragmented QRS indicates long-term CRT-induced LV remodeling [12].
Furthermore, other studies have shown that QRS widening is associated with suboptimal CRT response and also with worsening of cardiac function [13]. Thus, the correction for electrical conduction impairment, as expressed by the QI, may reflect the quality of electrical resynchronization. The QI study may also have direct clinical applicability.
The QI is within everyone’s reach, very simple to calculate and interpret and could therefore also be used during left ventricular lead positioning to identify, if possible, the most suitable branch of the coronary sinus. Additionally, with the modern quadripolar CRT catheter, it can be used to select the most appropriate pacing electrode and/or configuration to decrease the rate of non-responders. Identifying the stimulation sites that can generate the highest QRS contraction, along with other potential predictors of implantation, such as inter-electrode distance or electrical firing delay, may be a better way to increase the likelihood of reverse remodeling and clinical response [14-16].
Another contributor to reverse remodeling, as shown in our study, is the female sex which appears to be responsible for greater remodeling of the left ventricle; for example, we found that women with QI > 12.25% had a statistically significant improvement in LVEF at follow-up compared to men with QI > 12.25% emphasizing not only the importance of CRT implantation in women but also showing that the remodeling response is generally better and the results are similar. It is no less important to consider that in our study only 29% were women and this probably reflects a possible underuse of CRT as reported in a recent survey by the European Society of Cardiology (ESC) [17]. Although our study shows that women show a greater response to CRT in terms of reverse left ventricular remodeling, which is likely due to the higher prevalence of non-ischemic cardiomyopathy, that doesn’t mean CRT wouldn’t benefit men with ischemic cardiomyopathy. As our QI results show and the study by Martens et al. supports, the risk of outcome appears to be similar for women and men, regardless of their HF etiology and despite differences in left ventricular remodeling [18].
Conclusion
The study group was relatively small, in fact although the data were collected prospectively, the design of the study is observational. Future studies, using large cohorts, should focus on which measure is most informative for predicting CRT response in ischemic and non-ischemic etiology and possibly consider individualized measures of electrical dyssynchrony adjusted for body/heart size or mass.
Electrical dyssynchrony plays a major role on CRT outcomes such as the etiology or sex of the patient with heart failure. We currently know several measures of electrical dyssynchrony and these are used in studies and clinical practice such as the QRS area, QRS duration, QRS morphology, apical oscillation and, more recently, the QRS area / LVEDV ratio in which electrical dyssynchrony is indexed to the size of the heart.
This study suggests the possible role of QI as a quick and easy parameter to obtain early prediction of CRT response. In this way we could reduce the non-responder population, optimize the device or the drug therapy during or immediately after the implant, search for a possible different pacing position or configuration of the left catheter during the implant, better titrate the drug therapy. Further studies are useful, if possible, with data collected from three or more centers in an observational method, in order to demonstrate the strength of this index.
Authors contributions
All authors contributed equally to the manuscript and read and approved the final version of the manuscript.
- Carità P, Corrado E, Pontone G, Curnis A, Bontempi L, Novo G, Guglielmo M, Ciaramitaro G, Assennato P, Novo S, Coppola G. Non-responders to cardiac resynchronization therapy: Insights from multimodality imaging and electrocardiography. A brief review. Int J Cardiol. 2016 Dec 15;225:402-407. doi: 10.1016/j.ijcard.2016.09.037. Epub 2016 Sep 17. PMID: 27776243.
- Yu CM, Bleeker GB, Fung JW, Schalij MJ, Zhang Q, van der Wall EE, Chan YS, Kong SL, Bax JJ. Left ventricular reverse remodeling but not clinical improvement predicts long-term survival after cardiac resynchronization therapy. Circulation. 2005 Sep 13;112(11):1580-6. doi: 10.1161/CIRCULATIONAHA.105.538272. Epub 2005 Sep 6. PMID: 16144994.
- Menet A, Guyomar Y, Ennezat PV, Graux P, Castel AL, Delelis F, Heuls S, Cuvelier E, Gevaert C, Le Goffic C, Tribouilloy C, Maréchaux S. Prognostic value of left ventricular reverse remodeling and performance improvement after cardiac resynchronization therapy: A prospective study. Int J Cardiol. 2016 Feb 1;204:6-11. doi: 10.1016/j.ijcard.2015.11.091. Epub 2015 Nov 18. PMID: 26649446.
- Stabile G, Bertaglia E, Botto G, Isola F, Mascioli G, Pepi P, Caico SI, De Simone A, D'Onofrio A, Pecora D, Palmisano P, Maglia G, Arena G, Malacrida M, Padeletti L. Cardiac Resynchronization Therapy MOdular REgistry: ECG and Rx predictors of response to cardiac resynchronization therapy (NCT01573091). J Cardiovasc Med (Hagerstown). 2013 Dec;14(12):886-93. doi: 10.2459/JCM.0b013e3283644bb2. PMID: 24149063.
- Chaturvedi V. Current research on the relevance of electrocardiography in cardiac resynchronization therapy. Indian Pacing Electrophysiol J. 2015 Oct 19;15(2):145-7. doi: 10.1016/j.ipej.2015.09.001. PMID: 26937104; PMCID: PMC4750123.
- Rickard J, Popovic Z, Verhaert D, Sraow D, Baranowski B, Martin DO, Lindsay BD, Varma N, Tchou P, Grimm RA, Wilkoff BL, Chung MK. The QRS narrowing index predicts reverse left ventricular remodeling following cardiac resynchronization therapy. Pacing Clin Electrophysiol. 2011 May;34(5):604-11. doi: 10.1111/j.1540-8159.2010.03022.x. Epub 2011 Jan 28. PMID: 21276023.
- Coppola G, Bonaccorso P, Corrado E, Ciaramitaro G, Ajello L, Nugara C, Assennato P. The QRS narrowing index for easy and early identification of responder to cardiac resynchronization therapy. Int J Cardiol. 2014 Jan 1;170(3):440-1. doi: 10.1016/j.ijcard.2013.11.057. Epub 2013 Dec 4. PMID: 24342404.
- Coppola G, Ciaramitaro G, Stabile G, DOnofrio A, Palmisano P, Carità P, Mascioli G, Pecora D, De Simone A, Marini M, Rapacciuolo A, Savarese G, Maglia G, Pepi P, Padeletti L, Pierantozzi A, Arena G, Giovannini T, Caico SI, Nugara C, Ajello L, Malacrida M, Corrado E. Magnitude of QRS duration reduction after biventricular pacing identifies responders to cardiac resynchronization therapy. Int J Cardiol. 2016 Oct 15;221:450-5. doi: 10.1016/j.ijcard.2016.06.203. Epub 2016 Jul 1. PMID: 27414720.
- Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F; Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006 Aug 15;145(4):247-54. doi: 10.7326/0003-4819-145-4-200608150-00004. Erratum in: Ann Intern Med. 2008 Oct 7;149(7):519. Erratum in: Ann Intern Med. 2021 Apr;174(4):584. PMID: 16908915.
- Kligfield P, Gettes LS, Bailey JJ, Childers R, Deal BJ, Hancock EW, van Herpen G, Kors JA, Macfarlane P, Mirvis DM, Pahlm O, Rautaharju P, Wagner GS; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society; Josephson M, Mason JW, Okin P, Surawicz B, Wellens H. Recommendations for the standardization and interpretation of the electrocardiogram: part I: The electrocardiogram and its technology: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation. 2007 Mar 13;115(10):1306-24. doi: 10.1161/CIRCULATIONAHA.106.180200. Epub 2007 Feb 23. PMID: 17322457.
- Packer M. Proposal for a new clinical end point to evaluate the efficacy of drugs and devices in the treatment of chronic heart failure. J Card Fail. 2001 Jun;7(2):176-82. doi: 10.1054/jcaf.2001.25652. PMID: 11420770.
- Yang XW, Hua W, Wang J, Liu ZM, Ding LG, Chen KP, Zhang S. Regression of fragmented QRS complex: a marker of electrical reverse remodeling in cardiac resynchronization therapy. Ann Noninvasive Electrocardiol. 2015 Jan;20(1):18-27. doi: 10.1111/anec.12172. Epub 2014 Jul 7. PMID: 25040593; PMCID: PMC6931476.
- Bax JJ, Bleeker GB, Marwick TH, Molhoek SG, Boersma E, Steendijk P, van der Wall EE, Schalij MJ. Left ventricular dyssynchrony predicts response and prognosis after cardiac resynchronization therapy. J Am Coll Cardiol. 2004 Nov 2;44(9):1834-40. doi: 10.1016/j.jacc.2004.08.016. PMID: 15519016.
- Sweeney MO, Hellkamp AS, van Bommel RJ, Schalij MJ, Borleffs CJ, Bax JJ. QRS fusion complex analysis using wave interference to predict reverse remodeling during cardiac resynchronization therapy. Heart Rhythm. 2014 May;11(5):806-13. doi: 10.1016/j.hrthm.2014.01.021. Epub 2014 Jan 22. PMID: 24462523.
- Singh JP, Fan D, Heist EK, Alabiad CR, Taub C, Reddy V, Mansour M, Picard MH, Ruskin JN, Mela T. Left ventricular lead electrical delay predicts response to cardiac resynchronization therapy. Heart Rhythm. 2006 Nov;3(11):1285-92. doi: 10.1016/j.hrthm.2006.07.034. Epub 2006 Aug 10. Erratum in: Heart Rhythm. 2006 Dec;3(12):1515. PMID: 17074633.
- Stabile G, D'Onofrio A, Pepi P, De Simone A, Santamaria M, Caico SI, Rapacciuolo A, Padeletti L, Pecora D, Giovannini T, Arena G, Spotti A, Iuliano A, Bertaglia E, Malacrida M, Botto GL. Interlead anatomic and electrical distance predict outcome in CRT patients. Heart Rhythm. 2015 Nov;12(11):2221-9. doi: 10.1016/j.hrthm.2015.05.020. Epub 2015 May 19. PMID: 26001509.
- Dickstein K, Normand C, Auricchio A, Bogale N, Cleland JG, Gitt AK, Stellbrink C, Anker SD, Filippatos G, Gasparini M, Hindricks G, Blomström Lundqvist C, Ponikowski P, Ruschitzka F, Botto GL, Bulava A, Duray G, Israel C, Leclercq C, Margitfalvi P, Cano Ó, Plummer C, Sarigul NU, Sterlinski M, Linde C. CRT Survey II: a European Society of Cardiology survey of cardiac resynchronisation therapy in 11 088 patients-who is doing what to whom and how? Eur J Heart Fail. 2018 Jun;20(6):1039-1051. doi: 10.1002/ejhf.1142. Epub 2018 Feb 19. PMID: 29457358.
- Martens P, Nijst P, Verbrugge FH, Dupont M, Tang WHW, Mullens W. Profound differences in prognostic impact of left ventricular reverse remodeling after cardiac resynchronization therapy relate to heart failure etiology. Heart Rhythm. 2018 Jan;15(1):130-136. doi: 10.1016/j.hrthm.2017.08.021. Epub 2017 Aug 24. PMID: 28843420.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
