Journal of Clinical Microbiology and Biochemical Technology
Prevalence and molecular analyses of extended spectrum β-lactamase producing uropathogens among pregnant women, Jigawa state, Nigeria
Eze L Chinyere1*, Sani M Nura1, Gumel M Ahmad1, Amoo F Kemi1 and Mujahid N Sani2
2Department of Microbiology, Kano University of Science and Technology, Wudil Kano, Nigeria
Cite this as
Chinyere EL, Nura SM, Ahmad GM, Kemi AF, Sani MN (2020) Prevalence and molecular analyses of extended spectrum β-lactamase producing uropathogens among pregnant women, Jigawa state, Nigeria. J Clin Microbiol Biochem Technol 6(1): 033-038. DOI: 10.17352/jcmbt.000041The Extended Spectrum β-Lactamase (ESBL)-producing uropathogens are of serious clinical concern worldwide. They are plasmid-mediated enzymes that are capable of hydrolysing virtually all β-lactam antibiotics including oxyimino-cephalosporins and monobactams. In this study, the uropathogens and risk factors for Urinary Tract Infections (UTI), their antibiotic susceptibility patterns, and plasmid studies of ESBL-producing uropathogens prevalent in pregnant women attending to Rasheed Shekoni Teaching Hospital (RSTH) and Dutse General Hospital (DGH) were investigated. During the current work, midstream urine samples were collected aseptically from 200 pregnant women and then cultured. The antibiotic susceptibility testing and ESBL detection were done using the standard methods. Identification of the recovered bacterial pathogens were carried out using the standard procedures and then confirmed through molecular analyses. The prevalence of UTI and ESBL production in this study was 37.0% and 17.6%, respectively. In pregnant women, the prevalence of UTI was higher in DGH (27.0%) than in RSTH (10.0%). The most predominant isolated uropathogens were Alcaligenes sp. (58.1%) followed by Klebsiella pneumoniae (28.3%). The most sensitive antibiotic was ceftazidime (31.2%). Molecular enumeration using 16S rRNA partial sequencing revealed the presence of 750bp .There is an urgent need to enlighten the pregnant women on the hazardous effects of self-medication, over-the-counter prescription, possible risk factors of UTI and the importance of personal hygiene during antenatal clinic, in order to prevent the emergence and spread of antibiotic resistant bacteria.
Introduction
Beta-lactamases are enzymes produced by bacteria that breakdown a particular class of antibiotics called the beta-lactams. As the bacteria developed resistance to one type of beta-lactam antibiotic, new antibiotic derivatives (cephalosporins, carbapenems and monobactams), which inturn made the bacteria to continually evolved and developed new set of enzymes described as extended-spectrum beta-lactamases (ESBLs) that can break down the newer derivatives . ESBLs are plasmid mediated enzymes that confer resistance to penicillin, third generation cephalosporins and aztreonam but are said to be inhibited by clavulanic acid [1]. The global increase in antibiotic resistance among these uropathogens are due to their ability to hydrolyse virtually all the beta-lactam antibiotics including the 3rd generation cephalosporins and carbapenems by producing ESBLs such as CTX-M enzymes (CTX-M-14 and CTX-M-15) or AmpC β-lactamases and OXA-type beta-lactamases [2,3].
In Nigeria, there have been reports on multi-drug resistance and various infections with ESBL producing organisms including recent study on plasmid profile of ESBL producing bacterial isolates causing urinary tract infection among pregnant women in Uyo [4].
ESBL producing bacteria such as E. coli, Klebsiella sp, Proteus sp and P. aeruginosa, have been found to continuously develop resistance to several antibiotics, which has resulted to wide spread cases of chronic urinary tract infections (UTI) in pregnant women. It has been widely reported that the bacteria harbour antibiotic resistant genes which can be horizontally transferred to other bacteria thus call for urgent research on their prevalence and management [5].
This study, intend to report on the prevalence and molecular typing of ESBL uropathogens among pregnant women, thereby providing an avenue for policy makers and researchers to build on effective management policies and public health strategies.
Materials and methods
Study site
This study was carried out at the Rasheed Shekoni Teaching Hospital and Dutse General Hospital, Jigawa State, Nigeria. According to National Population Commission, Jigawa state is one of the developing cities in the North-west of Nigeria, and is one of the less populated state in the Northern region.
Study population
The studied populations include samples from 200 pregnant women randomly collected attending Rasheed Shekoni Teaching Hospital and Dutse General Hospital Jigawa State, Nigeria. About 100 samples were collected from each hospital individually.
Samples collection
Structured questionnaires were used to collect demographic and clinical information from all patients. Clean catch midstream urine was aseptically collected and processed immediately into the Microbiology laboratory.
Specimen processing and isolation of the uropathogens
Laboratory analysis of the urine samples including macroscopic examination was conducted, to check out the color, turbidity and presence of blood. The uncentrifuged urine samples were mixed by rotating the plastic container before inoculating the surface of MacConkey agar and nutrient agar, followed by incubation at 37˚C for 24 h [6].
Identification and characterization of the bacterial isolates
Further studies and identification of the bacterial isolates was based on colony morphology, Gram staining, and biochemical characterization using standard method [6].
The antibiotic susceptibility tests
The antibiotic susceptibility test was carried out using the Kirby-Bauer disc diffusion method; isolated colonies was inoculated in Muller Hinton Agar plate and spread evenly over the surface, then the disc of commercially available discs (Oxoid Uk)) with standard antibiotic concentrations of Ceftazidime (30µg), Cefotaxime (30µg), Tetracycline (30µg), Augmentin (30µg), Ofloxacin (5µg) and Gentamicin (10µg) were placed on the culture plate and incubate at 37˚C. After 24h the zone of inhibition around the discs were observed following recommendations of the Clinical and Laboratory Standard Institute [7].
Determination of β-lactamase production by the bacterial isolates
The double-disc synergy test was used for confirming the extended spectrum β-lactamase (ESBL) production by the bacterial isolates. Augmentin (Amoxicillin/ clavulanic acid) was placed in the center of ceftazidime and ceftriazone in muller hinton agar plate inoculated with the bacterial strain, the plates were incubated at 37˚C for 24h. The antibiotics used were cell wall synthesis inhibitors. The isolates showing inhibition zone diameter of ≤ 22 mm with Ceftazidime (30 µg), ≤ 27 mm with Ceftriaxone (30 µg) and ≤ 27 mm with Augmentin (30 µg) were identified as potential ESBL producers [7].
Molecular analysis
These isolates were subjected to molecular characterization using 16S rRNA amplification and sequencing. The product of PCR amplicons was separated using gel electrophoresis. The sequence was parsed for genomic analogy using EMBL Blastn database, in reference to Sun [8]. The MEGA-X software was used to construct the phylogenetic neighbor joining tree for species relationship. In addition, the evolutionary distance was established using bootstrapping.
Statistical analysis
The data obtained were analyzed using Statistical Package for Social Sciences (SPSS 20), all observed data was recorded as ±SEM (Standard Error of Means). Statistical significant established at 95% confidence interval P < 0.05.
Results
The prevalence of UTI among the pregnant women was found to be 37.0%.
Demographic date of patient in relation to UTI
The prevalence of UTI is highest in DGH 54(27.0 %) than in RSTH 20(10 %), as clear in (Table 1). The age group UTI distribution within the studied population showed that the prevalence is lowest (4.5 %) in age group of 31-40, and highest (27.5 %) in age group of 21-30. Results showed that there is no statistical significance difference in the prevalence of UTI(P<0.05) within the age group (X2= 5.759; P =0.056). However, highest incidence of UTI is recorded in second trimester (19.0 %) followed by first trimester (10.0 %), and is least in the third trimester (8.0%).
Prevalence of ESBL production among the bacterial uro pathogens
The recorded prevalence of UTI among the pregnant women is 74(37.0%) presented in Table 2. Alcaligenes sp. (58.1%) is the predominant one followed by K. pneumonia (28.3%). Of the total of 74 isolates, only 17.6% are ESBL producers.
Susceptibility pattern of the isolated uropathogens to the selected antibiotics
The antibiotic susceptibility assay revealed the increased resistance of the uropatogens against Ofloxacin, Gentamicin and Tetracycline (Table 3). The most effective antibiotic is ceftazidime (31.1%) that caused inhibition to almost all of the isolated bacterial uropathogens.
Result of ESBL producers
The result of the screening tests for possible ESBL production showed that only 8(18.6) of Alcaligenes, 4(19.0) of Klebsiella pneumoniae and 1(16.7) 0f Myroides were confirmed phenotypically to be ESBL producers (Table 2) in reference to CLSI Performance Standard for Antimicrobial Susceptibility Testing which shows inhibition zone diameter of ≤ 22 mm with Ceftazidime (30 µg), ≤ 27 mm with Ceftriaxone (30 µg) and ≤ 27 mm with Augmentin (30 µg).
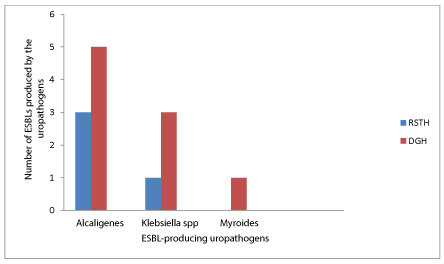
Comparative distribution of the ESBL-producing isolates between the two hospitals
Comparative distribution of the ESBL-producing bacterial isolates between the two hospitals is shown in Figure 1. Of the 13 ESBL-producing isolates, 9 isolates (5 Alcaligenes, 3 Klebsiella pneumoniae and 1 Myroides ) are from Dutse General Hospital (DGH), while 4 isolates ( 3 Alcaligenes and 1 Klebsiella pneumoniae ) are from Rasheed Shekoni Teaching Hospital (RSTH).
Molecular analysis
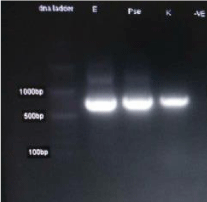
Gene extraction and amplification
The product of PCR amplicons were separated using gel electrophoresis are presented in Figure 2 and DNA base pair of 750pb
Post PCR phylogenetic analysis
The results for phylogenetic analaysis and molecular systematics of the isolates were presented in Figures 3-5 through 5. The evolutionary distance was presented using boostraping.
Discussion
This study, observed high prevalence of Gram-negative bacilli 70(94.5%) compared to that of Gram-positive cocci 4(5.4%). Among the bacterial isolates, Alcaligenes (58.1%) was the most prevalent uropathogen followed by Klebsiella pneumoniae (28.3%), Myroides (8.1), Staphylococcus aureus (5.4%). This is consistent with the reports of Otajevwo [9] which recorded Alcaligens sp and Klebsiella sp as the most prevalent uropathogens. The result differs from reports of Aboderin [10] which recorded Pseudomonas aeruginosa and Klebsiella sp and that of Oluremi [11] which revealed 85.0% of Gram-negative bacilli of which E. coli and Klebsiella sp as the most prevalent uropathogens. Although klebsiella sp are commonly prevalent in all the study but the variation in other microorganisms isolated further reveals the different species of uropathogens prevalent within the location of study.
The highest incidence was observed among age group of 21-30(27.5%) and the lowest seen in 31-40(4.5%). This could probably be due to the fact that greater number of the subject fall within ages 21-30 which represents sexually and reproductively active age group. The analysis showed that there was no statistical significant difference (X2=5.759 P=0.056) in the prevalence of UTI between the age groups. This study observed that multigravida had an increased risk factor of developing bacteriuria among pregnant women, which could be as a result of their reproductive system becoming more vulnerable to infections after successive pregnancies. Although this differs with that of Ranjan [12] who reported highest incidence in primigravida and lower incidence in multigravida (Table 1). More so, in this study, highest incidence of UTI is seen in second trimester (19.0%) followed by first trimester (10.0%) and least is seen in third trimester (8.0%), the result was in contrast with Ranjan [12] whose highest incidence of UTI is seen in third trimester (48.0%) followed by second trimester (45.0%). These variations could be a mere occurrence resulting from subjects’ exposure level and personal hygiene. The result of this study is not significant at (p<0.05) which means there is no association between gravidity and incidence of UTI in pregnancy. The socio-demographic data analyzed statistically showed no significant relationship with UTI (P<0.05).
The prevalence of UTI was found to be highest in DGH 54(27.0%) than RSTH 20(10.0%). The reason for high prevalence of UTI in DGH could be due to socio economic status and poor genital hygienic practice. Pearson Chi-square analysis showed that the difference in the prevalence of UTI between the two hospitals was statistically significant (X2=24.796, P<0.001, OR=0.213).
The prevalence rate of urinary tract infection (UTI) as revealed by this study was 37.0%. This was close to that obtained from similar UTI studies by Ranjan [12] recorded 35% prevalence rate at SVR maternity Hospital Bhimavaram, India while Akinola [13] recorded 40.0% prevalence rate among antenatal patients in University of Ilorin Teaching Hospital and Rekha [14] recorded 30.5% prevalence rate in Paropakar Maternity and Women’s Hospital, Thapathali, Kathmandu, Nepal. Similarly, Muhammad [15] recorded lower prevalence rate of 15.8% at Murtala Muhammed Specialist Hospital, Kano and Omonigho [16] who also reported prevalence rates of 22.3%. Much higher prevalence rates have been reported by authors; Okonko [17] recorded prevalence rate of 47.5% at antenatal clinics at Oluyoro Catholic Hospital Ibadan and Kolawole [18] recorded prevalence rate of 60% among outpatients attending Dalhatu Araf Specialist Hospital, Lafia, Nasarawa State, Nigeria while Gayathri [19] recorded prevalence rates of 56% among pregnant women at kanayo specialist and General hospital Onitsha. Of the 74 isolated uropathogens tested against the antibiotics, 9(20.9%) strains of Alcaligenes, 3(14.3%) of Klebsiella pneumoniae and 2(33.3%) of Myroides sp was sensitive to all the selected antibiotics used (Table 3).
Of the 70 isolated Gram-negative bacteria, ESBL production was seen in 13(17.6%) isolates of which 8(18.6%) were Alcaligenes, 4(19.0%) were of Klebsiella sp and 1(16.7%) was Myroides sp. Comparatively, the prevalence of ESBLs in urinary isolates was higher in Klebsiella spp (19.0%) followed by Alcaligenes (18.6%) in this study. Prevalence rates of 12.6%, 20.0% and 21.6 % was obtained by Dong [20], Onwuezobe [4], and Motayo [21] respectively. The antibiotic susceptibility testing revealed increasing resistance with Ofloxacin, Gentamicin and Tetracycline that resisted strains of all isolated uropathogens (Table 3). This low sensitivity of these drugs may be due to development of resistance genes by pathogens as a result of antibiotic abuse or self-medication [21-26].
In this study, the most effective antibiotic is ceftazidime that was sensitive to strains of all isolated uropathogens. The possible reason for the clinical effectiveness of ceftazidime compared to other antibiotics may be due to its limited use and abuse by patients. The molecular enumeration of ESBL-producing uropathogens was observed using 16S rRNA partial sequencing analyses [26-33].
Conclusion
The observed prevalence of UTI among pregnant women in this study was 37.0%. Past history of UTI, contraceptive use and cleaning method after urination or defecation contributed to constitute risk factors for acquiring UTI and the predominant uropathogens are Alcaligenes, Klebsiella species, S. aureus and Myroides. The prevalence of ESBL-producing Gram-negative uropathogens was 17.6%.
The ESBL-producing bacteria among UTI pregnant was found to be associated with risk factors such as past histories of exposure to a specific antibiotic, UTI and recent antibiotic use.
The authors wish to acknowledge the contributions of management and staff of Rasheed Shekoni specialist hospital and Dutse General Hospital, and the Department of Microbiology and Biotechnology, Federal University Dutse, for their support and cooperation towards success of the current research.
- Paterson DL, Bonomo RA (2005) Extended-spectrum β-lactamases: A clinical update. Clin Microbiol Rev 18: 657-686. Link: https://bit.ly/3b5QNAB
- Laupland KB, Church DL, Vidakovich J (2008) Community-onset Extended-Spectrum Beta-Lactamase (ESBL) Producing Escherichia coli: Importance of International Travel. J Infect 57: 441-448. Link: https://bit.ly/3hwwz5e
- Woodford N, Ward ME, Kaufmann ME (2004) Community and Hospital Spread of Escherichia coli Producing CTX-M Extended-Spectrum Beta-Lactamases in the UK. J Antimicrob Chemother 54: 735-743. Link: https://bit.ly/2YzHiVc
- Onwuezobe IA, Orok FE (2015) The Bacterial Isolates and Plasmid Profiles of Extended Spectrum Beta-Lactamases Producers causing Urinary Tract Infections among Pregnant Women in Uyo, Nigeria. J Biosci Med 3: 25-30. Link: https://bit.ly/3grSaui
- Piddock LJV (2006) Clinically Relevant Chromosomally Encoded Multidrug Resistance Efflux Pumps in Bacteria. Clinical Microbiology Review 19: 382-402. Link: https://bit.ly/3gs8Fq0
- Hamza S, Abdulhadi SK (2016) Beta- Lactamase Production Among Uropathogens in Patient Attending Some Hospitals in Kano, Nigeria. International Journal of Biomedical Materials Research 4: 58-63. Link: https://bit.ly/31rqzW0
- CLSI Performance Standard for Antimicrobial Susceptibility Testing (2014) Twentyfourth Informational Supplement, CLSI Document M100-S20, Wayne, PA: Clinical and Laboratory Standard Institute. Link: https://bit.ly/2QlbPkO
- Sun Y, Zeng Z, Chen S, Ma J, He L, et al. (2010) High prevalence of blaCTX-M extended-spectrum b-lactamase genes in Escherichia coli isolates from pets and emergence of CTX-M-64 in China. Clin Microbiol Infect 16: 1475-1481. Link: https://bit.ly/2EmbvzW
- Otajevwo FD (2013) Urinary Tract Infection among Symptomatic Outpatients visiting a Tertiary Hospital Based in Midwestern Nigeria. Glob J Health Sci 5: 187-99. Link: https://bit.ly/3letqcw
- Aboderin OA, Abdu A, Odetoyinbo BW, Lamikanra A (2009) Antimicrobial Resistance in Escherichia coli Strains from Urinary Tract Infections. J Natl Med Assoc 101: 1268-1273. Link: https://bit.ly/2EetyZ5
- Oluremi BB, Idowu AO, Olaniyi JF (2011) Antibiotic Susceptibility of Common Bacterial Pathogens in Urinary Tract Infections in a Teaching Hospital in South Western Nigeria. African Journal of Microbiology Research 5: 3658-3663. Link: https://bit.ly/32pjHYe
- Ranjan A, Srimath TK, Nandini M, Sumalatha C, Rashidah KA (2017) Prevalence of UTI among pregnant women and its complications in newborns. India J Pharm Pract 10: 45-49. Link: https://bit.ly/31nkgTc
- Ajayi AB, Nwabuisi C, Aboyeji AP, Ajayi NS, Fowotade A, et al. (2012) Asymtomatic Bacteriuria in Antenatal Patients in Ilorin, Nigeria. Oman Med J 27: 31-35. Link: https://bit.ly/34C9ESe
- Rekha T, Pramila L, Megha R, Genesh PA (2015) Prevalence of Extended Spectrum Beta Lactamase Producing Uropathogens in Pregnant women. Asian J Pharm Clin Res 8: 207-210. Link: https://bit.ly/2QjVn4B
- Muhammad A Muhammad SA (2017) Prevalence of Urinary Tract Infection among pregnant women in Kano; North Archives of reproductive medicine and sexual health 2: 23-29.
- Omonigbo SE, Obasi EE, Akvkalia RN (2001) Invitro resistance of urinary isolates of Escherichia coli and Klebsiella species to Nalidixic acid. Niger Journal Microbiology 15: 22-29.
- Okonko IO, Ijandipe LA, Ilusanya OA, Donbrave OB, Erembi J (2009) Incidence of Urinary Tract Infection among Pregnant Women in Ibandan, South-Western Nigeria. African Journal of Biotechnology 8: 6649-6657. Link: https://bit.ly/3li5Ewr
- Kolawole AS, Kolawole OM, Kandaki-Olukemi YT, Babatunde SK, Durowade KA (2009) Prevalence of Urinary Tract Infections(UTI) among Patients Attending Dalhatu Araf Specialist Hospital, Lafia, Nasarawa State, Nigeria. International Journal of Medicine and Medical Sciences 1: 163-167. Link: https://bit.ly/3gygo6k
- Gayathri C, Nwachukwu E, Onyebuchi O (2018) Prevalence of Urinary Tract Infections in Pregnant Women in Onisha, Nigeria. J Bacteriology Mycology Open Access 6: 284-285. Link: https://bit.ly/34qhKgW
- Dong SL, Chung BL, Seung-Ju L (2010) Prevalence and Risk Factors for ESBL-producing Uropathogens in Patients with Urinary Tract Infections. Korean Journal of Urology 51: 492-497. Link: https://bit.ly/3grRzsy
- Motayo BO, Akinduti PA, Adeyakimi FA, Okerentugba PO, Nwanze JC, et al. (2013) Antibiogram and Plasmid Profiling of Carbapenemase and Extended Spectrum Beta-Lactamase Producing E. coli and Klebsiella pneumonia in Abeokuta, South Western, Nigeria. Afr Health Sci 13: 1091-1097. Link: https://bit.ly/2YzGyiS
- Aibinu IE, Ohagbulam VC, Adenipekun EA, Ogunsola FT, Odugbemi TO, et al. (2003) Extended spectrum beta-lactamase enzymes in clinical isolates of Enterobacter species from Lagos, Nigeria. J Clin Microbiol 41: 2197-2200. Link: https://bit.ly/3aVDMJO
- Abubakar EM (2009) Antimicrobial Susceptibility Pattern of Pathogenic Bacteria causing Urinary Tract Infections at the Specialist Hospital, Yola, Adamawa State, Nigeria. Journal of Clinical Medicine and Research 1: 001-008. Link: https://bit.ly/32ryHEW
- Anuja P, Shah MD (2015) Overview of Urinary Tract Symptoms. Merck Manual: Kenilworth, USA.
- Chander A, Shrestha CA (2013) Prevalence of extended spectrum beta lactamase producing Escherichia coli and Klebsiella pneumoniae urinary isolates in a tertiary care hospital in Kathmandu, Nepal. BMC Res Notes 6: 487. Link: https://bit.ly/2Qk8NgX
- Chedi BAZ, Wannang NN, Halliry MA, Bichi LA (2009) A Seven Month Retrospective Study of Urinary Tract Infection Among Patients at Aminu Kano Teaching Hospital, Kano, Nigeria. Bayero Journal of Pure and Applied Sciences 2: 95-98. Link: https://bit.ly/3jdc4uU
- Cheesbrough M (2010) District Laboratory Practice in Tropical Countries, part 2, New York, USA: Cambridge University Press 62-118.
- Chernecky CC, Barbara JB (2001) Laboratory Tests and Diagnostic Procedures. 3rd edition. Philadelphia, PA: W.B. Saunders Company.
- Gangoue-Pieboji J, Bedenic B, Koulla-Shiro S, Randegger C, Adiogo D (2005) Extended spectrum-beta-lactamase-producing enterobacteriaceae in Yaounde, Cameroon. J Clin Microbiol 43: 3273 –3277. Link: https://bit.ly/3huQ3qM
- Kollef MH (2003) The importance of appropriate initial antibiotic therapy for hospital- acquired infections. Am J Med 115: 582-584. Link: https://bit.ly/3hsuw28
- Mulvey MA, Schilling JD, Martinez JJ, Hultgren SJ (2000) Badbugs and Beleaguered Bladders: Interplay between Uropathogenic Escherichia coli and Innate Host Defenses. Proceedings of the National Academy of Sciences, USA 97: 8829-8835. Link: https://bit.ly/2Etnvzs
- Raphael E, Wong LK, Riley LW (2011) Extended-spectrum Beta-lactamase gene sequences in gram-negative saprophytes on retail organic and nonorganic spinach. Appl Environ Microbiol 77: 1601–1607. Link: https://bit.ly/3lk6OHQ .
- Sarier M, Seyman D, Tekin S, Duman I, Uygun B, et al. (2017) Comparision of Ureteral Stent Colonization Between Deceased and Live Donor Renal Transplant Recipients. Transplant Proc 49: 2082-2085. Link: https://bit.ly/3jgfoW9

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
