Journal of Civil Engineering and Environmental Sciences
Membrane-assisted reactor for the direct conversion of CO2 to DME/MeOH
Francesco Frusteri*, Giuseppe Bonura, Catia Cannilla, Serena Todaro and Alessandro Cajumi
Cite this as
Frusteri F, Bonura G, Cannilla C, Todaro S, Cajumi A (2022) Membrane-assisted reactor for the direct conversion of CO2 to DME/MeOH. J Civil Eng Environ Sci 8(2): 068-070. DOI: 10.17352/2455-488X.000053Copyright License
© 2022 Frusteri F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Several strategies are currently underway to investigate alternative routes to efficiently use CO2 as a carbon source for the production of alternative fuels for energy end transportation [1,2]. Among several suggested approaches, the direct catalytic conversion of CO2/CO/H2 mixtures to dimethyl ether (DME) is receiving particular attention, representing an important breakthrough in terms of economy and process efficiency with respect to the classic dual-step process involving first the production of methanol (MeOH) over Cu-based catalysts and then the subsequent dehydration to DME over acidic systems. In a direct one-step process, the catalytic system should so contain a dual functionality integrated either in a mechanical mixture of a methanol synthesis catalyst (like Cu-ZnO-Al2O3 or Cu-ZnO-ZrO2) and a zeolite (e.g., HZSM-5) or in a hybrid system combining the metal-oxide(s) and acidic sites directly in one solid [3-6]. Despite the interesting results recently achieved over the hybrid systems in the experimental range of 220-260 °C and 3.0-5.0 MPa [7], however, up to now all the investigated catalysts show some limits mainly associated with the thermodynamic restrictions which level of the achievement of CO2 conversion at around 20% with DME selectivity close to 60% [8]. This behavior is related to the water formation prompted both by the water gas shift and dehydration reactions. Besides , negatively affecting the reaction equilibrium, the water presence also compromises the stability of both the methanol synthesis catalyst and the acid functionality [9].
Recently, to overcome the problem linked to the presence of water, the research addressed the possibility to adopt technical solutions, compatible with the reaction conditions, and suitable to determine an effective process intensification. As a rule, by using a conventional plug flow reactor, to achieve DME selectivity close to 90% the CO2 conversion never exceeds 10%, resulting too low for an industrial application [10]. Therefore, the research is going on by following different approaches mostly focused either on the development of innovative effective catalysts at low temperature (i.e., < 220 °C) so to deliver a superior activity-selectivity pattern, or by operating with a multi-pass recycle reactor and an intermediate condensation of DME and MeOH. This last approach can really allow determining a significant increase of the total CO2 conversion as high as 70% (3% - 5% Conv. per pass), paralleled by a DME selectivity close to 60% [11]. Naturally, the use of a high recirculation ratio, as well as the cooling of the reaction mixture for the product separation, involves a significant energy expenditure preventing a large-scale application.
Other viable solutions include the use of water absorbent materials that require to be cyclically regenerated [12], but more promising is the use of selective membranes which should ensure efficient removal of water in the presence of gas mixtures containing H2.
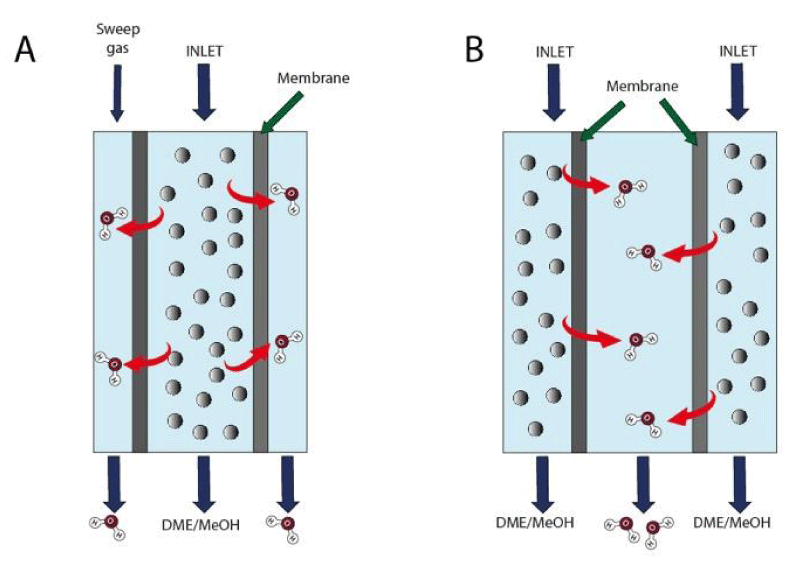
As follows, the most significant papers recently published and dealing with the use of membranes are considered in this mini-review. In Figure. 1, two alternative reactor configurations typically proposed are shown. Usually, the membrane is a ceramic tube covered by a porous layer permeable to water but not to MeOH and/or DME. The catalyst can be housed inside (A) or outside the membrane (B).
Among the papers recently published, several studies have focused on modeling before validating the process performance in an experimental environment [13-14]. Even if these approaches are merely theoretical, being based on well-defined reaction conditions and membrane properties (permeability, selectivity), most of them have contributed to providing fruitful insights on the limits of application and potentiality of such membrane technology. Indeed, the membrane must be not only stable under severe reaction conditions, but it must possess some specific features, mainly related to water permeability (~4.10-7 mol/Pa/m2/s) or to product selectivity (i.e., H2/CO/CO2, 50/30/30 respectively), making easily evident the difficulty behind the development of a membrane with tailored characteristics suitable for operation at high temperature (> 200 °C) and high pressure (3.0 - 5.0 MPa). Anyhow, the modeling studies also confirm that, by favoring the in-situ removal of water, the use of a membrane reactor can significantly shift the equilibrium of the water-limited reaction paths, so determining an effective increase of the values of CO2 conversion and DME/MeOH yield.
Once one understood the feasibility of such a membrane-assisted reaction system, it is necessary to understand the real difficulties to overcome for an application on large scale requiring high performance and stability. The most effective membranes actually proposed are mainly based on zeolites. In particular, the best results in terms of permeability and selectivity have been obtained by using the LTA-based frameworks. Some experiments carried out by using a mechanical mixture of Cu-ZnO-ZrO2/SAPO-11 catalyst and LTA zeolite as membrane [15] (see configuration A in Figure 1), revealed that, independently of temperature and pressure, CO2 conversion and DME selectivity attain higher values in a membrane permeate flux reactor, although this increase appears not too relevant: CO2 conversion rises from 20% to 25%, favoring an increase of oxygenates yield from 10% to 15%. In any case, a major benefit of better water management is an extended catalyst lifetime according to a reduced deactivation rate. Other authors [16], by using Cu-ZnO-Al2O3-ZrO2 as a catalyst and a hydrophilic LTA membrane characterized by an H2O/H2 separation factor of 50, claim extraordinary data both in terms of CO2 conversion (35%) and methanol selectivity (100%) under CO2 hydrogenation to methanol at 260 °C and 3.0 MPa, such values resulting much higher compared with those obtained by using different reactors (i.e., catalytic fixed bed reactor, packed-bed membrane reactor, catalytic non-permselective membrane reactor). Furthermore, endurance tests confirmed that water removal from the reaction system is helpful to avoid catalyst deactivation and by-product formation [17].
In conclusion, from the few available data, it is clear that the idea of using a water perm-selective membrane in an equilibrium-limited reaction, like the direct catalytic conversion of CO2 to DME/MeOH, performed at 200-300 °C and 3.0-5.0 MPa, represents a real challenge. The development of a solid membrane characterized by a high water permeation and high selectivity to H2, CO, and CO2 is a complex matter and in any case, the deterioration during time could constitute an additional problem to be taken into due consideration.
The authors acknowledge the Italian Ministry of University and Reseach (MUR) for financial support of this work, through the PON2014-2020 (PNR2015-2020) project “Gassificazione di Rifiuti Organici Umidi con Acqua Supercritica per Produzione di Biometano e GNL – WWGF” (ARS01_00868).
- Fawzy S, Osman AI, Doran J, Rooney DW. Strategies for mitigation of climate change: A review. Environ. Chem. Lett. 18. 2020; 2069–2094.
- Zhang Z, Wang T, Blunt MJ, Anthony EJ, Park AA, Hughes RW, Webley PA, Yan J. Advances in carbon capture. utilization and storage. Appl. Energy 278:2020; 115627–115680.
- Liu Z, An X, Song M, Wang Z, Wei Y, Mintova S, Giordano G, Yan Z. Dry gel assisting crystallization of bifunctional CuO–ZnO–Al2O3/SiO2–Al2O3 catalysts for CO2 hydrogenation. Biomass Bioenergy 163:2022; 106525-106532.
- Catizzone E, Freda C, Braccio G, Frusteri F, Bonura G. Dimethyl ether as circular hydrogen carrier: Catalytic aspects of hydrogenation/dehydrogenation steps. J. Energy Chem. 58:2021; 55–77.
- Bonura G, Migliori M, Frusteri L, Cannilla C, Catizzone E, Giordano G, Frusteri F. Acidity control of zeolite functionality on activity and stability of hybrid catalysts during DME production via CO2 hydrogenation. J. CO2 Util. 24:2018; 398-406.
- Catizzone E, Bonura G, Migliori M, Frusteri F, Giordano G. CO2 Recycling to Dimethyl Ether: State-of-the-Art and Perspectives. Molecules 23 (2018) 31-58.
- Bonura G, Todaro S, Frusteri L, Majchrzak-Kucęba I, Wawrzyńczak D, Pászti Z, Tálas E, Tompos A, Ferenc L, Solt H, Cannilla C, Frusteri F. Inside the reaction mechanism of direct CO2 conversion to DME over zeolite-based hybrid catalysts. Appl. Catal. B 294: 2021; 120255-120264.
- Cara C, Seccia F, Lai S, Mameli V, Skrodczkyd K, Russo PA, Ferrara F, Rombia E, Pinna N, Mureddu M, Cannas C. On the design of mesostructured acidic catalysts for the one-pot dimethyl ether production from CO2. J. CO2 Util. 62:2022; 102066-102075.
- Bonura G, Cannilla C, Frusteri L, Catizzone E, Todaro S, Migliori M, Giordano G, Frusteri F. Interaction effects between CuO-ZnO-ZrO2 methanol phase and zeolite surface affecting stability of hybrid systems during one-step CO2 hydrogenation to DME. Catal. Today 345:2020; 175–182.
- Gutiérrez-Martín F, Rodríguez-Antón LM. Power-to-SNG technologies by hydrogenation of CO2 and biomass resources: A comparative chemical engineering process analysis. Int. J. Hydrogen Energy 44:2019; 12544-12553.
- Ateka AA, Ereña J, Bilbao J, Aguayo AT. Strategies for the Intensification of CO2 Valorization in the One-Step Dimethyl Ether Sintesis Process. Ind. Eng. Chem. Res. 59:2020; 713-722.
- Delgado Dobladez JA, Águeda Maté VI, Álvarez Torrellas S, Larriba M, Muñoz GP, Alberola Sánchez R. Comparative simulation study of methanol production by CO2 hydrogenation with 3A, 4A and 5A zeolites as adsorbents in a PSA reactor. Sep. Purif. Technol. 262:2021; 118292-118302.
- Koybasi HH, Hatipoglu C, Avci AK. Sustainable DME synthesis from CO2-rich syngas in a membrane assisted reactor-microchannel heat exchanger system. J. CO2 Util. 52:2021; 101660-101668.
- Salehi MS, Askarishahi M, Gallucci F, Godini HR. Selective CO2-Hydrogenation using a membrane reactor. Chem. Eng. & Process. Process Intensif. 160:2021; 108264-108273.
- Hamedi H, Brinkmann T. Valorization of CO2 to DME using a membrane reactor: A theoretical comparative assessment from the equipment to flowsheet level. Chem. Eng. J. Advances 10:2022; 100249-100267.
- Rodriguez-Vega P, Ateka A, Kumakiri I, Vicente H, Ereña J, Aguayo AT, Bilbao J. Experimental implementation of a catalytic membrane reactor for the direct synthesis of DME from H2+CO/CO2. Chem. Eng. sci 234:2021; 116396-116409.
- Yue W, Li Y, Wei W, Jiang J, Caro J, Huang A. Highly Selective CO2 Conversion to Methanol in a Bifunctional Zeolite Catalytic Membrane Reactor. Angew Chem Int Ed Engl. 2021 Aug 9;60(33):18289-18294. doi: 10.1002/anie.202106277. Epub 2021 Jul 9. PMID: 34111327.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
