Journal of Biology and Medicine
Preparation, production and characteristics of sugarcane vinegar
Jyotsana Singh, Neha Bisht and Amar P Garg*
Cite this as
Singh J, Bisht N, Garg AP (2023) Preparation, production and characteristics of sugarcane vinegar. J Biol Med 7(1): 008-016. DOI: 10.17352/jbm.000036Copyright License
© 2023 Singh J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Sugarcane is one of the main crops worldwide, and it has an important impact on environmental issues. Sugarcane is used in daily routine life in many ways like as vinegar, jiggery, juices etc. In India sugarcane crop is best cultivated in the west U.P. Sugarcane original vinegar drink with high nutritional quality was produced from fresh sugarcane juice using the yeast culture and acetic acid bacteria by fermentation techniques such as submerged alcoholic fermentation followed by acetic fermentation at room temperature. Refined sugarcane is the primary product of sugarcane juice, during its processing, various other valuable products are also obtained in an unrefined form such as brown sugar, molasses, jaggery and vinegar. Sugarcane juice is widely used in the treatment of jaundice, hemorrhage, dysuria, and other urinary disease. Nowadays sugarcane vinegar is also used in Indian kitchens commonly in pickles, salads, etc. Vinegar is extremely useful for human health including antimicrobial activity, blood pressure reduction, antioxidant activity, reduction in the effects of diabetes, and prevention of cardiovascular disease. Phenolic acid in vinegar can scavenge superoxide anion and free radicals in vivo resulting in a potent antioxidant activity.
Introduction
Sugarcane (S. officinarum) is one of the most important crops in the world. In 2016, a total of 26,774,304 ha was harvested with 1.93% of the world’s harvested area, which places it as the 12th most important crop globally [1]. For the same year, sugarcane production was 1,890,661,751 tons, placing it as the most important crop in the world in terms of volume and representing 21.1% of the total world crop production. The countries with the largest production volume in 2017 were: Brazil (41% of world production), India (16%), China (6%) and Thailand (6%). The remainder was produced by 100 countries [2]. Sugarcane produced essential products such as sugar, ethanol, jaggery and vinegar [3,4].
The literature regarding sugarcane is abundant. Most of the previous reviews regarding this crop focus on products [5], or by-products such as ethanol [6]; many publications are not specific to sugarcane, that is they focus on comparing sugarcane with other crop or products [7,8]. In other reviews, the process [9,10], its applications [11] and its implications [12] are discussed.
The perennial grass, indigenous to tropical South Asia and Southeast Asia is S. officinarum Linn. It has a thick longitudinal stalk, which is generally three to five meters in height, approximately 5cm in diameter, and is characterized by its sweet taste due to its high sucrose content. It is renowned for chewing and noble cane. Tropical and subtropical regions are suitable for the growth of sugarcane. It will require well-drained soil of pH 7.5- 8.5 and high organic matter, along with a hot and humid environment [13]. Sugarcane has been used in various parts of the world for curing various diseases. In the Ayurvedic system of medicine, sugarcane is used either as a single drug or in combination with some other plant materials [14,15].
Sugarcane crop and its products
In 2020, global production of sugarcane was 1.87 billion tonnes, with Brazil producing 40% of the world’s total, India 20% and China producing 6% (Table 1).
Worldwide, 26 million hectares were devoted to sugarcane cultivation in 2020 (FAOSTT). The average worldwide, yield of sugarcane crops in 2020 was 71 tonnes per hectare, led by Peru with 132 tonnes per hectare (FAOSTT). The theoretically possible yield for sugarcane is about 280 tonnes per year, and small experimental plots in Brazil have demonstrated a yield of 236-280 tonnes of sugarcane per hectare [16,17].
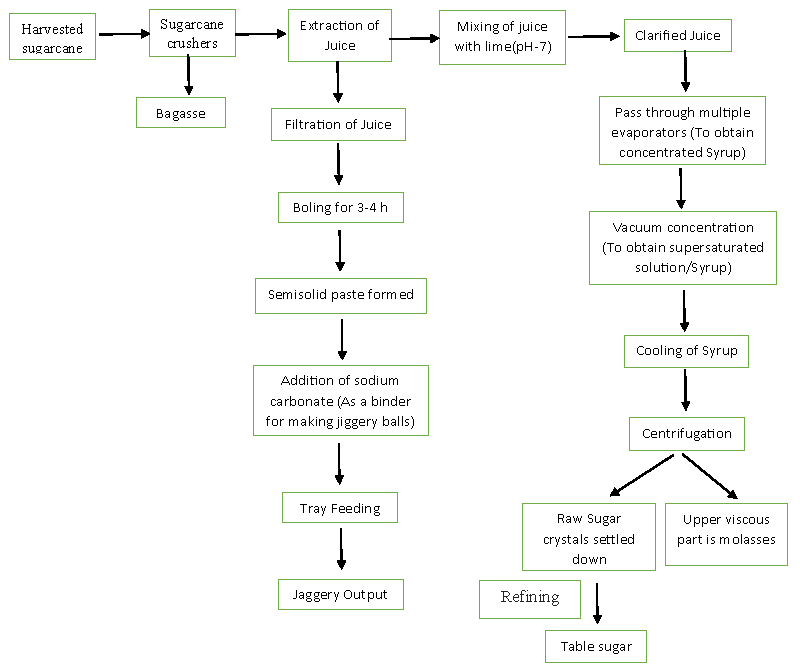
From 2008 to 2016, the production of standards-compliant sugarcane experienced a compound annual growth rate of about 52%, while conventional sugarcane increased at less than 1% [18]. Sugarcane crop is cultivated for the production of sugar, but the processing of sugarcane yields various valuable products such as bagasse [19], Brown sugar, molasses, syrup, vinegar and jaggery along with sugar (table sugar). The processing of sugarcane in a large-scale industry for the production of sugar is shown in Figure 1. After refining vacuum-concentrated cane juice, sugar is obtained. However, other sugarcane products such as brown sugar, jaggery and molasses are obtained in an unrefined form [20]. On account of the unrefined form of these products, there must be the presence of some phenolic compounds, which enhance their nutritional and medicinal value [21]. Now a day’s sugarcane vinegar is a wide use used product because of its nutritive value. In daily routine sugarcane vinegar is used with different vegetables like white radish, onion and green chili etc. Sugarcane vinegar also showed antimicrobial activity against so many food-borne bacteria. Natural vinegar is a fermented product involving two successive biochemical processes firstly the conversion of sugar to ethanol i.e. primary fermentation and finally to acetic acid i.e. secondary fermentation. Sugarcane vinegar has a high content of sugar and is a potential substrate for making vinegar through alcoholic and acetous fermentation. Fermented vinegar has massive antioxidant potential. However, its use in our country is negligible, primarily due to a lack of awareness of its benefits and the cost difference between fermented and synthetic vinegar. The present industries dealing with the production of natural vinegar still use the traditional batch fermentation which generally spans 4-5 weeks [22,23].
One of the most commonly consumed fruit and vegetable products, vinegar is typically made using modern food processing methods or traditional fermentation methods. It is becoming more and more popular due to its beneficial health effects. Vinegar is the world’s oldest change of state ingredient and food preservation methodology. To keep with Vinegar Institute [24], vinegar’s use springs back over 10,000 years. Traditionally vinegar is generated from raw products holding starch additionally sugar. Figure 2 represents the production of vinegar. In two-stage fermentation initially, ethanol is produced and at that time ethanoic acid is produced. During a traditional method, vinegar production takes an elongated quantity for fermentation of nearly about 30 days yet because it acts as starter culture in vinegar production at a commercial platform, commercial production is completed in 24 hours.
The formation and chemical reaction of vinegar
Tan [25]; Bhat, et al. [26] proposed the combination of acetic acid as well as yeast (produced due to longer fermentation time having harmless slime) called as mother of vinegar. For preservation purposes, most commercial vinegar producers are pasteurized as well as filtration vinegar before starting the packing operation to protect it from forming. The chances of producing a mother are high while storing, which is non-toxic. That tart flavor, overpowering smell as well as sharp odor of vinegar is due to volatile natural acids present within acetic acid results in such things [27]. This vinegar has various constituents like vitamins, minerals, and amino acids, as well as phenolic compounds and non-volatile natural acids [28,29]. In 1822, Dobereier established the theory of producing acid from alcohol [30] and the equation of the process is shown below from Kehrer 1921 [30].
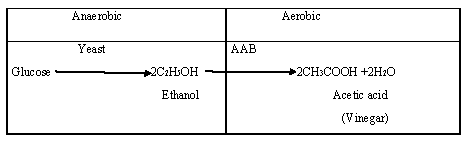
Vinegar production methods can range from traditional methods employing wood casks (Orleans Process) and surface culture (Generator Process) to submerged fermentation [31]. Vinegar is an important ingredient in many food products. The need for large amounts of vinegar demands industrial fermentation systems that are capable of producing volumes that are reliably controlled [32]. Aerobic and anaerobic fermentation of glucose represents in Figure 3. Many technical devices have been developed to improve the industrial production of vinegar. Generally, those improvements increase the speed of the transformation of ethanol into acetic acid in the presence of acidic acid bacteria [33].
The chemical reaction of vinegar
Processes method of vinegar: Vinegar is obtained from the fermentation of ethanol by acetic acid bacteria, the product is obtained as a result of the impartial oxidation of alcohol in fermenting sugar-containing fruit or sugarcane juice, molasses, a fermented mash of malted grain, honey, syrup, etc.
The three common methods used for vinegar production are the generator or trickling method, the submerged fermentation method, and the Orleans traditional method. Traditionally natural spontaneous fermentation is also used. The Generator method is the quicker method and it is commercially used for the production of vinegar.
Orleans process
It is the traditional and slow method used in France since 1670, for the acetifying wine known as the French or Orleans process. In this process, alcohol solutions less than 5% in wine can’t be acetified easily. Below this strength, phosphates and nitrogenous substances must be added to the mash and the products have to be sold under the name “spirit vinegar”. The Orleans process was the only method of pure wine vinegar [34] and was reported to be the best process to produce fine-quality table vinegar (Hickey and Vaughn 1954). In these processes, wood barrels from [35] are used and filled with alcohol fermenting liquid to approximately ¾ full.
First, holes are drilled at the ends of the barrel a few inches above the liquid surface, which is left open and covered with a fine screen. Secondly, approximately 20% - 25% of fresh vinegar is acidifying the liquid to the point of optimum growth for the vinegar bacteria [35]. Vinegar bacteria settle into the liquid from the air and form a gelatinous slime layer on top of the liquid [36]. The liquid is fermented for about 1 to 3 months at 70℉ to 85℉ (Hickey and Vaughn 1954). After this time, ¼ to 1/3 of the vinegar may then be drawn off for bottling purposes and an equivalent amount of alcoholic liquid added [35]. Constantly alcohol is added to the vinegar or the oxidation of acetic acid begins [35].
Generator fermentation
Early in the nineteenth century, a vinegar-making system called the trickly method (now called generator fermentation or quick process) was developed by German chemist Schutzenbach in 1832 (Hickey and Vaughn 1954). In this method, a thick coating of bacteria was formed around a non-compacting material [36]. The non -compacting material was packed into large upright wood tanks [35] of 2000 cubic feet capacity above a perforated wood grating floor. The wood shavings from [36] are generally made of air-dried beech wood sliced to form a coil about 2 inches long and 1¼ inches in diameter.
Re-circulated fermenting or mash trickles over the packing material toward the bottom while air moves from the bottom through inlets toward the top. The rate of acetification is dependent upon oxygen concentration [35]. A limited air supply means limited acetic acid production and lower generator temperature while an overabundant air supply creates overproduction and higher generator temperatures. The generators must be closely monitored to prevent over-oxidation or unacceptable temperatures (Hassack 1922). The process takes about 3 to 7 days. Final vinegar product i.e. 2/3rd withdrawn from the tank and followed by the addition of fresh mash [35]. Replacement mash is slowly poured into the tank until the working level for acetification of the solution and a beginning temperature of 70℉ (21.10C) are reached. The optimum temperature for generator operation is 85℉ to 90℉ (30 0C to 32.2 0C) (Hickey and Vaughn 1954). Each gallon of 190-proof alcohol oxidized to acetic acid releases about 30000 to 35000 Btu (32000000 to 37000000 Joules) (Hickey and Vaughn 1954). The optimized temperature is 86°F (30°C) for Acetobacter from preventing overheating and the inactivation of a bacterial temperature control system is necessary [36].
Submerged fermentation
Today, the most common production method is submerged culture [32] which improves the general fermentation conditions like aeration, stirring, heating, etc [37]. As generator culture systems are slow and expensive, submerged culture fermenters have become widely used at industrial scales [37]. In this process, the mash is stirred and aerated frequently (DeLey and Swings 1984). The fermenters are usually fitted with a heat exchanger for the maintenance of the optimum temperature during the fermentation process [32]. The typical operation mode in industrial submerged cultures [38] is semi-continuous [39]. Successive discontinuous cycles of acetification develop during the operation process. At the end of every cycle, a given volume of acetic acid is discharged and refilled with mash [40]. The best temperature for industrial production of 11% to 12% vinegar was 86 °F (30 °C) [38,41]. A temperature greater than 86°F will damage the bacteria and finally affects the concentration of acetic acid production (Fregapane, et al. 2001).
Vinegar bacteria
After stuff preparation, the alcoholic fermentation and acetification processes play a key role in vinegar production. Hoping on the environmental factors (temperature, pH, water activity) or the nutrients (carbon sources) and additionally the microbial diversity present inside the staple, totally different bio-transformations might happen. Microbial species concerned inside the fermentations could vary from yeast and carboxylic acid bacteria (LAB) to molds and AAB. The microorganisms concerned within the elaboration of vinegar square measure primarily yeasts and AAB. The previous being blame for alcoholic fermentation, and also the latter is required for the acetification [42-44]. Transformation of alkyl group alcohol towards carboxylic acid with the incorporation of species of bacterium named Acetobacterit’s results in the production of vinegar. Hence, any material that contains saccharides having the potential to produce vinegar undergoes fermentation treatment having a differential concentration combination of alcohol and water.
The preparation of the raw material is one of the critical steps in the production of vinegar, depending on the raw material the processing differs [45]. When compared fruits require less preparation as compared to seeds, which is easier for storage as well as for preservation after the harvest. Fruits are rich in water content, highly perishable, and need quick processing. To prevent the growth of pathogenic microorganisms basic food safety handling practices, storage, and processing are essential steps. The quality of the final product could be altered due to the growth of pathogenic microorganisms, and may even produce dangerous toxins such as aflatoxin. The alcoholic fermentation and acetification processes play a vital role in the production of vinegar after raw materials. Various environmental factors like temperature, pH, water activity, nutrients, and microbial diversity present in raw material alter the bio-transformations process. A wide range of fermentation microbial species are involved which may vary from yeast and LAB (Lactic Acid Bacteria) to molds and AAB (acetic acid bacteria). Mainly AAB and yeasts are involved in the elaboration of vinegar.
Microorganisms involved in the vinegar production
Yeasts: One of the most important microorganisms during fermentation influences the flavor, qualities, and speed of the whole process [46]. Yeasts are ‘unicellular Ascomycetous or Basidiomycetous fungi, whose vegetative growth results predominantly from budding or fission’. Yeasts do not form their sexual states within or upon a fruiting body [47]. Optimization of alcoholic fermentation as a process where the sugar as a substrate was converted into ethanol in the presence of a yeast belonging to the Class Saccharomycetes; Phylum Ascomycota and was responsible for fermentation [48]. Several studies revealed that yeasts had a high tolerance to the acidity that facilitated their survival and growth in fruit juices that have pH values below the tolerance level for several other microorganisms. Monosaccharides such as glucose, fructose, and mannose substrate are used for yeast metabolism that metabolized two molecules of pyruvate in the glycolysis which is also called the Embden-Meyerhof-Parnas (EMP) pathway. Pyruvate was further reduced to C2H5OH (ethanol) and CO2 (carbon dioxide) with the help of enzymes like pyruvate decarboxylase and alcohol dehydrogenase. Theoretically, the yield of ethanol was estimated to be about 65% (v/w) with the initial glucose content but due to the loss of glucose for the production of minor compounds and growth actual conversion efficiency was reduced to about 60%. Fleet, et al. [49] explored the Saccharomyces genus commonly used in the beverage industry due to their high capacity to ferment sugars which allowed them to colonize sugar-rich media amongst other yeasts that were not tolerant to alcohol. Several studies reported the imposition of Saccharomyces cerevisiae along with AF was directly proportional to the presence of ethanol in the anaerobic condition the use of sulfites and the high concentration of sugar during harvesting in the must. Joshi, et al. [50] stated that the species of Saccharomyces cerevisiae, Saccharomyces bayanuscommonly used for cider production and the choice of yeast strain as a mother culture have a high impact on the profile of fermented beverages, especially its flavor. The rate of ethanol production and its content sugars content, tannins, esters, methanol, and volatile acids were some of the quality characteristics affected by specific yeast strains. Kocher, et al. [51] reported the conversion of sugarcane juice to ethanol by Saccharomyces cerevisiae. Using adsorbents like bagasse, corn cobs, and wood shavings this ethanol was used for vinegar production and entrapped in calcium alginate cells of Acetobacter aceti NRRL 746. All three adsorbed carrier materials were statistically similar for the production of acetic acid and produced acidity in the range of 5.9% - 6.7% in submerged fermentation after 28 days.
Acetic Acid Bacteria (AAB)
The classification of Acetic Acid Bacteria (AAB) on the basis of the ninth edition of Bergey’s Manual of Systematic Bacteriology classified it in the family of Acetobacteriaceae and Gluconobacter. They are Gram’s-negative, catalase-positive, and oxidase negative with the morphologies of ellipsoidal to rod-shaped, motile due to the presence of flagella that could be either in polar or peritrichous position. They vary in size between 0.4 μm - 1 μm wide and 0.8 μm - 4.5 μm long observed as individual cells in pairs or in chains. AAB shows a strict aerobic metabolism with a terminal electron acceptor as oxygen [52]. Gullo and Giudici [53] study reported the presence of AAB in the environment as raw material cannot grow during alcoholic fermentation because of its anaerobic conditions. When alcoholic liquid is exposed to oxygen the growth of AAB started on the surface. The growth of most of the AAB was observed between pH 5.4 - 6.3, and can’t grow at pH lower than 4 but some isolates at pH values of 2.0-2.3 in media containing acetate and were aerated were also isolated [54]. The optimal temperature was 25 oC - 30 oC, but their growth was also observed between 38 oC – 40 oC and poor growth at temperatures lowers then 10oC. Yamada and Yukphan [55] reported that the AAB was usually found in substrates that contain sugar and/or ethanol. The substrates include flowers, food, fruits, and also fermented beverages such as juices of fruits, wine, cider, beer, cocoa, and vinegar. Gullo, et al. [56] reported clear differences in the growth of AAB species isolated from different sources like fruits, flowers, and various fermented foods with different morphology characters in different culture media depending on the available nutrients. Garcia-Garcia, et al. [57] conducted studies on the acetous fermentation in which the conversion of ethanol into acetic acid by acetic acid bacteria belonging to the family Acetobacteriaceae and the genera Acetobacter and Gluconobacter. The total chemical reaction was as follows:
Ethanol + Oxygen → Acetic acid + Water + 493 kJ
CH3CH2OH O2 CH3COOH H2O
The two steps involve the oxidation of ethanol to acetic acid with the help of enzymes Alcohol Dehydrogenase (ADH) and Aldehyde Dehydrogenase (ALDH). The initial steps involve oxidation to acetaldehyde through ADH which is finally oxidized to acetic acid by ALDH. The reaction was exothermic which increase the temperature in the medium. Further oxidation of acetic acid to carbon dioxide in the tri-carboxylic cycle which was totally an unwanted process in the production of vinegar, occur only when the concentration of ethanol was limited and the process called over-oxidation which was caused by bacteria belonging to Acetobacter with the help of enzymes that were non-functional in species of Gluconobacter.
Several studies showed that the ethanol content is totally affected by AAB at the beginning as well as the end of the fermentation process Raspor and Goranovic [58]. Higher initial ethanol concentration is inversely proportional to bacterial growth, because of the antimicrobial effect of ethanol. When the concentration of acetic acid increases during fermentation, the pH decreased which reduces the bacterial activity, and also limits the concentration of acetic acid.
Qualitative components of vinegar
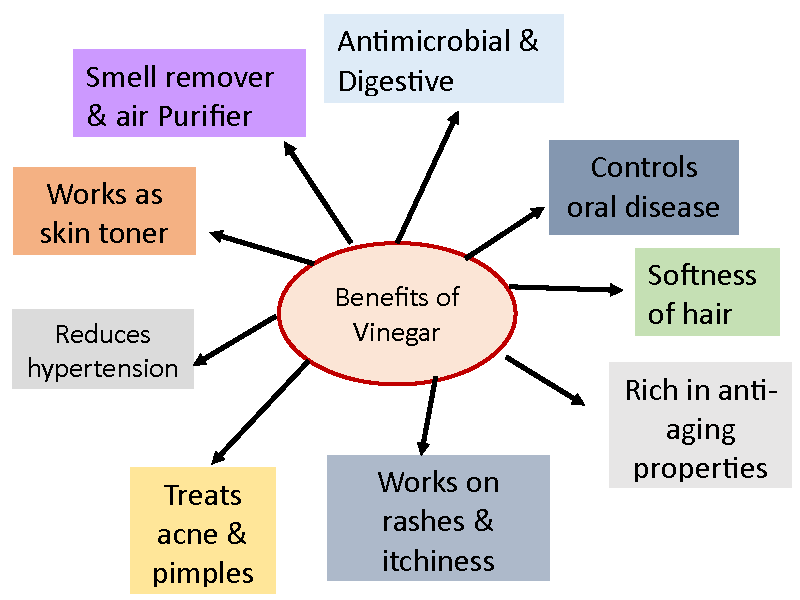
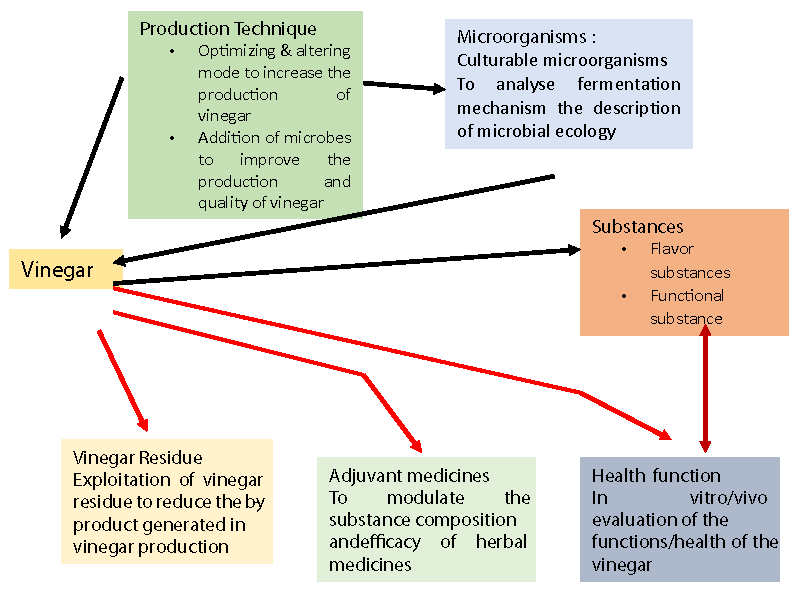
Sugarcane is the foremost virtual important sugar crop around the world because it stores 15% - 18% sucrose within the juicy stalk. Also, to give sugar, sugarcane juice is extremely salutary to mortal human health because it contains numerous amino acids like aminoalkanoic acid, alanine and citric acid; vitamins such as vitamin A, vitamin B1, vitamin B2, vitamin B3 and ascorbic acid, niacin, riboflavin; and essential nutrients like calcium, phosphorus, manganese, zinc, especially iron (9 mg/kg) [59]. Sugarcane vinegar is produced from sugarcane juice through the processes of alcoholic turmoil and ethanolic acid turmoil [8]. Literature has shown that total organic acids [60] and total polyphenol content [61] in sugarcane vinegar are 3.65% and 132.08µg/mL, singly. Sugarcane vinegar, with its own health-promoting promoting parcels, can be good volition to apple cider vinegar. Additionally, to sugars, sugarcane juice contains amino acids, vitamins like niacin and riboflavin, and minerals like calcium, phosphorus, manganese, zinc, and iron (> 9 mg/kg) [62]. The benefits of sugarcane vinegar are summarized in Figure 4 and its logic model of related themes in current vinegar research areas in Figure 5.
Nutrients
1. Sugar: The Codex Standard for Sugars [63] describes refined white sugar, intended for human consumption, as purified and crystallized sucrose (saccharose) with a polarisation note of less than 99.7%. Generally speaking, refined white sugar contains about 99.93% sucrose, with minor amounts of water, inverted or reducing sugars (glucose and fructose), ash, color components pulse other organic non-sugar components [64]. Although these minor components typically make up less than 0.1% of sugar content, they may affect the quality of the sugar and its behavior during storage [65]. The sucrose content of raw sugar varies but is mainly in the range of 97% to 99.5% sucrose.
2. Sugarcane juice: The sugar content is heavily influenced by the maturity of the cane at harvest, with sucrose content increasing with maturity and glucose and fructose content generally decreasing [66]. The protein content is negligible. In terms of the total amino acid content, the most abundant are aspartic acid, glutamic acid, and alanine [65].
3. Whole cane: The most important constituent in sugarcane is sucrose, which is typically measured in the plant stalk. Sucrose content can be quite variable, typically ranging from 9% to 20% (fresh weight basis) [67]. Sucrose content in the stalk reaches up to 60%, on the basis of dry weight basis. Reported ranges for dry matter sucrose content of verities grown in Australia include 39.2% - 59.7% [67] and 30% - 55% [68].
4. Vinegar: Vinegar is a natural product rich in bioactive compounds such as phenols, flavonoids, and organic acids. Several factors are responsible for the quality of vinegar such as origin, environmental conditions, production methods, processing, and storage conditions. We investigate the quality of different vinegar as well as their phytochemical properties and antimicrobial activity on selective food-borne pathogens. Vinegar is commonly used in the pickling of fruits and vegetables and in other food condiments. Although useful as a food ingredient for flavor and functional properties, the potential health benefits of vinegar varieties are leading researchers to further consider this long-used foodstuff [25,69]. The sugarcane vinegar is sweet for slowing down the sugar absorption from blood and is a superb source for diabetic patients. It also reduces salt intake and helps in maintaining pressure. It also improves gastrointestinal function and maintains the gut microbiome.
Conclusion
Kinds of vinegar are extremely useful for human health including antimicrobial activity, blood pressure reduction, antioxidant activity, reduction in the effects of diabetes, and prevention of cardiovascular disease. Used in the preservation of a wide variety of products, due to low pH and acetic acid content. It is also used for the preservation, or pickling, of a wide variety of food such as vegetables, meat, fish production, and spiced fruits. Phenolic acid in vinegar can scavenge superoxide anion and free radicals in vivo resulting in a potent antioxidant activity. According to my results that vinegar is more effective with eatables. Other positive health effects of daily consuming vinegar reported include improving blood glucose response which would be beneficial to diabetic patients.
The authors are grateful to Shobhit Institute of Engineering & Technology, Deemed to be-University, Modipuram, Meerut-250110 for financial help and facilities to complete this research work.
- Chen H, Chen T, Giudici P, Chen F. Vinegar Functions on Health: Constituents, Sources, and Formation Mechanisms. Compr Rev Food Sci Food Saf. 2016 Nov;15(6):1124-1138. doi: 10.1111/1541-4337.12228. Epub 2016 Sep 28. PMID: 33401833.
- FAO. FAOSTAT. http://www.fao.org/faostat/en/#data/QC.
- Jorrat MDM, Araujo PZ, Mele FD. Sugarcane water footprint in the province of Tucumán, Argentina. Comparison between different management practices. J Clean Prod. 2018; 188: 521–529.
- Singh AK. Overview of Vinegar Production-Palarch’s. Journal of Archaeology of Egypt/Egyptology. 2020; 17(6).
- Loh YR Sujan D, Rahman ME, Das CA. Review sugarcane bagasse-The future composite material: A literature review. Resour Conserv Recycl. 2013; 75: 14-22.
- Waclawovsky AJ, Sato PM, Lembke CG, Moore PH, Souza GM. Sugarcane for bioenergy production: an assessment of yield and regulation of sucrose content. Plant Biotechnol J. 2010 Apr;8(3):263-76. doi: 10.1111/j.1467-7652.2009.00491.x. PMID: 20388126.
- Chandra R, Takeuchi H, Hasegawa T. Methane production from lignocellulosic agricultural crop wastes: A review in context to second generation of biofuel production. Renew. Sustain. Energy Rev. 2012; 16: 1462–1476.
- Cheng JJ, Timilsina GR. Status and barriers of advanced biofuel technologies: A review. Renew Energy. 2011; 36: 3541–3549.
- White JE, Catallo WJ, Legendre BL. Biomass pyrolysis kinetics: A comparative critical review with relevant agricultural residue case studies. J Anal Appl Pyrolysis. 2011; 91: 1-33.
- Bessou C, Basset-Mens C, Tran T, Benoist A. LCA applied to perennial cropping systems: A review focused on the farm stage. Int J Life Cycle Assess. 2013; 18: 340–361.
- Larson ED, Williams RH, Leal MRLV. A review of biomass integrated-gasifier/gas turbine combined cycle technology and its application in sugarcane industries, with an analysis for Cuba. Energy Sustain Dev. 2001; 5: 54-76. Doi: http://doi.org/10.1016/S0973-0826(09)60021-1
- Le Gal PY, Lyne PWL, Meyer E, Soler LG. Impact of sugarcane supply scheduling on mill sugar production: A South African case study. Agric Syst. 2008; 96: 64–74.
- Koh HL, Chua TK, Tan CH. Singapore: World Scientific Publishing; A Guide to Medicinal Plants: An Illustrated Scientific and Medical Approach. 2009; 13.
- Anis M, Iqbal M. Antipyretic utility of some Indian plants in traditional medicine. Filoterpia. 1986; 57:52–5.
- Vedavthy S, Rao KN, Rajiah M. Nagarajun N. Folklore information from Rysalasenna region, Andhra Pradesh for family planning and birth control. Int J Pharmacognosy. 1991; 29:113–6.
- Bogden AV. Tropical Pasture and Fodder Plants (Tropical Agriculture)Longman Group (Far East), Limited. ISBN 978-0582466760.
- Duke, J (1983). "Saccharumofficinarum L." Purdue University
- Voora V, Bermudez S, Larrea C. "Sugar Coverage". International Institute for Sustainable Development. 2019.
- Xu F, Sun RC, Sun JX, Liu CF, He BH, Fan JS. Determination of cell wall ferulic and p-coumaric acids in sugarcane bagasse. Analytica Chimica Acta. 2005; 552:207-17
- Harish Nayaka MA, Sathisha UV, Manohar MP, Chandrashekar KB, Dharmesh MS. Cytoprotective and antioxidant activity studies ofjaggery sugar. Food Chem. 2009;115: 113‑8.
- Chen ZY, Jiao R, Ma KY. Cholesterol‑lowering nutraceuticalsand functional foods. J Agric Food Chem. 2008; 56: 8761‑73.
- Lea AGH. Cider vinegar. In Processed apple products, ed. D.L. Downing. New York: Van Nostrand Reinhold. 1989.
- Fregapane G, Rubio-Fernandez H, Nieto J, Salvador MD. Wine vinegar production using a noncommercial 100-litre bubble column reactor equipped with a novel type of dynamic sparger. Biotechnol Bioeng. 1999 Apr 20;63(2):141-6. doi: 10.1002/(sici)1097-0290(19990420)63:2<141::aid-bit2>3.0.co;2-6. PMID: 10099590.
- Vinegar Institute. 5775 G Peachtree-Dunwoody Rd., Suite 500 Atlanta, GA 30342.2005. http://www.versatilevinegar.org/index.html
- Tan SC. Vinegar fermentation (Master of Science Thesis). Lousiana State University Dept. of Food Science Baton Rouge. 2005; 101s.
- Bhat SV, Akhtar R, Amin T. An overview on the biological production of vinegar. International Journal of Fermented Foods. 2014; 3(2):139
- Johnston CS, Gaas CA. Vinegar: medicinal uses and antiglycemic effect. MedGenMed. 2006 May 30;8(2):61. PMID: 16926800; PMCID: PMC1785201.
- Natera R, Castro R, de Valme García-Moreno M, Hernández MJ, García-Barroso C. Chemometric studies of vinegars from different raw materials and processes of production. J Agric Food Chem. 2003 May 21;51(11):3345-51. doi: 10.1021/jf021180u. PMID: 12744665.
- Morales ML, Tesfaye W, García-Parrilla MC, Casas JA, Troncoso AM. Evolution of the aroma profile of sherry wine vinegars during an experimental aging in wood. J Agric Food Chem. 2002 May 22;50(11):3173-8. doi: 10.1021/jf011313w. PMID: 12009982.
- Kehrer CL. The chemistry of vinegar. Journal of Food Product and the American Vinegar Industry. 1921; 1: 5-20.
- Morales ML, Gustavo A, Gonzalez Jose A, Troncoso Ana M. Multivariate analysis of commercial and laboratory produced sherry wine vinegar: influence of acetification and aging. Journal of Food Technology. 2001; 212: 676-682
- De Ory L, Romero LE, Cantero D. Maximum yield acetic acid fermenter. Bioprocess Engineering. 1999; 21: 187-190.
- Tesfaye W, Morales LM, Gacia-Parrilla MC, Troncoso AM. Wine vinegar: technology, authenticity and quality evaluation. Journal of Food Science and Technology. 2002; 13: 12-21.
- Mitchell CA. Oxon BA. Vinegar: Its manufacture and examination. Exeter Street, Strand: Charles Griffin and Company. 1916; 100.
- Cruess WV. Commercial fruit and vegetable products: Chapter 21 – Vinegar manufacture. 1st ed. New York: McGraw-Hill Book Company, Inc. 1958; 681-707.
- Peppler Hendry J, Beaman Robert G. Microbial technology. In: Yeoman.Chapter 13 vinegar fermentation. 1st ed. Illinois: Reinhold Publishing Corporation. 1967; 344-359.
- HROMATKA O, EBNER H. Untersuchungen über die Essiggärung. III. Uber den Einfluss der Belüftung auf die submerse Gärung [Studies on acetic fermentation. III. Effect of aeration on submerged fermentation]. Enzymologia. 1951 Aug 15;15(2):57-69. Undetermined Language. PMID: 14906335.
- Adam MR. Vinegar in microbiology of fermented foods. 1st ed. New York: Elsevier Applied Science Publishers. 1985; 147.
- De Ory I, Romero Luis E, Cantero D. Optimum starting-upprotocol of a pilot plant scale acetifier for vinegar production. J Food Eng. 2002; 52: 31-37.
- De Ory I, Romero Luis E, Cantero D. Operation insemi-continuous with a closed pilot plant scale acetifier for vinegar production. J Food Eng. 2004; 63: 39-45.
- Allgeier RJ, Hildebrandt FM. Newer developments in vinegar manufacture. Adv Appl Microbiol. 1960;2:163-82. doi: 10.1016/s0065-2164(08)70125-4. PMID: 13682571.
- Nanda K, Taniguchi M, Ujike S, Ishihara N, Mori H, Ono H, Murooka Y. Characterization of acetic acid bacteria in traditional acetic acid fermentation of rice vinegar (komesu) and unpolished rice vinegar (kurosu) produced in Japan. Appl Environ Microbiol. 2001 Feb;67(2):986-90. doi: 10.1128/AEM.67.2.986-990.2001. PMID: 11157275; PMCID: PMC92679.
- Haruta S, Ueno S, Egawa I, Hashiguchi K, Fujii A, Nagano M, Ishii M, Igarashi Y. Succession of bacterial and fungal communities during a traditional pot fermentation of rice vinegar assessed by PCR-mediated denaturing gradient gel electrophoresis. Int J Food Microbiol. 2006 May 25;109(1-2):79-87. doi: 10.1016/j.ijfoodmicro.2006.01.015. Epub 2006 Feb 24. PMID: 16499984.
- Wu D, Kimura F, Takashima A, Shimizu Y, Takebayashi A, Kita N, Zhang G, Murakami T. Intake of vinegar beverage is associated with restoration of ovulatory function in women with polycystic ovary syndrome. Tohoku J Exp Med. 2013 May;230(1):17-23. doi: 10.1620/tjem.230.17. PMID: 23666047.
- Solieri L, Giudici P. Vinegars of the world. Springer-Verlag. 2009; 17-39.
- Pretorius IS. Tailoring wine yeast for the new millennium: novel approaches to the ancient art of winemaking. Yeast. 2000 Jun 15;16(8):675-729. doi: 10.1002/1097-0061(20000615)16:8<675::AID-YEA585>3.0.CO;2-B. PMID: 10861899.
- Kurtzman CP, Fell JW. Definition, classification and nomenclature of the yeasts. In: Kurtzman, C. P., Fell, J. W. (Edition). The Yeasts, A Taxonomic Study. (3-5) 4th edition. Elsevier Science B. V., Amsterdam. 1998.
- Rainieri S, Zambonelli C. Organisms associated with acetic acid bacteriain vinegar production. In: Solieri L, Giudici P. (Edition) Vinegars of the WorldItaly: Springer-Verlag. 2009; 73-95.
- Fleet GH. Yeasts in fruit and fruit products. In: Boekhout T, Robert V. (Eds.). Yeasts in food: Beneficial and Detrimental aspects, Wood head Publishing Limited, Cambridge. 2003; 267-288.
- Joshi VK, Lal BB, Kakkar KS. Updating the technique of apple chops making and its utilization. Beverage and Food World 17(1) 2002: 21-24.
- Kocher GS, Kalra KL, Tewari HK. Production of vinegar from Cane juice. Electronic Proceedings of Symposium on Food and Nutritional Security: Technological Interventions and Genetical options. 2006; Sept 18-19, HPKV, Palampur, India.
- Gonzalez N, Hierro M, Poblet N, Rozes A, Mas S, Guillamon JM. Application of molecularmethodsforthedifferentiation of acetic acidbacteriainaredwine fermentation. Journal of Applied Microbiology. 2004; 96: 853-860.
- Gullo M, Giudici P. Acetic acid bacteria in traditional balsamic vinegar: phenotypic traits relevant for starter cultures selection. Int J Food Microbiol. 2008 Jun 30;125(1):46-53. doi: 10.1016/j.ijfoodmicro.2007.11.076. Epub 2007 Dec 5. PMID: 18177968.
- Du Toit WJ, Pretorius IS. The occurrence, control and esoteric effect of acetic acid bacteria in winemaking. International Journal of Food Microbiology. 2002; 52: 155-179.
- Yamada Y, Yukphan P. Genera and species in acetic acid bacteria. Int J Food Microbiol. 2008 Jun 30;125(1):15-24. doi: 10.1016/j.ijfoodmicro.2007.11.077. Epub 2007 Dec 5. PMID: 18199517.
- Gullo M, Caggia C, De Vero L, Giudici P. Characterization of acetic acid bacteria in "traditional balsamic vinegar". Int J Food Microbiol. 2006 Feb 1;106(2):209-12. doi: 10.1016/j.ijfoodmicro.2005.06.024. Epub 2005 Oct 7. PMID: 16214251.
- Garcia-Garcia I, Santos-Duenas IM, Jimenez-Ot C, Jimenez-Hornero JE, Bonilla- Venceslada JL.Vinegar Engineering. In: Solieri L, Giudici P. (Ed.) Vinegars of the World. 2009; 97-120. Italy: Springer-Verlag.
- Raspor P, Goranovic D. Biotechnological applications of acetic acid bacteria. Crit Rev Biotechnol. 2008;28(2):101-24. doi: 10.1080/07388550802046749. PMID: 18568850.
- Huang ME, Gao ZS, Zhang Y, Zhou JJ. Study on healthy beverage of Imperatacylinarica (L.) rhizome and sugarcane. Food and Fermentation Industries, 2006; 32(2): 141–143
- He J. Lao S B, Zheng F J, Chen G L. Simulataneous determination of 11 phenolic compounds in apple vinegar and sugarcane vinegar by HPLC-DAD. Science and Technology of Food Industry. 2017; 38(23):210-213.
- Chen GL, Zheng FJ Li, ZC Sun, J Lin B, Li YR. Production and characteristics of high-quality vinegar from sugar cane juice. Sugar Tech. 2015; 17: 89-93.
- Maurício Duarte-Almeida J, Novoa AV, Linares AF, Lajolo FM, Inés Genovese M. Antioxidant activity of phenolics compounds from sugar cane (Saccharum officinarum L.) juice. Plant Foods Hum Nutr. 2006 Dec;61(4):187-92. doi: 10.1007/s11130-006-0032-6. Epub 2006 Nov 22. PMID: 17123161.
- Codex Alimentarius Commission (2001) “Codex Standard for Sugars”, Codex Standard Series No. 2121999, www.codexalimentarius.net/download/standards/338/CXS_212e_u.pdf.
- Clarke MA. “Sugarcane Processing: Raw and Refined Sugar Manufacture”, In: Chemistry and Processing of Sugar beet and Sugarcane (eds. MA. Clarke& MA. Godshall), Elsevier Science Publishers BV, Amsterdam, 1988; 162-175.
- Van der Poel PW, Schiweck H, SchwartzT. Sugar Technology: Beet and Cane Sugar Manufacture, 1998; Verlag Dr. Albert Bartens KG, Berlin.
- Qudsieh HYM, Yusof S,Osman A, Rahman RA. Physico-chemical Changes in Sugarcane (Saccharum officinarum var. Yellow Cane) and the Extracted Juice at Different Portions of the Stem during Development and Maturation. Food Chemistry. 2001; 75: 131-137.
- Berding N. Clonal Improvement of Sugarcane Based on Selection for Moisture Content: Fact or Fiction. In: Proceedings of the 1997 Conference of the Australian Society of Sugar Cane Technologists (ed. B.T. Egan), Watson Ferguson and Company, Brisbane, 1997; 245-253.
- Inman-Bamber NG, Bonnett G.D, Spillman MF, Hewitt ML, Xu J. Source-sink Differences in Genotypes and Water Regimes Influencing Sucrose Accumulation in Sugarcane Stalks. Crop & Pasture Science. 2009; 60: 316-327.
- Turker I. Vinegar Technology and Lactic Acid Fermentations in Technology. In; Turker I editor. Ankara, Turkey: Ankara Univ. Schoolbook of faculty of Agriculture, 1963; Ankara Univ. Press.
- Sugarcane production in 2020, Crops/Regions/World list/Production Quantity (pick lists) (h ttp://www.fao.org/faostat/en/#data/QC). UN Food and Agriculture Organization, Corporate Statistical Database (FAOSTAT). 2022.
- Carnacini A, Gerbi V, Zeppa G. Rapid extraction of volatile compounds in wine and vinegar using extrelut resin. Italian Journal of Food Science. 1992; 4:259-267.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
