Journal of Biology and Medicine
Histomorphological characterization of Moringa- oleifera oil and walnut oil on cadmium induced lateral geniculate body damage in adult wistar rats (Rattus novergiccus)
Omotoso OD1,3, Adelakun SA2*, Idomeh IJ1 and Ogbonna E4
2Department of Human Anatomy, School of Health and Health Technology, Federal University of Technology, Akure, Nigeria
3Department of Anatomy, Faculty of Basic Medical Sciences, Kogi State University, Anyigba, Nigeria
4Department of Pharmacology and Therapeutics, Faculty of Basic Clinical Sciences, Kogi State University, Anyigba, Nigeria
Cite this as
Omotoso OD, Adelakun SA, Idomeh IJ, Ogbonna E (2019) Histomorphological characterization of moringa- oleifera oil and walnut oil on cadmium induced lateral geniculate body damage in adult wistar rats (rattus novergiccus). J Biol Med 3(1): 001-007. DOI: 10.17352/jbm.000005Background: Cadmium (Cd) has been reported to cause distinct neurotoxic effects in adult and newborn animals. This study was designed to investigate some of the effects caused by cadmium on the lateral geniculate body of adult male wistar rats and the ameliorative properties of antioxidants present in Moringa oleifera seed oil and walnut oil extracts.
Methods: Seven groups of five animals each were used in this experiment. Group A received 3 ml of 0.9% normal saline; group B received 2.5 mg/kg bw of 3CdSO4.8H2O, group C received 5 mg/kg bw vitamin C & 6 mg/kg bw vitamin E, group D received 5 mg/kg bw vitamin C & 6cmg/kg bw vitamin E + 2.5mg/kg bw Cd, group E received 2.5 mg/kg bw Cd + 4 mg/kg bw Moringa oleifera Oil, group F received 2.5 mg/kg bw Cd + 4 mg/kg bw walnut oil, while group G received 2.5 mg/kg bw Cd + 2 mg/kg bw walnut + 2 mg/kg bw Moringa oleifera oil concomitantly for 3 weeks, histomorphological investigations were carried out in the brain tissues.
Results: Light microscopy examination revealed marked changes in the structure of the studied tissues of Cd administered animals. However, administration of vitamin C & E, walnut and Moringa oleifera seed oil was seen to counteract the changes of the measured parameters and restore the damaged tissues associated with Cd administration to appear nearly like those of the control group A.
Conclusion: Walnut and Moringa oleifera seed oil administration attenuated morphological changes induced by cadmium in the lateral geniculate body of the brain of the adult male wistar rats.
Introduction
Cadmium is produced mainly as a by-product from mining, smelting, and refining of sulfide ores of zinc and it is one of the heavy metals, which is highly toxic to human, plants and animals, the metal is of special concern because it is non-degradable and therefore persistent [1]. The main anthropogenic pathway through which cadmium enters environment is via wastes from industrial processes such as electroplating, smelting, alloy manufacturing, pigments, plastic, cadmium-nickel batteries, fertilizers, pesticides, mining, pigments and dyes, textile operations and refining [2]. All over the world, cadmium contaminated waste waters and effluents are being generated either directly due to cadmium production or through secondary sources [3].
A major past disaster ‘Itai-Itai’ due to contamination of cadmium in Jintsu river of Japan is well known [4]. Various regulatory bodies have set the maximum limits for the discharge of toxic heavy metals in the aquatic systems [5]. However, the metal ions that are being added to the water stream are at a much higher concentration than the prescribed limits by industrial activities, thus leading to the health hazards and environmental degradation [6]. The major exposure route for humans and animals is via respiratory exposure (inhalation) and by dietary exposure through the ingestion of food and drink [7,8]. Dermal exposure to cadmium is not a significant health concern due to limited exposure and minimal absorption by the skin [9]. Cadmium is found in most foods, beverages and animal products [10].
Moringa oleifera is a tropical multipurpose tree that naturally grows in India, South Saharan, Africa and South-America [11]. Almost every part of the plant (leaves, flowers, seeds, roots and bark) can be used as food or with medicinal and therapeutic purposes [12], especially in developing countries. Moringa oleifera seeds also contain between 30-35 % (w/w) of vegetable oil known as “Behen” or “Ben” oil [13]. This oil resembles olive oil in its fatty acid composition and is oleic acid-rich, which makes it suitable for edible purposes [14]. The shelled Moringa oleifera seeds have been found effective for the removal of heavy metals such as cadmium by absorption [15]. It has a high concentration of antioxidant, antiseptic and anti-inflammatory properties [16,17]. The antioxidants and nutrients present in the Moringa oleifera oil help to curb the activity of the free radicals [18]. Moringa oleifera seed oil has also been said to aid neurological development in infants [19]. In particular, this plant family is rich in compounds containing the simple sugar, rhamnose, and it is rich in a fairly unique group of compounds called glucosinolates and isothiocyanates [20]. For example, specific components of Moring preparations that have been reported to have hypotensive, anticancer, and antibacterial activity include 4-(4’-O-acetyl-α-L rhamnopyranosyloxy)benzylisothiocyanate,4-(α-L-rhamnopyranosyloxy) benzyl isothiocyanate,niazimicin,pterygospermin, benzylisothiocyanate, and 4-(α-L-rhamnopyranosyloxy) benzyl glucosinolate [21]. While these compounds are relatively unique to the Maringa family, it is also rich in a number of vitamins and minerals as well as other more commonly recognized phytochemicals such as the carotenoids (including β-carotene or pro-vitamin A) [22].
The black walnut tree (Juglansnigra L, Juglandaceae) is native in southeastern Europe, Asia Minor, India and China. Some species of the Walnut tree is now cultivated throughout Europe, North America, North Africa and East Asia [23]. The beneficial action of walnut oil on skin is known for centuries and it is widely used in cosmetic manufacturing industry [24]. The walnut oil is a component of dry skin creams, ant wrinkle and antiaging products, because it presents moisturizing properties as well as free radical scavenging capacity [25]. The major constituents of the oil are triacylglycerols; free fatty acids, diacylglycerols, monoacylglycerols, sterols, sterol esters, and phosphatides are all present in only minor quantities [26]. Numerous studies have shown that adding walnuts to the diet produces favorable health outcomes, including lowering LDL cholesterol, improving LDL: HDL ratios, and reducing inflammation associated with increased heart disease risk [27,28]. Omega-3 is a very important nutrient, which can prevent many diseases [29]. Walnuts have a high concentration of these good fats, which lower the risks of cardiovascular diseases, promote better cognitive function and have anti-inflammatory property that protect against asthma, rheumatoid arthritis and other skin diseases related to inflammation like psoriasis and eczema [30]. Walnuts can lower the cholesterol level, due to the nutrients it contains, like antioxidants, phenols, vitamin E, gallic acid and ellagic acid [30]. Ellagic acid in walnuts is an antioxidant compound that boosts the immune system [31]. Its anticancer properties also help in fighting the dreaded disease [32].
This study was designed to investigate some of the effects caused by cadmium on the lateral geniculate body of adult male wistar rats and the ameliorative properties of antioxidants present in Moringa oleifera seed oil and walnut oil extracts.
Materials and Methods
Plants source
The Moringa oleifera seeds were obtained from a local market in Jos, Plateau State, Nigeria and walnuts were procured from a local market in Ilorin, Kwara State, Nigeria. These seeds were then taken to a Mr Jeromes a Botanist in the Department of Biology, Bingham University Karu, Nasarawa State for proper identification.
Animal care
All experimental investigations were done in compliance with humane animal care as studied in the “Guide to the care and use of laboratory animals resources”. National research council, DHHS, Pub. No NIH 86-23 [33].
Conditioning of animals
The study was carried out on thirty-five (35) adult male wistar rats of body weight 150-200g. The rats were reared in the animal holdings of Bingham University Karu, Nasarawa State, Nigeria. The rats were allowed to gain maximum acclimatization before the actual commencement of the experiment. The rats were housed five in a standard laboratory cage, at a constant room temperature in a 12 hs light: 12 hs dark (lights on 8 a.m.–8 p.m.) cycle. They were fed with 100g standard rat diet chow daily containing proteins, carbohydrate, fats, vitamins and minerals which was purchased from vital feeds and grand cereals Ltd (Jos, Nigeria) to avoid changes in dietary compositions and weight variability and 200 ml of normal drinking tap water was provided ad libitum. They were weighed routinely every day.
Animal grouping
The thirty-five (35) male wistar rats were randomly divided into groups, A, B, C, D, E, F and G of five (5) animals each.
Oil extraction and preparation
The fresh walnuts and Moringa oleifera seeds weighing 1500 g each were oven dried at 400C for 7days and were blended separately with nuts and seeds blender. The molten Moringa oleifera seeds and walnuts were then independently poured into an oil extraction machine that separated the oil from the residue at high temperature and pressure. The oil obtained was then kept at about 60C-100C under reduced pressure in order to maintain the constant temperature of the oil.
Drug preparation
The cadmium sulphate solution was prepared by dissolving the 2.5 mg of cadmium sulphate salt (CdSO4) in 20 ml of 0.9% w/v normal saline solution. The ascorbic acid (vitamin C) was prepared by dissolving 5mg of vitamin C in 10ml of 0.9% w/v normal saline solution. The vitamin E (Alpha tocopherol) was prepared by dissolving 6mg of vitamin E in 20 ml of olive oil.
Animal treatment
The animals were treated as shown in the table 1:
Control groups
The animals were grouped into three control groups namely; the normal control (A), the negative control (B) and the positive control (C). The normal control group A animals received 3ml of 0.9% w/v normal saline solution orally for a period of three (3) weeks. The negative control group B animals were induced intraperitoneally with 2.5 mg/kg 3CdSO4.8H2O and after 72 hs of injection, the animals received 3ml of 0.9% w/v Normal saline solution for a period of three (3) weeks. The positive control group C animals received 5 mg/kg body weight and 6 mg/kg body weight of vitamins C and E orally respectively for a period of three (3) weeks.
Prophylactic treatment group
The group D animals were the prophylactic group that received 6 mg/kg body weight of vitamin E and 5 mg/kg body weight of vitamin C for the period of three (3) weeks followed by the intraperitoneal injection of 2.5 mg/kg 3CdSO4.8H2O for 72 hs.
Therapeutic treatment groups
The group E, F and G animals served as the therapeutic treatment group. The animals in each group were injected intraperitoneally with 2.5mg/kg 3CdSO4.8H2O, after 72hours of injection, the animals in Group F and G received 4mg/kg body weight of Moringa oleifera Seed oil and 4ml/kg body weight of walnut oil respectively for a period of three (3) weeks. The animals in group G received Moringa oleifera seed oil and walnut oil at 2 mg/kg body weight for a period of three (3) weeks.
Experimental design
The animals were administered orally with the 2 mg/kg Moringa oleifera seed and walnut oils, 5 mg/kg vitamin C, 6 mg/kg vitamin E and 3 ml of 0.9% w/v normal saline solution according to their body weights treated as shown in the table above for three (3) weeks using an oral cannular with a ball point at the tip. The animals were held with a glove with the left hand such that the neck region is held by the fingers to still the neck while being fed with the cannular; treatment was done every morning before the animals were fed. Twenty-four (24) hours after the last administration; the animals were sacrificed by cervical dislocation. They were laid in a supine position on the dissecting board and pinned through the fore and hind paws. A midline incision through the skin and muscles of the abdominal wall were made from the xiphisternum to the pubic symphisis, extended laterally at the lower end to achieve adequate exposure of the thoracic and abdominal cavities. The skulls of the animals were fractured open using brain forceps and the whole brain was carefully removed and weighed. The lateral geniculate body was then excised and fixed in 10% formol calcium for 72hs. This was done for all the animals in each of the groups labelled Groups A to G. The tissues were processed for histological analysis.
Histological preparation of tissue
After the brains of the wistar rats were harvested, they were placed in specimen bottles containing 40% formol calcium to prevent autolysis and after 24 hs, the brains were dissected to remove the lateral geniculate body. The processes involved include:
Fixation- The brain was fixed in 40% formol calcium to preserve the cell and tissue components in a life-like state as much as possible, and also to prevent autolysis or decomposition by bacteria.
Dehydration- The tissue was passed through ascending grades of alcohol (70%, 80%, 90% and absolute 100%) to gradually remove its water content.
Clearing- The sample was placed in a clearing agent i.e. xylene to remove the alcohol. This improves the refractive index of the tissue.
Infiltration- This is the immersion of the tissue in molten paraffin wax to fill up the holes left by the alcohol so as to give the tissue support.
Embedding- The tissue is placed into an embedding mould which is filled with more paraffin wax and allowed to solidify. This is done so as to make the tissue compact for sectioning. The block is trimmed to remove excess wax.
Sectioning- The block of tissue was placed in a microtome and trimmed to expose the surface. The microtome was set to 3-5 micron and the tissue was sectioned. The sections were picked with forceps and placed in a water bath to float out and spread well. It was then picked up with a slide and the slide placed on a hot plate for the tissue to stick to the slide.
Staining- The slides were arranged on a staining rack and dewaxed in 2 changes of xylene for 10 minutes. It was hydrated in descending grades of alcohol and then rinsed. The stain was then applied and the slides left to dry.
Statistical analysis
Data for the oxidative enzyme tests were expressed as Mean ± Standard Error of Mean (S.E.M). The data was analyzed in SPSS 17.0 software (SPSS Incorporation, Chicago, USA) to determine the analysis of variance. Except where otherwise stated, P ≤ 0.05 was taken as the level of significance.
Results
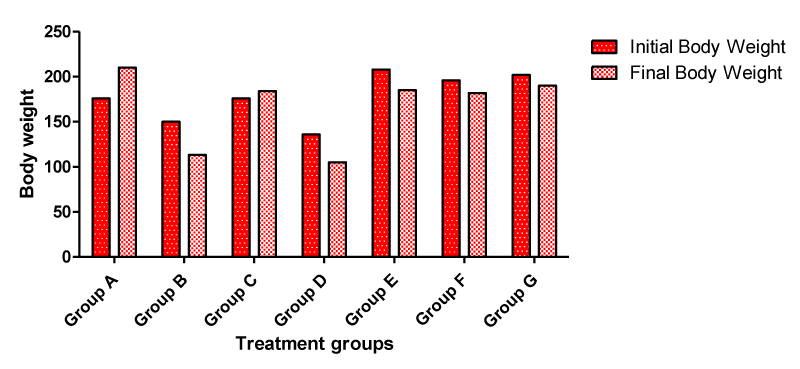
Body weight
Following the administration of cadmium, Moringa oleifera seed oil and walnut oil to the experimental animals in the treatment groups, the average body weight (Figure 1) in each of the experimental groups A, B, C, D, E, F and G were calculated at the end of the experimental procedures. Anorexia (a reduction in food consumption and body weight) was observed in all the rats in the treatment groups administered 2.5 mg/kg 3CdSO4.8H2O and others groups that received cadmium plus Moringa oleifera seed oil, walnut oil, vitamin C and vitamin E respectively except group C animals that received 5 mg/kg bw vitamin C & 6 mg/kg bw vitamin E. Since food consumption was reduced, similar reductions in body weight were observed during the study for all the treatment groups except group C which shows increased in body weight when compared with the initial body weight of the same animal group. All control group rats showed an increase in body weight relative to initial body weight of the same animal group.
Histological observations of the lateral geniculate body tissues
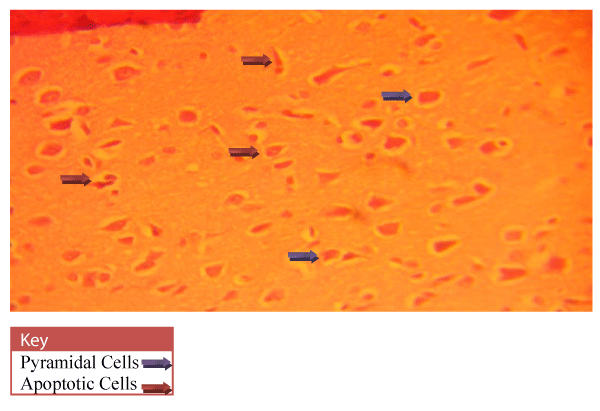
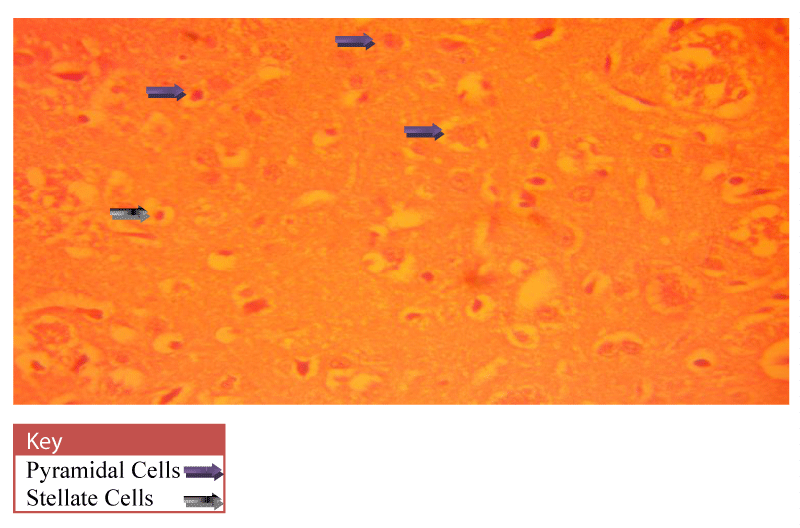
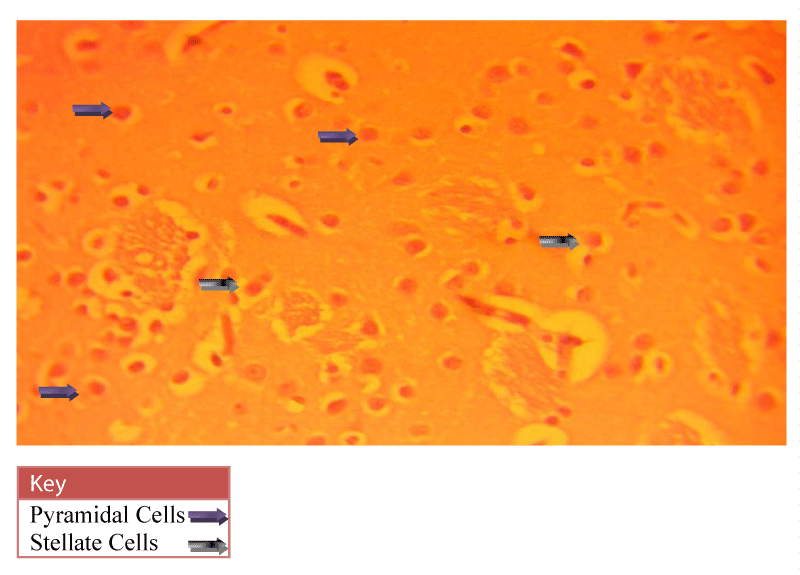
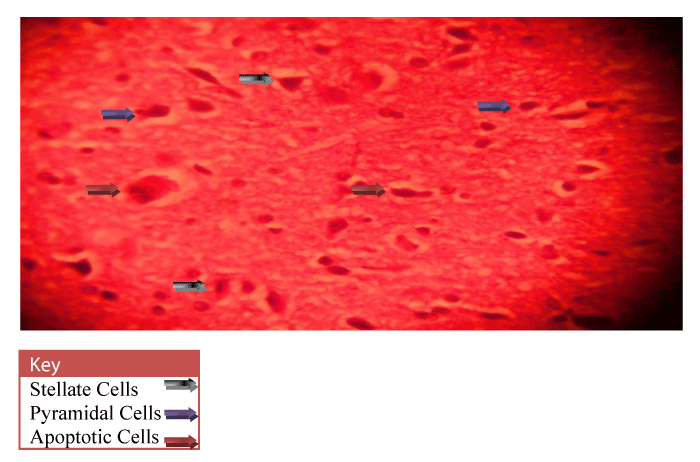
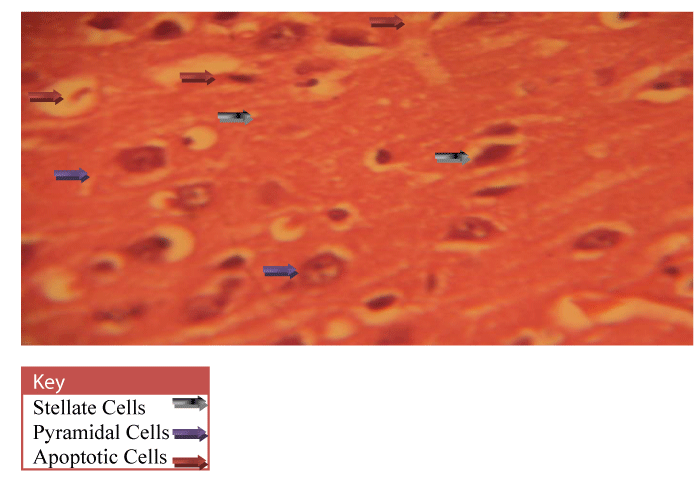
Neurohistological assessment of the lateral geniculate body of the animals in the treatment groups showed varying degrees of degenerative, deleterious and regenerative effects on the neurons of the lateral geniculate body. The administration of cadmium was observed to have induced histomorphological changes in the lateral geniculate body of the brain involving the different types of neurons (Figure 2) when compared to the normal morphology of the lateral geniculate body of the rat brains in the normal control group A (Figure 3). The neurons were observed to have undergone either degenerative or apoptotic changes. The degenerative changes manifested by shrinkage, neurofibrillary tangles, senile plaques and deeply stained neurons in the studied regions. However, in figure 4-8, the neurons showed cellular regeneration after they had undergone either degenerative or apoptotic changes manifested by shrinkage, neurofibrillary tangles, senile plaques and deeply stained neurons in the studied regions. This shows that the administration of the vitamin C and E antioxidants and the antioxidant containing Moringa oleifera oil and walnut oil extracts actually helped to curb the deleterious effects of the toxic cadmium.
The results obtained are shown in figures 2-8: Microscopic examination of the lateral geniculate body.
Discussion
Cadmium (Cd) is known to produce a variety of health hazards in humans and experimental animals due to its ability to induce severe alterations in various organs and tissues including the nervous system, following either acute or chronic exposure [34]. It promotes an early oxidative stress and afterward contributes to the development of serious pathological conditions [35].
In this study there was a decrease in final body weight of the animals in cadmium groups when compare with the initial body weight of the same animal group (Figure 1) which complied with the report of Stohs et al., [36] that loss of weight and appetite is imperative due to excessive accumulation of Cadmium.
In present study, the administration of cadmium was observed to have induced histomorphological changes in the lateral geniculate body of the brain involving the different types of neurons (Figure 2), when compared to the normal morphology of the lateral geniculate body of the rat brains in the normal control group A (Figure 3). The neurons were observed to have undergone either degenerative or apoptotic changes. The degenerative changes manifested by shrinkage, neurofibrillary tangles, senile plaques and deeply stained neurons in the studied regions since cell death which occurred pathologically is regarded as necrotic and could result from extrinsic implications or disturbances to the cell is toxic or traumatic [37]. Processes involved in cellular necrosis which may lead to cell death include disruption of the structural and functional potentials of the various membranes within the cell. Necrosis of the cell is not induced by intrinsic stimuli to the cells as observed in programmed cell death, but by an abrupt environmental disturbances and deviation from the normal physiological conditions, factors and functions [38]. The type of cell loss and the particular part of the organ affected determines the symptoms associated with individual disease [39]. Thus histomorphology alterations observed in this study as a results of histological damage to lateral geniculate body of male rats could have been as a result of direct toxicity of the cadmium or could have resulted from the release of toxic substances from other organs like liver and kidneys into the blood which may have circulated to the brain. This study shows that administration of the cadmium confers toxic and disruptive interference on cellular integrity of the lateral geniculate body in rats and led to oxidative damage of the neurons. This confirms the suggestion made by Stohs and Bagchi, [40] that cadmium is a potent cell poison and its toxicity was mediated by the oxidative damage of essential cellular macromolecules [40]. However, in figure 4-8, the neurons showed cellular regeneration after they had undergone either degenerative or apoptotic changes manifested by shrinkage, neurofibrillary tangles, senile plaques and deeply stained neurons in the studied regions. This shows that the administration of the vitamin C and E antioxidants and the antioxidant containing Moringa oleifera and walnut oil extracts actually helped to curb the deleterious effects of the toxic cadmium.
In figure 3, the lateral geniculate body cells of the group A (Normal control) animals at 400X magnification showed the cell’s normal architecture. The pyramidal cells and stellate cells which are the predominant cells in this region were seen to be visibly intact and undergoing cell division. In figure 4, neuronal cells of the group C (Vitamin E & C) animals at 400X magnification were seen to be intact with fine layered architecture (Pyramidal and stellate cells) and with visible cell regeneration occurring as some neuronal cells contained extended axons at their ends. This shows that the antioxidant activities of the vitamins are actively boosting the immunity of the cell in order to prevent or fight against oxidative stressors and damage. In figure 5, the neuronal cells of the group D (Vitamin C & E + cadmium) animals also shows the normal layered architecture, but, a few cells show signs of axonal degeneration indicating that apoptosis had occurred and presence of numerous cells with extended axonal ends indicate cell regeneration. This implies that the antioxidant properties of the vitamins actually curb the effects of the toxic cadmium. This however confirms the suggestion of Peters et al., [41] that free radical scavengers and antioxidants, such as glutathione, vitamin E, and vitamin C are capable of protecting against cadmium toxicity [41].
In figures 6-8 the neuronal cells of the Group E (Cadmium + Moringa oleifera seed oil), Group F (Cadmium + walnut oil) and Group G (Cadmium + walnut oil + Moringa oleifera seed oil) animals at 400X magnification shows the normal layered architecture with visible pyramidal cells of the lateral geniculate body of the brain. Presence of a few shrunken pyknotic cells and signs of axonal degeneration indicate the occurrence of apoptosis. But presence of numerous cells with extended axonal ends indicates that cell regeneration also occurred after. This implies that the animal exposure to cadmium resulted in damage of the nerve cells and fibres evident by the presence of senile plaques but the antioxidant activity of the Walnut and Moringa oleifera seed oil helped to minimize the damage caused by cadmium. Also, figure 8 (Group G) animals that were given a combination of both the walnut and Moringa oleifera seed oil showed better recovery as their cytoarchitecture was similar to the normal control group A animal (Figure 3) indicating that the strong scavenging activity of the antioxidant almost totally mopped up the free radicals in the cell and mediated cell regeneration. This thus confirms the suggestion made by Law et.al [42], that antioxidants have therapeutic importance in neurological disorders where oxidative stress is involved [42].
Conclusions
In conclusion, in the present investigation, the vitamin C and E antioxidant contents of Moringa oleifera seed oil and walnut oil helped to curb the morphopathological changes caused by the cadmium induced damage to the lateral geniculate body of the rats’ brain. Also, the combined action of the antioxidant contents of both oils evidently attenuated the morphological changes induced by cadmium.
- Ramirez DC, Giménez MS (2002) Lipid modification in mouse peritoneal macrophages alter chronic cadmium exposure. Toxicology 172: 1-12. Link: https://goo.gl/zW4S4p
- Wu Q, Magnus JH, Hentz JG (2010) Urinary cadmium, osteopenia, and osteoporosis in the US population. Osteoporos Int 21: 1449-1454. Link: https://goo.gl/B37RZu
- Mortaheb RH, Kosuge H, Mokhtarani B, Amini MH, Banihashemi HR (2009) Study on removal of cadmium from wastewater by emulsion liquid membrane. J. Hazard. Mater 165: 630–636. Link: https://goo.gl/FjnQ1P
- Tokyo KM (1989) A case study of restoration and prevention from cadmium pollution caused by zinc mine and refinery in Japan. In: Proceedings of international conference on base metals technology. 431-439.
- Zhang W, Pang F, Huang Y, Ping Y, Lin W (2008) Cadmium exerts toxic effects on ovarian steroid hormone release in rats. Toxicology Letter 182: 18-23. Link: https://goo.gl/AwKhu7
- Katerina A, Kiril L, Gordana R, Aleksandar TD, Anita G (2018) Removal of Heavy Metal Ions from Wastewater using Conventional and Nano sorbents: A Review. Journal of Chemical Technology and Metallurgy 53: 202-219. Link: https://goo.gl/nPvUrf
- WHO (1992) Cadmium. Geneva, World Health Organization (Environmental Health Criteria 134).
- IARC (International Agency for Research on Cancer) (1993) Beryllium, Cadmium, Mercury and Exposures in the Glass Manufacturing Industry. IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans. 58: 444. Link: https://goo.gl/vBdzd7
- Wesbter RC, Maibach HI, Sedik L, Melendres J, DiZio S, et al. (1992) In vitro percutaneous absorption of Cadmium from water and soil into human skin. Fundam Appl Toxicol 19: 1-5. Link: https://goo.gl/eMcpwX
- Degraeve N (1981) Carcinogenic, teratogenic and mutagenic effects of cadmium. Mutation Res 86: 115-135. Link: https://goo.gl/x92Vkj
- Jahn SAA (1988) Using Moringa seeds as coagulants in developing countries. Journal of American Water Works Association 80: 43–50. Link: https://goo.gl/uBG1Xa
- Anwar F, Latif S, Ashrat M, Gilani AH (2007) Moringa oleifera: A food plant with multiple medicinal uses. Phytother. Res 21: 17-25. Link: https://goo.gl/NxEWFR
- Nepolean P, Anitha J, Emilin RR (2009) Isolation, analysis and identification of phytochemicals of antimicrobial activity of Moringa oleifera Lam. Current Biotica 3: 33-39. Link: https://goo.gl/GWBRvH
- Bennett RN, Mellon FA, Foidl N, Pratt JH, DuPont MS, et al. (2003) Profiling glucosinolates and phenolics invegetative and reproductive tissues of the multipurpose trees Moringa oleifera L. (Horseradish tree) and Moringa stenopetala L.” Journal of Agricultural and Food Chemistry 51: 3546-3553. Link: https://goo.gl/a4tayQ
- Sharma P, Kumani P, Srivastava MM, Srivastava S (2006) Removal of Cadmium from aqueous system by shelled Moringa oleifera Lam. seed powder. J. Biores. Tech 97: 299-305. Link: https://goo.gl/z2fs69
- Singh BN, Singh B, Singh R, Prakash D, Dhakarey R, et al. (2009) Oxidative DNA damage protective activity, antioxidant and anti-quorum sensing potentials of Moringaoleifera. Food and Chemical Toxicology 47: 1109-1116. Link: https://goo.gl/PGzqaY
- Thilza I, Sanni S, Isah Z, Sanni F, Talle M, et al. (2010) In vitro antimicrobial activity of water extract of Moringaoleifera leaf stalk on bacteria normally implicated in eye diseases. Acad arena 2: 80-82. Link: https://goo.gl/8jHWKx
- Sharma V, Paliwal R, Sharma P, Sharma S (2011) Phytochemical analysis and evaluation of antioxidant activities of hydro-ethanolic extract of Moringaoleifera Lam. Pods. J Pharmacy Res 4: 554-557. Link: https://goo.gl/KMegQJ
- Walter A, Samuel W, Peter A, Joseph O (2011) Antibacterial activity of Moringa oleifera and Moringa stenopetala methanol and N-hexane seed extracts on bacteria implicated in water borne diseases. Afr. J Microbiol. Res 5: 153–157. Link: https://goo.gl/mf7aiv
- Fahey JW, Zalcmann AT, Talalay P (2001) The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 56: 5-51. Link: https://goo.gl/j3RvaM
- Fahey JW (2005) Moringa oleifera: A Review of the Medical Evidence for Its Nutritional, Therapeutic, and Prophylactic Properties. Part 1.” Johns Hopkins School of Medicine, Trees for Life Journal 1: 5. Link: https://goo.gl/yFVvTf
- Mahajan SG, Banerjee A, Chauhan BF(2009) Inhibitory effect of n-butanol fraction of Moringa oleifera Lam. Seeds on ovalbumin-induced airway inflammation in a guinea pig model of asthma. International Journal of Toxicology 28: 519-527. Link: https://goo.gl/kMZquZ
- Pollegioni P, Woeste K, Chiocchini F, Del Lungo S, Ciolfi M, et al. (2017) Rethinking the history of common walnut (Juglans regia L) in Europe: Its origins and human interactions. P LoS One 12: e0172541. Link: https://goo.gl/fAPfyN
- Espin JC, Solere-Rivers C, Wichers HJ (2000) Characterization of the total free radical scavenger capacity of vegetable oils and oil fractions using 2,2-diphenyl-1-picrylhydrazyl radical. J Agric.Food chem 48: 648-656. Link: https://goo.gl/FPMgLN
- Claire EB, Jessica AG, Sheila GW, Chung-Yen OC, Jeffrey BB, et al. (2013) Acute Consumption of Walnuts and Walnut Components Differentially Affect Postprandial Lipemia, Endothelial Function, Oxidative Stress, and Cholesterol Efflux in Humans with Mild Hypercholesterolemia. The Journal of Nutrition 143: 788–794. Link: https://goo.gl/QFBRPs
- Prasad RBN (1994) Walnuts and Pecans.Encyclopaedia of Food Science, Food Technology and Nutrition, Academic Press, London 4828–4831. Link:
- McKay DL, Berkowitz JM, Blumberg JB, Goldberg JP (2004) Communicating cardiovascular disease risk due to elevated homocysteine levels: Using the EPPM to develop print materials. Health Educ and Behav 31: 355-371. Link: https://goo.gl/nEGfBy
- Reiter RJ, Manchester LC, Tan DX (2005) Melatonin in walnuts: influence on levels of melatonin and total antioxidant capacity of blood. Nutrition 21: 920-924. Link: https://goo.gl/tBFuSU
- Masters C (1996) Omega-3 fatty acids and the peroxisome. Mol Cell Biochem 165: 83-93. Link: https://goo.gl/5iWFkJ
- Seed Guides (2013) Walnut Health Benefits, Walnut Nutrition, Side Effects and Facts. Link: https://goo.gl/DcUiq6
- Bae JY, Choi JS, Kang SW, Lee YJ, Park J, et al. (2010) Dietary compound ellagic acid alleviates skin wrinkle and inflammation induced by UV-B irradiation. Exp Dermatol 19: e182-190. Link: https://goo.gl/pobAcz
- Papoutsi Z, Kassi E, Chinou I, Halabalaki M, Skaltsounis LA, et al. (2008) Walnut extract (Juglans regia L) and its component ellagic acid exhibit anti-inflammatory activity in human aorta endothelial cells and osteoblastic activity in the cell line KS483. Br J Nutr 99: 715-22. Link: https://goo.gl/Lpb6Vw
- National Research Council (1985) Guide to the care and use of Laboratory Animals Resources. DHHS, Pub. No NIH 86–23.
- Manca D, Ricard AC, Trottier B, Chevalier G (1991) Studies on lipid peroxidation in rat tissues following administration of low and moderate doses of cadmium chloride. Toxicology 67: 303-323. Link: https://goo.gl/5cfx3P
- Bagchi D, Josh SS, Bagchi M (2000) Cadmium and chromium induced oxidative stress, DNA damage, and apoptotic cell death in cultured human chronic myelogenous Leukemic K562 cells, promylocytic leukemic HL-60 cells, and normal human peripheral blood mononuclear cells. J Biochem Mol Toxicol 14: 33-41. Link: https://goo.gl/zUqmnN
- Stohs SJ, Bagchi D, Hassoun E, Bagchi M (2000) Oxidative mechanism in the toxicity of chromium and cadmium ions. J Environ Pathol Toxicol Oncol 19: 201-213. Link: https://goo.gl/Mo4Dyi
- Farber JL, Chein KR, Mittnacht S (1981) The pathogenesis of Irreversible cell injury in ischemia. Am J Pathol 102: 271-281. Link: https://goo.gl/EhFT1d
- Ito U, Sparts M, Walker JR, Warzo I (2003) Experimental cerebral ischemia in magolian gerbils (1), light microscope observations. Acta Neuropathol. (USA) 32: 209-223. Link: https://goo.gl/nqokL8
- Waters CM, Lawson S, Moser B, Laidlaw SM, Cooper AJ, et al. (1994) Glutamate induced apoptosis of striatal cells in rodent model for Parkinsonism. Neuroscience 63: 1-5. Link: https://goo.gl/sGY1Rj
- Stohs SJ, Bagchi D (1995) Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med 18: 321-336. Link: https://goo.gl/MkAV9W
- Peters JM, Duncan JR, Wiley LM, Keen CL (1995) Influence of antioxidants on cadmium toxicity of mouse preimplantation embryos in vitro. Toxicology 99: 11-18. Link: https://goo.gl/PJXHnf
- Law A, Gauthier S, Quuirion R (2001) Neuroprotective and neurorescuing effects of isoform-secific nitric oxide synthase inhibitors, nitric oxide scavenger, and antioxidant against betaamyloid toxicity. Br J Pharmacol 133: 1114-1124. Link: https://goo.gl/3km4FZ
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
