International Journal of Veterinary Science and Research
Review of bacteriophage and its applications
Soressa Bakala Gamachu* and Motuma Debalo
Cite this as
Gamachu SB, Debalo M (2022) Review of bacteriophage and its applications. Int J Vet Sci Res 8(3): 133-147. DOI: 10.17352/ijvsr.000126Copyright License
© 2022 Gamachu SB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.The most prevalent and ubiquitous organisms in the world are bacteria-killing viruses called bacteriophages. The aim of this paper is to highlight some application areas of bacteriophages. There are both virulent and temperate bacteriophages in the environment, but only virulent bacteriophages are used for treatment, known as phage therapy. Since their discovery, bacteriophages have been considered a vital weapon to fight human and animal illnesses of bacterial origin. Currently, the emergence of growing microbial resistance to antibiotics and attention to bacteriophage use in treatment has all but grown up again. Correspondingly, the applications of bacteriophages for biocontrol purposes have become a remarkable opportunity in a number of fields, including farms and food production. Despite their treatment effects, bacteriophages also maintain a vital relationship with their hosts through moderating microbial populations and promoting their evolution by horizontal gene transfer. Additionally, they serve as vehicles for the transfer of vaccinations, the detection of dangerous microorganisms and systems for displaying proteins and antibodies. Bacteriophages are a varied collection of viruses that are simple to handle, making them suitable for use in treatments and biotechnology research. Hence, the expansion of various phage companies for phage production and the use of phage cocktails for the treatment of various bacterial diseases at different stages is recommended.
Introduction
Viruses are regarded as intracellular parasites that require a specific host cell for replication. A type of virus that only targets and replicates within bacterial cells is called a bacteriophage [1]. Bacteriophages consist of a genetic material, which can be either deoxyribonucleic acid or ribonucleic acid, surrounded by a protein coat, or capsid. Phages are structurally simple and extremely diverse, representing the most abundant living organisms in the environment. It’s expected that there are between 1031–1032 bacteriophages helping to manage bacteria populations within natural ecosystems within the world at any given time [2]. Additionally, it has been claimed that they are in charge of eliminating 20% – 40% of the bacteria on marine surfaces once every 24 hours [3].
As natural adversaries of their host, bacteria, bacteriophages are helpful to humans in the fight against bacterial illness. Bacteriophages can infect bacteria either lyrically or lysogenically after adhering to receptors on their surface and introducing their genetic material. A lytic infection occurs when a bacteriophage multiplies, lysing the bacterial target while continuing to attack additional bacteria. During a lysogenic infection, a DNA bacteriophage inserts its genetic material into the bacterial genome and also passes on its genome to daughter cells as the bacterium grows. The incorporated phage DNA may extract itself from the bacterial chromosomes in response to changes in the environment, creating lytic phage particles [4-6].
After bacteriophages were developed in the early 20th century, several researchers speculated about their capacity to destroy bacteria, which would surely make them useful therapeutic agents. But after World War II, when antibiotics were found, this natural potential medicinal agent received little attention and was only used as a research tool several times (Clark and March 2016). Bacteriophages are accepted as harmless, effective [7] and innovative alternatives to the use of chemotherapies nowadays [8,9]. This would make it possible to stop the development of bacterial antibiotic resistance. Additionally, bacteriophages are recognized as effective agents for bio-decontamination against undesirable bacteria in hospitals, along the food supply chain and in flocks and livestock. [10,11].
Even though they are solely bacteria’s natural enemies, bacteriophages still have a vital relationship with their hosts because they control microbial populations and encourage horizontal gene transfer to speed up bacterial evolution [12]. Bacteriophages have contributed significantly to the fields of molecular biology and biotechnology and continue to do so. They have helped to unravel many molecular biology riddles. Bacteriophages are receiving greater attention than ever before in today’s technologically advanced world due to their potential for use as antibacterials, diagnostic tools (phage typing), phage display systems and delivery methods for vaccines (Clark and March 2016).
Phage molecular biology research has recently fueled numerous biotechnological applications in a wide range of sectors, such as vaccine development, medicinal administration, bacterial detection systems, novel antibiotics against antibiotic-resistant bacteria, etc. Another possible area of use is the use of bacteriophages as natural antimicrobials in food to prevent undesirable microorganisms, which is probably acceptable to consumers [10,13]. Hence, the objectives of this paper are:
Hence, the objectives of this paper are:
- To review the role of bacteriophages in the treatment of diseases caused by different bacteria as well as in the bacterial diagnosis,
- To review the role of bacteriophages in vaccine administration.
Literature review
Taxonomy
In order to approve and coordinate the taxonomic coding and nomenclature of viruses, the International Committee on Taxonomy of Viruses (ICTV) was established in 1973. Notably, classification is crucial for the following reasons: finding novel bacteriophages, figuring out how phages are related to one another and maintaining bacteriophage databases and collections. Additionally, it can be utilized to identify either hazardous bacteriophages in the biotechnology and fermentation industries for control and eradication purposes or beneficial bacteriophages with medicinal and industrial applications [14].
Based on the type of encapsulated nucleic acid and the appearance of their virion, bacteriophages are divided into 13 families [15]. There are nine families with double-stranded DNA: the Corticoviridae (icosahedra capsid with lipid layer), Fuselloviridae (pleomorphic, envelope lipids, no capsid), Lipothrixviridae (enveloped filaments, lipids),Myoviridae (contractile tail), Plasmaviridae (pleomorphic, envelope, lipids, no capsid), Podoviridae (short, non-contractile tail), Rudiviridae (helical rods), Siphoviridae (long, non-contractile tail) and Tectiviridae (icosahedral capsid with inner lipoprotein vesicle). Microviridae (icosahedral capsids) and Inoviridae (rod-shaped with helical symmetry) were seen in single-stranded DNA. Cystoviridae (enveloped, icosahedral capsid, lipids) and Leviviridae (quasi-icosahedral capsid) are two families that have double-stranded RNA and single-stranded RNA, respectively. According to Ackermann [16], tail phages, which are a subclass of the Caudovirales order, account for more than 96% of all bacteriophages. Additionally, they have a wide range of characteristics, including size and fine structure, DNA content and composition, the way proteins are made, serology, host range and physiology [14].
The three major families that make up the Caudovirales account for 60% of the described phages: Siphoviridae, Myoviridae and Podoviridae [14,17,18]. However, only three to four percent of the phages studied (polyhedral, filamentous and pleomorphic) belong to the ten families studied and some of these families are extremely small [16].
Bacteriophage life cycle
Because they lack their own metabolism, bacteriophages must totally rely on the machinery used by their bacterial hosts to produce energy and proteins in order to reproduce [19]. Adsorption (attachment), infection, multiplication and discharge (release) are typically the four steps that distinguish bacteriophage replication cycles from those of their bacterial hosts [20].
The attachment phase relies on the presence of specific receptors on the surface of the bacterial host cell, to which the phage can attach. The phage is delivered to the host receptor by random diffusion. Following attachment, the infection phase occurs because the phage genetic material passes into the host cell by a mechanism likened to injection [21]. After-infection, the beginning of the multiplication phase relies on the phage species. Virulent bacteriophages are known to require bacterial replication machinery for the assembly of the progeny of the phage within the bacteria. Several copies of those new phages are produced until a critical mass is reached, triggering the lysis of the bacteria’s semipermeable membrane and therefore the release of the new phage progeny to reinitiate the lytic cycle (Figure 1) [19,22]. This critical mass frequently depends on factors such as specific bacteriophage characteristics, the strain of bacteria infected by the virus, and therefore the environment within which the bacteriophage-bacteria interaction occurs [19]. Phage lytic enzymes (endolysins or lysins) play a major role in breaking down bacterial peptidoglycan during the ultimate stage of the lytic cycle. Additionally, some phages with filamentous morphology are capable of fleeing from the host cell via cell membrane extrusion without causing the destruction of the host [15].
On the other hand, lysogenic phages are found to integrate their genetic material into that of the host, forming an entity known as a prophage (Figure 1). This results in the vertical transmission of the virus’s genetic information to daughter cells of the bacteria through a biological process and it is also possible for viral genes and proteins to be expressed. Occasionally, the lysogenic phage’s genetic material stays inside the host bacteria’s cells as a distinct plasmid rather than integrating into its chromosome, where it might continue to pass from one bacterial generation to the next. The cycle can change from lysogenic to lytic as a result of stresses such as chemicals, UV light, or damage to the host DNA [23].
Because of the difference between phages, it’s important to see which are the most appropriate bacteriophages for every potential application [24]. Given the antibacterial activity of virulent phages, they’re considered suitable for biocontrol purposes, whereas temperate phages don’t seem to be usable because of the high probability that they’re going to cause horizontal gene transfer between bacteria [25], which is potentially related to the chance of favoring the spread of antimicrobial resistance or other dangerous genes between microbes through bacterial transduction. Non-lytic phages, on the other hand, are frequently as important in biomedical research, being used in phage display techniques [26] and potentially providing the ability to observe tumors [27] and treat some diseases [28].
Application of bacteriophages
Therapeutic agents: In order to combat bacterial illnesses or to cure environments contaminated with dangerous bacteria, viruses’ host specificity offers an alluring approach. Although there is little research on the potential benefits of viral treatment, studies have demonstrated that illnesses in humans, plants, livestock, and aquacultured fish can be successfully treated using this technology [29,30].
The ability of bacteriophages to infect and kill bacteria led to the exploration of their therapeutic potential against bacterial pathogens in a clinical approach known as bacteriophage therapy/phage therapy, almost immediately after their innovation, with the first therapeutic use in humans described in 1919, just two years after their innovation by d’Herelle [30,31]. Phages can support the progress of an inflammatory response against bacteria via the breakdown of bacterial cell walls, which triggers the immune system [32]. Thus, bacteriophage therapies, i.e., the administration of bacteriophage cocktails to patients infected with bacteria, besides the direct removal of bacteria cells, further stimulate the immune system to fight against infection [33].
A wide variety of bacterial infection diseases, including cholera, dysentery, typhoid fever, skin and surgical site infections, peritonitis, septicemia, and external otitis, have been treated by phages before the discovery of antibiotics [34]. Nevertheless, an incomplete understanding of bacteriophage biology, together with the discovery and increased use of antibiotics during the 1940s and 1950s, as well as several other factors, led to degeneration in the clinical use of bacteriophages in Western Europe and North America. In contrast, phage therapy continued to be employed in the previous Soviet Union and certain Eastern European countries (e.g., Poland), where therapeutic phage preparations are still readily available in pharmacies today [30,33,35].
Two main advances played a major role in reviving the interest in bacteriophages as antimicrobial agents. First, the appearance and extensive distribution of antimicrobial resistance (AMR) bacterial pathogens have narrowed our therapeutic options. The situation is further worsened by the limited number of new antimicrobial drugs entering the market. Also, most novel antibiotics approved in recent years are modifications of existing drugs and, therefore, are at advanced risk of being rendered ineffectual in a short period [36]. Secondly, there has been an enlarged appreciation of the injury broad-spectrum antibiotics can impose upon the microbiome. In fact, antibiotic-mediated microbiome agitation is now believed to contribute to several non-communicable and chronic diseases [37].
Bacteriophages are classes of viruses that can attack and destroy bacteria without any impact on human or animal cells. As a result, it is supposed that they can be used, alone or in combination with antibiotics, to treat infections caused by bacteria [38]. Hyman [39] suggested the following features of bacteriophages be used for therapeutic purposes: (a) the bacteriophage should be lytic and be able to cause broad cytotoxicity to the target bacterium. (b) It should be completely lytic and should not become a lysogenic phage. (c) The bacteriophage should have the ability to transduce the host bacteria. (d) It should have the desired host range. (e) It should be checked for toxin genes that can affect the patient. Myoviridae, Siphoviridae, and Podoviridae families are used frequently for bacteriophage therapy [40,41]. There are around 800 phages against pathogens like Escherichia, Klebsiella, Enterococcus, Pseudomonas, Staphylococcus, and Salmonella. [42].
Phage therapy may not completely replace antibiotics at this time, but there is optimism that it will be used in conjunction with antibiotics, particularly for strains that are resistant to antibiotics. Phages will be much more reliable when used externally and where the immune system gives them a chance by favoring them to persist within the body for a little while (Clark and March 2016).
Clinical Cases Treated with Bacteriophages
In human medicine, bacteriophages are utilized both topically and internally to treat various problems and diseases that are resistant to antibiotic treatment. Patients suffering from ulcers received effective treatment from bacteriophage moistened in biodegradable films and the wounds swiftly healed [43]. Numerous studies have demonstrated the effectiveness of local and topical phage treatments. Phage cocktails have been used locally to treat infections such as conjunctivitis, otitis, gingivitis, furunculosis, decubitis ulcer, open wound infection, burns, osthitis (caused by fractures) and chronic suppurative fistulae caused by Staphylococci, Klebsiella, Pseudomonas, Proteus and Escherichia [44-47]. A commercial product called PhagoBioDerm, which targets P. aeruginosa, S. aureus, and Streptococcus spp. and contains phages as well as ciprofloxacin, can be applied directly over infected wounds. Goode, et al. [48] eliminated Salmonella contamination on chicken skin by using a lytic bacteriophage.
A P. aeruginosa that had caused skin infections was treated with phage therapy by Vieira, et al. [49]. Phage treatment resulted in a four-orders-of-magnitude reduction in P. aeruginosa 709 levels in human skin. S. aureus, which produces wound abscess, may be successfully combated by sewage-derived phage [50]. According to studies, mice that get an intra-peritoneal injection of a bacteriophage to treat P. aeruginosa infection experience less infection [51]. E. coli-induced diarrhea in calves can be avoided by giving them bacteriophages orally. Similarly, oral phage therapy could help stop the sepsis brought on by P. aeruginosa [52]. Additionally, intramuscular treatments against E. coli in chickens have been performed, and they were successful in preventing septicemia [53]. Mice were saved from death by phages against vancomycin-resistant Enterococcus faecium [54]. In mouse models, phages against Acinetobacter baumanii, P. aeruginosa, and S. aureus also performed well [55]. As a result, Salmonella Typhimurium, one of the main threats to the swine sector, has been reduced in prevalence as a result of the use of phage [56,57].
Advantages and limitations of phage therapy
Bacteriophage treatment has been used in animals, plants, and humans with a diverse degree of accomplishment. Bacteriophages have various potential benefits over antibiotics, but at the same time, they do have drawbacks as well (Table 1). The main advantage of bacteriophages is their specificity for target host bacteria, which significantly reduces the destruction of the host’s normal flora. The bacteria to be targeted must be recognized first, or else a mixture of bacteriophages should be used. Phages are self-limiting, i.e., they require their hosts to be continually growing; their persistence in the environment for a long period of time depends on their specific host availability (Clark and March 2016). Duplication at the site of infection is another merit of bacteriophages. They are safe with no or few side effects [35]. If bacteria become resistant to bacteriophages, bacteriophages will naturally change to infect the resistant bacteria, reducing the likelihood of bacterial escape (Hausler, 2007).
After their administration, bacteriophages can disperse very rapidly in the body, reaching almost every organ; but the immune system quickly clears systemic bacteriophages, which poses yet another problem for their acceptance as therapeutic agents [58,59]. One of the serious concerns about the use of bacteriophage therapy in vivo is a strong antibody response, which would clear the bacteriophages more quickly and thus the use of bacteriophages for a prolonged period of time would not be possible (Clark and March 2016).
Other limitations of bacteriophages as therapeutic agents are their narrow host ranges and the fact that bacteriophages are not permanently virulent under certain physiological situations. During the preparation of bacteriophage stocks, it must be ensured that bacteriophage preparations are free of bacteria and bacterial toxins in order to prevent secondary infections. But sterilizing bacteriophages could deactivate them. Bacteriophages may convey toxic properties to the bacteria, resulting in virulence [60]. One way around this is the use of the bacteriophage lytic enzyme endolysins, rather than administering the entire virion [61,62]. Likewise, genetically improved bacteriophages can be used, which will only deliver the DNA essential for making antibacterials that would be specific to the target bacteria [63].
Status of phage therapy in Ethiopia
Although bacteriophages are widely used as treatment agents for pathogenic bacteria in developed countries, this application potential is not widely practiced in Ethiopia. Only limited investigation has been conducted in our country regarding the therapeutic potential of bacteriophages, focusing on the isolation of bacteriophages and assessment of their activity against biofilms of uropathogenic Escherichia coli in Jimma Town, South Western Ethiopia (Gudina et al., 2018).
As-biocontrol agents in food industry
Foodborne infections from microbial sources remain a severe food safety problem worldwide. In addition to being of substantial public health significance, the economic impact of foodborne bacterial illness is significant [64]. Contamination prevention and the endowment of a safe food supply is one of the extraordinary priority areas to control and bound the foodborne pathogen outbreaks under the “One Health” approach [65]. Nevertheless, the challenge is not a direct one to solve quickly or simply. The epidemiology of foodborne pathogens is difficult and involves multiple routes of transmission from food animals and agricultural produce to consumers. For example, animals are often asymptomatic carriers of Shiga-toxin producing Escherichia coli (STEC), Salmonella, Campylobacter and Listeria, and they can spread the pathogens to other food animals, crops, slaughter facilities, and, in some instances, directly to humans. Food processing facilities can also harbor foodborne pathogens as biofilms, which can potentially transfer to food products and reach consumers [66].
Traditional pathogen sanitization procedures in food processing facilities emphasize the use of chemicals, physical destruction techniques, and irradiation to reduce the microbial load in those facilities and the foods produced in them [67]. For instance, numerous harsh chemical sanitizers, such as chlorine and peracetic acid, are commonly used to reduce microbial contaminants in many fresh fruits and vegetables as well as ready-to-eat (RTE) food products [68]. Heat pasteurization is often used to decrease bacterial numbers, generally in liquids and dairy items, such as milk. High-pressure processing (HPP) is also used to successfully reduce pathogens in liquid products, as well as pre-cooked, meant to be frozen meals [69,70]. This technique exposes foods to high pressure to deactivate microbes. Irradiation has been accepted as a means of reducing the burden of pathogenic organisms in foods since 1997 [71].
Conversely, no single approach is 100% effective, and the aforementioned methods also have some substantial disadvantages. For example, many chemical sanitizers corrode and harm food processing materials [72,73] and may have toxic chemical residues that may damage the environment. Pasteurization and HPP are not appropriate for fresh produce and meat products as they can harmonically affect the organoleptic properties and/or the nutritional content of some foods [69,70]. Irradiation, which can deleteriously affect the appearance of some foods, also has low customer acceptance, which is compounded by a labeling requirement for many food items treated with radiation [74]. Food sustainability and safety are challenges that continue to dominate the food industry worldwide. The shift in Western countries towards consuming foods that are produced by natural means adds significant pressure to produce foods that are safe, natural, free from chemical preservatives, and of acceptable quality to meet consumer demands [75].
The food industry has repeatedly had to contribute to efforts to avoid infectious diseases and the problems associated with antibiotic resistance in human pathogens originating in food animals. Regardless of the many advances in technological methods for the detection and removal of foodborne pathogens at each stage of the food production process, good manufacturing practices, quality control and sanitation and changes in animal agriculture and agronomic processes, microbial safety problems are still predominant. In addition, the controlled use of certain antibiotics during food animal production, together with the lack of development of novel antimicrobials, has put further strain on the food production sector and as such, there is a need for the development of alternative antibacterial approaches at the production level to maintain safety standards, control foodborne pathogens and limit their negative impact on the food industry and on human health [76].
The natural specificity of phages to attack and destroy their target bacteria, in addition to the fact that they are abundant in the surrounding environments and are harmless to humans and animals, makes them valuable candidates for use in both the detection and control of pathogens at each stage of the food production process from farm-to-fork. In recent years, a number of bacteriophage-based products have gone into commercial use to control some of the leading foodborne pathogens, including Listeria monocytogenes, Escherichia coli, and Salmonella serovars [76,77]. The application of bacteriophages is harmless and related to the use of antibiotics [78]. Phages have been used since the 1980s to control and exclude bacterial contaminants from food surfaces, food-borne spoilage bacteria, and bacteria causing gastrointestinal diseases [57], as well as to disinfect raw food. Due to their specificity, phages are smart for the sanitization of ready-to-eat foods (RTE) such as milk, vegetables, and meat products [79].
In addition to direct treatment of food, bacteriophage use is also indicated for decontaminating material surfaces in farms or food-processing facilities to significantly decrease pathogen colonies on surfaces and the development of biofilm, helping to reduce the risk of transmission of pathogens along the supply chain. In fact, biofilm represents one of the most important sources of contamination with pathogens in farms or industrial settings, contributing to the transmission of pathogens to dairy products and, along the food chain, to consumers [78].
The idea of preventing pathogens in food by means of bacteriophages can be achieved at all stages of production in the classic ‘farm to fork’ approach throughout the complete food chain. Phages are suitable (i) to prevent or reduce colonization and diseases in livestock (phage therapy), (ii) to decontaminate carcasses and other raw products, such as fresh fruit and vegetables and to disinfect equipment and contact surfaces (bacteriophage sanitation and biocontrol); and (iii) to extend the shelf life of perishable manufactured foods as natural preservatives (biopreservation). Phages should also be considered in hurdle technology in combination with different preservation methods [80,81].
Merits and limitations of using bacteriophages as biocontrol agents
The fact that bacteriophages are presently being used as bio-control agents in sectors of food production [82] shows their advantage as efficacious complementary approaches for controlling specific dangerous pathogens in food in many circumstances. The fact that they have GRAS status supports their safety for food applications, and indeed, there is no known negative side effect of using lytic bacteriophages on humans or animals. Bacteriophages are natural, and low-cost to produce [83,84]. While a suitable propagating host must be selected to ensure endotoxin and virulence factor contamination of the preparation does not occur, the commercialization timeframe is less stringent than what might be required for human therapeutic applications [85].
Additionally, bacteriophages are highly specific for their target bacterial host and, as such, have no substantial impact on consumers’ resident microflora. Bacteriophages also don’t impact the sensory and quality characteristics of food [73]. Contrasting chemical biocides or antibiotics that have the ability to leak into food products and persist in the environment; bacteriophages persist in high numbers for a short time without their host. Commercial bacteriophage products are 100% natural and non-genetically modified organisms (GMOs). They’re generally kosher, halal, and permitted in organic foods, with several officially certified as such [73].
Generally, there are two main scientific challenges in bacteriophage-mediated biocontrol in food. Initially, the constituents of the bacteriophage product must have a wide enough host range to kill all members of the target pathogenic genus or species. Secondly, the bacteriophages need to be applied such that the particles physically come into contact with all or most of the target bacterial cells in order to work. It is also important that users of bacteriophage products in the food sector understand that individual products don’t guarantee the full well-being of foods if the foods are contaminated by a different foodborne pathogen (e.g. a pathogen not targeted by the phage product applied to the food) [86].
Bacterial detection (phage typing)
Bacteriophage-based approaches for bacteria detection were developed using the bacteriophages’ typical capacity to grab host bacteria. Only the recognition of a suitable and workable host offers the chance for virion proliferation and virus life cycle completion. This offers an advantage over other widely used techniques, such as PCR or mass spectroscopy-based ones, which might produce false positive results when dead bacteria are present. Bacteriophages are far less expensive and simpler to prepare than antibodies, while conventional biochemical or culturing approaches take a lot longer to produce results [87,88].
Due to the fact that bacteriophages only infect live hosts, bacterial cells may be quickly and precisely identified using bacteriophage detection methods. Bacteriophages can be adjusted, lysed, isolated, and extracted from their hosts to permit detection in a variety of methods. The three main categories of phage-mediated detection techniques are reporter bacteriophages, bacteriophage amplification, and bacteriophage capture [89].
Genetically modified reporter phages allow the entry of reporter genes into the bacterial host, where the gene is produced, a signal is picked up, and the pathogen is recognized. In order to enable gene expression when the phage infects live hosts, reporter genes encoding luciferases or fluorescent proteins have been introduced into the phage genome [90].
The formation of offspring phages or the demise of the bacterial host is used as a detection signal in bacteriophage multiplication assays [91]. Typically, the development of plaques on a Petri plate is used to gauge the bacteriophage’s growth. Plaques develop when infected hosts lyse, releasing offspring phage that allows bacterial reinfection. Bacteriophage amplification requires phage infection, extracellular phage chemical inactivation using virucide and plaque detection using a rapidly proliferating “reporter” organism/lawn. Each plaque is thought to represent the initially infected bacterium. Plaques can then be removed and analyzed using PCR for further specificity [92,93].
Phage capture uses a phage’s features to specifically bind bacteria, such as endolysins or tail spikes. Endolysins are phage-produced enzymes that help lyse bacteria by dissolving their cell walls. They have two domains: one breaks down the cell wall, while the other, the cell wall binding domain (CWBD), selectively identifies regions of the host cell wall. To enable infection, phage tail spikes bind and selectively attach to host cells. Tail spikes have been used to collect particular bacteria from a variety of matrices, such as food and processing machinery [89].
Phage display system
Despite the fact that natural phages have been successfully used to lower bacterial resistance, some researchers have gone above and beyond to uncover additional antibacterial strategies based on bacteriophages. This results in bacteriophage modifications by gene engineering, the main benefit of which is increased precision [94]. However, bacteriophage engineering has further benefits, such as obtaining phage elements that can recognize bacterial hosts, altering phage hosts, or enhancing their activity (Hauser, 2016).
George P. Smith introduced the idea of bacteriophage display for the first time in 1985 (Figure 2) [95]. It is a molecular technique used to produce polypeptides with novel properties. The desired protein is expressed on the surface of the bacteriophage particle when the DNA encoding the polypeptide is linked with genes for the bacteriophage coat protein [95,96]. Although the E. coli filamentous phage M12 is frequently utilized, the phage display technique also makes use of lambda and T7 [97,98]. Detecting and isolating peptides with high specificity and affinity for target proteins can be done using bacteriophage display libraries. These peptides can be employed in medication development as tools for comprehending molecular recognition and reducing receptor mimics [96].
Possibly different phage kinds are utilized in phage display systems, but filamentous phages are the most beneficial because they enable the genetic material extension by merely enlarging their filament size. The phage’s interior structures are unharmed during the process of delivering genetic material. Lysogenic filamentous phages do not kill bacterial cells in order to complete their life cycle and release new virions. The standard phage for this technique is M13. The most intriguing gene in phage display is gene VIII, which codes for important structural proteins and can show short peptides and produce a large number of desired compounds. In contrast, gene III only produces a small number of minor proteins and is more suited for producing big peptides than other genes [99].
The development of vaccines, the advancement of new medications, the study of protein-protein interactions, the selection and modification of substances of interest, the development of monoclonal antibodies with the desired specificity for therapeutic use, the creation of libraries of peptides and other substances, in epitope mapping (as antivenom), or the production of food as biocontrol, all benefit greatly from the use of bacteriophage display. Similarly, selecting and isolating antibodies against desirable antigens or other targets, followed by the creation of an antibody library, is a highly helpful strategy [100].
Bacterial evolution vehicles
Bacteriophages play a significant role in horizontal gene transfer among bacteria. When bacteriophages multiply, they can occasionally encapsidate host bacterial DNA to generate transducing particles. Transduction is the process by which foreign DNA (plasmid or bacterial) is transmitted into bacterial cells with the help of a bacteriophage. In theory, transducing particles are similar to mature bacteriophage particles, but when they infect other cells, they release bacterial DNA rather than a viral genome. The DNA can then replicate as a plasmid or be recombined into the chromosome in the new host cell. Genetic transduction is the term for this process of bacterial DNA transfer from one bacterium to another [101].
Transducing particles’ genetic cargo can have a significant impact on the bacteria they transfer to. For instance, genes encoding for virulence or antibiotic resistance might confer new traits and open up new ecological niches, hastening the rise of new strains that are progressively more virulent and resistant to drugs. Although there are a number of horizontal gene transfer processes, phage transduction is frequently considered to be the primary method by which bacteria obtain the genes necessary for quick adaptation to changing environmental obstacles [101].
Generalized transduction
In Salmonella phage P22, generalized transduction was the first mode of bacteriophage-mediated gene transfer to be identified and discovered [102]. It is how bacteriophages package and transfer any type of bacterial DNA (chromosomal or plasmid) to another bacterium. These so-called pseudoviruses are nevertheless capable of binding to cells and ejecting packaged DNA into a new host. Depending on the kind of donor DNA, the transduced molecules can either be integrated or stay free to continue reproducing as plasmids in the cytoplasm (plasmid or chromosomal). This form of transduction can take place during the lytic phase of both the lytic and lysogenic life cycles (Figure 3) [103].
Specialized transduction
The second transduction mechanism described and discovered in the coli phage was specialized transduction [104]. Specialized transduction, in contrast to generalized transduction, is unique to lysogenic bacteriophages and entails the prophage DNA excision from a particular integration site as well as a neighboring region of the host genome (Figure 3). Site-specific recombination (mediated by phage integrases) or homologous recombination (mediated by bacterial recombinases) causes the integration of the phage DNA into the host genome, typically at specified places, after packing into virions and infection of a new host cell [105]. The term “lysogenic conversion” refers to the phenotypic alterations that occur as a result of the prophage’s integration into the host genome [106]. Typically, lysogenic conversion increases the pathogenicity of bacteria to improve fitness [107]. The environment’s presence of bacteria, bacterial viruses and bacteriophages as well as horizontal gene transfer enables the bacterial host to diversify its gene pool, which aids in environmental adaptability [108]. Therefore, bacteria can inhabit new ecological niches thanks to bacteriophage-mediated gene transfer, which influences subsequent gene transfer with local populations and increases bacterial diversity [19].
Vehicles for vaccines delivery (vaccination tools)
accines are biological agents that stimulate the immune system of the host to provide both therapy and disease prevention. They are made from disease-causing agents (bacteria, viruses, etc.). Conventional vaccines of the live attenuated or inactivated microbe variety have advanced greatly and some of them have been applied in clinical settings [109]. Newly developed vaccines are based on selected target antigens, derived from an infectious microorganism, a tumor cell, an allergen, or an auto-antigen. The target molecule may be administered as a purified protein or as a peptide (s) or may be expressed from plasmid DNA or a recombinant virus [110].
Bacteriophages have been shown to have the capacity to deliver vaccines both directly and indirectly. They can first display vaccine antigens as fusions to their coat proteins utilizing bacteriophage display, which causes the desired immune response [111]. Additionally, a DNA vaccine expression cassette can be delivered to eukaryotic cells using the phage particle as a gene delivery vehicle [112].
When compared to phage DNA vaccines, bacteriophage display vaccines are more stable since they are based on a virion that contains the gene that codes for the antigen being exhibited. On the other hand, phage DNA vaccines contain DNA that has the antigen gene cloned in a eukaryotic cassette inside the virion. These vaccinations produce a stronger immunological response than traditional vaccines do. These vaccinations are being studied for use against things including bacteria, autoimmune disorders, cancer, fungi, parasites and even contraceptive vaccines [113]. Studies have also looked into using vaccines to prevent or lessen the development of antibiotic resistance. This may be novel because when a patient is exposed to a pathogen, they trigger an immune response that prevents or lessens the sickness. Additionally, if vaccination rates rise among the populace, herd immunity may result, in protecting others who aren’t vaccinated [114]. Phage display, like other techniques, has several shortcomings that need to be examined. The peptide’s ability to bind to its target may be lost during the transfer to a soluble medium. Additionally, there is a chance that peptides’ in vivo and in vitro functionalities will differ, which could have an adverse impact on the patient. Due to proteolysis, peptides are also unstable and their capacity to trigger immunological reactions might provide a challenge for their use. However, various new methods are being explored to address these obstacles. Many of them try to reduce immunogenicity and increase peptide affinity and half-life using protein engineering or nanotechnology [115].
Purified phage particles are administered to the host after the vaccine gene is cloned in a lambda bacteriophage under the direction of a eukaryotic expression cassette. The coat shields DNA from deterioration, and because it behaves like a virus particle, it would direct the vaccination toward the cells that present the antigen [116]. In mice and rabbits, the antibody response was vastly superior when compared to the usual DNA vaccine [117]. A DNA vaccine encapsulated in a phage particle under a eukaryotic promoter and a phage display version of the same antigen present on the phage surface have recently been suggested as potential components of a hybrid phage [116]. A vaccination of this type would successfully target the humoral and cellular immune systems [59]. By adding particular protein sequences to target particular immune cell types, such as galactose residues that will target galactose-recognizing hepatic receptors in the liver, it can also be extended to the alteration of the phage vaccine’s surface. By separating peptides from the phage display libraries, Langerhans cells and dendritic cells [118] may both be targeted [119].
Gene delivery
The possible therapeutic gene delivery agents are phages [120,121]. Phage delivery of DNA vaccines, in which the phage coat shields the DNA inside from degradation after injection, is comparable to the use of phages for targeted gene delivery. Both, however, are conceptually distinct. Phages can target particular cell types because of their capacity to show foreign proteins on their surfaces, which is a requirement for effective gene therapy [116]. To show the targeting and processing molecules on the surfaces of phages, researchers have employed phage display and artificial covalent conjugation [122,123]. Targeting sequences, such as fibroblast growth factor, have been utilized to deliver phages to cells with the necessary receptors [124,125]. Protein sequences like the adenovirus penton base, which mediates entry, attachment, and endosomal release, are employed to increase the absorption and endosomal release of phages [126]. The absorption and nuclear targeting of modified phages like lambda have also been improved by the protein transduction domain of the human immunodeficiency virus (HIV) tat protein and the nuclear localization signal of the simian virus 40 (SV40) T antigen [127].
Other displayed peptides that can facilitate gene delivery via phages include integrin binding peptides that enhance binding and uptake [124] and DNA degradation reducing DNase II inhibitors [125]. To screen the ability of phages to target specific cells and tissues, phage display libraries have been used in mice many times and every time phages were found in specific tissues [128]. For instance, to isolate phages that target the liver, mice were inoculated with phage display libraries and phages were isolated after extracting the livers [116]. A similar in vitro strategy is used for the isolation of phage-displayed peptides that enhance cytoplasmic uptake into mammalian cells [129]. Therefore, phages once more demonstrated their versatility by making it possible to target particular tissues either by randomly scanning phage display libraries or by rational design [116].
Conclusion and recommendations
Generally, phages are a type of virus extensively disseminated in nature whose progression is firmly connected with the bacterial cell. They have been used for various types of applications starting from their discovery time and are still playing significant roles in modern biotechnology since their genomes are easily manipulated. Either lytic or lysogenic bacteriophages are estimated to be found everywhere the host bacteria live. Hence, it’s possible to get and isolate these viruses from any type of sample from which the hosts can be obtained. Phages have increased potential application in different parts of the world, especially in western countries, as therapeutic agents in different hospitals, clinics and food industries due to the rise in antimicrobial resistance strains of different bacteria pathogens. However, in developing countries, these phage futures are not well pursued or investigated. Bacteriophages look to be a great solution as an alternative treatment against bacterial diseases, but they are also used as interesting tools in vaccine delivery vehicles, modulating bacterial populations and assisting in diagnosis. Therefore, based on the above conclusion, the following points are forwarded as recommendations.
- It is recommended that bacteriophage therapy be expanded in various hospitals, clinics, and food industries to alleviate the burden of multi-drug-resistant bacterial pathogens.
- It is good if the building different phage companies are used for the production of different phage cocktails that have treatment effects will be constructed.
- It is recommended that more awareness be raised about bacteriophage research in order to develop monitoring and safety procedures that will lead to the widespread use of bacteriophages in various fields, including clinics.
- Mayer G, Bacteriology: “Bacteriophage” (Lecture Notes, Chapter 7). 2005; 2006: http://pathmicro.med.sc.edu/mayer/phage.htm.
- Suttle CA. Marine viruses--major players in the global ecosystem. Nat Rev Microbiol. 2007 Oct;5(10):801-12. doi: 10.1038/nrmicro1750. PMID: 17853907.
- Wittebole X, De Roock S, Opal SM. A historical overview of bacteriophage therapy as an alternative to antibiotics for the treatment of bacterial pathogens. Virulence. 2014 Jan 1;5(1):226-35. doi: 10.4161/viru.25991. Epub 2013 Aug 13. PMID: 23973944; PMCID: PMC3916379.
- Stone E, Campbell K, Grant I, McAuliffe O. Understanding and Exploiting Phage-Host Interactions. Viruses. 2019 Jun 18;11(6):567. doi: 10.3390/v11060567. PMID: 31216787; PMCID: PMC6630733.
- Dunne M, Hupfeld M, Klumpp J, Loessner MJ. Molecular Basis of Bacterial Host Interactions by Gram-Positive Targeting Bacteriophages. Viruses. 2018 Jul 28;10(8):397. doi: 10.3390/v10080397. PMID: 30060549; PMCID: PMC6115969.
- Kortright KE, Chan BK, Koff JL, Turner PE. Phage Therapy: A Renewed Approach to Combat Antibiotic-Resistant Bacteria. Cell Host Microbe. 2019 Feb 13;25(2):219-232. doi: 10.1016/j.chom.2019.01.014. PMID: 30763536.
- EFSA (European Food Safety Authority).Scientific Opinion of the Panel on Biological Hazards on a Request from European Commission on the Use and Mode of Action of Bacteriophages in Food Production. The EFSA Journal. 2009; 1076: 1-26.
- Mai V, Ukhanova M, Visone L, Abuladze T, Sulakvelidze A. Bacteriophage Administration Reduces the Concentration of Listeria monocytogenes in the Gastrointestinal Tract and Its Translocation to Spleen and Liver in Experimentally Infected Mice. Int J Microbiol. 2010;2010:624234. doi: 10.1155/2010/624234. Epub 2010 Jun 24. PMID: 20652074; PMCID: PMC2905708.
- Connerton PL, Timms AR, Connerton IF. Campylobacter bacteriophages and bacteriophage therapy. J Appl Microbiol. 2011 Aug;111(2):255-65. doi: 10.1111/j.1365-2672.2011.05012.x. Epub 2011 Apr 20. PMID: 21447013.
- Hagens S, Loessner MJ. Application of bacteriophages for detection and control of foodborne pathogens. Appl Microbiol Biotechnol. 2007 Sep;76(3):513-9. doi: 10.1007/s00253-007-1031-8. Epub 2007 Jun 7. PMID: 17554535.
- Hagens S, Loessner MJ. Bacteriophage for biocontrol of foodborne pathogens: calculations and considerations. Curr Pharm Biotechnol. 2010 Jan;11(1):58-68. doi: 10.2174/138920110790725429. PMID: 20214608.
- Clokie MR, Millard AD, Letarov AV, Heaphy S. Phages in nature. Bacteriophage. 2011 Jan;1(1):31-45. doi: 10.4161/bact.1.1.14942. PMID: 21687533; PMCID: PMC3109452.
- Strauch E, Hammerl J, Hertwig S. Bacteriophages: new tools for safer food? J Verbr Lebensm. 2007; 2:138–143.
- Kutter E, Sulakvelidze A. (Eds.) Introduction. In Bacteriophages: Biology and applications. 2005; 1-4. Boca Raton, FL: CRC Press.
- Hanlon GW. Bacteriophages: an appraisal of their role in the treatment of bacterial infections. Int J Antimicrob Agents. 2007 Aug;30(2):118-28. doi: 10.1016/j.ijantimicag.2007.04.006. Epub 2007 Jun 12. PMID: 17566713.
- Ackermann HW. 5500 Phages examined in the electron microscope. Arch Virol. 2007 Feb;152(2):227-43. doi: 10.1007/s00705-006-0849-1. Epub 2006 Oct 19. PMID: 17051420.
- Ackermann H, Dubow M. General properties of tailed phages. In Viruses of Prokaryotes. 1987; 2: 28.
- Lopes A, Tavares P, Petit MA, Guérois R, Zinn-Justin S. Automated classification of tailed bacteriophages according to their neck organization. BMC Genomics. 2014 Nov 27;15(1):1027. doi: 10.1186/1471-2164-15-1027. PMID: 25428721; PMCID: PMC4362835.
- Weinbauer MG. Ecology of prokaryotic viruses. FEMS Microbiol Rev. 2004 May;28(2):127-81. doi: 10.1016/j.femsre.2003.08.001. PMID: 15109783.
- Guttman B, Raya R, Kutter E. Basic Phage Biology. In: Kutter E, Sulakvelidze A, editors. Bacteriophages: CRC Press. 2005; 29-66.
- Kutter E, Raya R, Carlson K. Molecular Mechanisms of Phage Infection. In: Kutter E, Sulakvelidze A, editors. Bacteriophages: CRC Press. 2005; 165-213.
- Rakhuba DV, Kolomiets EI, Dey ES, Novik GI. Bacteriophage receptors, mechanisms of phage adsorption and penetration into host cell. Pol J Microbiol. 2010;59(3):145-55. PMID: 21033576.
- Mackey M, Santillan M, Tyran-Kaminska M, Zeron E. Simple Mathematical Models of Gene Regulatory Dynamics. In Lecture Notes on Mathematical Modelling in the Life Sciences; Springer: Berlin/Heidelberg, Germany. 2016; 87–97.
- Nilsson AS. Phage therapy--constraints and possibilities. Ups J Med Sci. 2014 May;119(2):192-8. doi: 10.3109/03009734.2014.902878. Epub 2014 Mar 30. PMID: 24678769; PMCID: PMC4034558.
- Kaźmierczak Z, Górski A, Dąbrowska K. Facing antibiotic resistance: Staphylococcus aureus phages as a medical tool. Viruses. 2014 Jul 1;6(7):2551-70. doi: 10.3390/v6072551. Erratum in: Viruses. 2015 Apr;7(4):1667. PMID: 24988520; PMCID: PMC4113783.
- Mimmi S, Maisano D, Quinto I, Iaccino E. Phage Display: An Overview in Context to Drug Discovery. Trends Pharmacol Sci. 2019 Feb;40(2):87-91. doi: 10.1016/j.tips.2018.12.005. Epub 2018 Dec 31. PMID: 30606501.
- Mimmi S, Maisano D, Nisticò N, Vecchio E, Chiurazzi F, Ferrara K, Iannalfo M, D'Ambrosio A, Fiume G, Iaccino E, Quinto I. Detection of chronic lymphocytic leukemia subpopulations in peripheral blood by phage ligands of tumor immunoglobulin B cell receptors. Leukemia. 2021 Feb;35(2):610-614. doi: 10.1038/s41375-020-0885-y. Epub 2020 Jun 1. PMID: 32483301; PMCID: PMC7862058.
- Pelechas E, Voulgari PV, Drosos AA. Preclinical discovery and development of adalimumab for the treatment of rheumatoid arthritis. Expert Opin Drug Discov. 2021 Mar;16(3):227-234. doi: 10.1080/17460441.2021.1846516. Epub 2020 Nov 18. PMID: 33183071.
- Sulakvelidze A, Barrow P. Phage therapy in animals and agribusiness. In Kutter, E. and Sulakvelidze, A. (Eds). Bacteriophages: Biology and applications. 2005; 335-380.
- Sulakvelidze A, Kutter E. Bacteriophage therapy in humans. In Bacteriophages: Biology and Application. E. Kutter, and A. Sulakvelidze, eds. 2005; 381-436.
- Summers WC. Bacteriophage therapy. Annu Rev Microbiol. 2001;55:437-51. doi: 10.1146/annurev.micro.55.1.437. PMID: 11544363.
- Hess KL, Jewell CM. Phage display as a tool for vaccine and immunotherapy development. Bioeng Transl Med. 2019 Sep 18;5(1):e10142. doi: 10.1002/btm2.10142. PMID: 31989033; PMCID: PMC6971447.
- Summers WC. The strange history of phage therapy. Bacteriophage. 2012 Apr 1;2(2):130-133. doi: 10.4161/bact.20757. PMID: 23050223; PMCID: PMC3442826.
- Wittebole X, De Roock S, Opal SM. A historical overview of bacteriophage therapy as an alternative to antibiotics for the treatment of bacterial pathogens. Virulence. 2014 Jan 1;5(1):226-35. doi: 10.4161/viru.25991. Epub 2013 Aug 13. PMID: 23973944; PMCID: PMC3916379.
- Sulakvelidze A, Alavidze Z, Morris JG Jr. Bacteriophage therapy. Antimicrob Agents Chemother. 2001 Mar;45(3):649-59. doi: 10.1128/AAC.45.3.649-659.2001. PMID: 11181338; PMCID: PMC90351.
- Talbot GH, Jezek A, Murray BE, Jones RN, Ebright RH, Nau GJ, Rodvold KA, Newland JG, Boucher HW; Infectious Diseases Society of America. The Infectious Diseases Society of America's 10 × '20 Initiative (10 New Systemic Antibacterial Agents US Food and Drug Administration Approved by 2020): Is 20 × '20 a Possibility? Clin Infect Dis. 2019 Jun 18;69(1):1-11. doi: 10.1093/cid/ciz089. PMID: 30715222.
- Dietert RR, Dietert JM. The Microbiome and Sustainable Healthcare. Healthcare (Basel). 2015 Mar 3;3(1):100-29. doi: 10.3390/healthcare3010100. PMID: 27417751; PMCID: PMC4934527.
- Domingo-Calap P, Delgado-Martínez J. Bacteriophages: Protagonists of a Post-Antibiotic Era. Antibiotics (Basel). 2018 Jul 27;7(3):66. doi: 10.3390/antibiotics7030066. PMID: 30060506; PMCID: PMC6163168.
- Hyman P. Phages for Phage Therapy: Isolation, Characterization, and Host Range Breadth. Pharmaceuticals (Basel). 2019 Mar 11;12(1):35. doi: 10.3390/ph12010035. PMID: 30862020; PMCID: PMC6469166.
- Kornienko M, Kuptsov N, Gorodnichev R, Bespiatykh D, Guliaev A, Letarova M, Kulikov E, Veselovsky V, Malakhova M, Letarov A, Ilina E, Shitikov E. Contribution of Podoviridae and Myoviridae bacteriophages to the effectiveness of anti-staphylococcal therapeutic cocktails. Sci Rep. 2020 Oct 29;10(1):18612. doi: 10.1038/s41598-020-75637-x. PMID: 33122703; PMCID: PMC7596081.
- Chen Y, Sun E, Song J, Tong Y, Wu B. Three Salmonella enterica serovar Enteritidis bacteriophages from the Siphoviridae family are promising candidates for phage therapy. Can J Microbiol. 2018 Nov;64(11):865-875. doi: 10.1139/cjm-2017-0740. Epub 2018 Jul 10. PMID: 29990444.
- Weber-Dąbrowska B, Jończyk-Matysiak E, Żaczek M, Łobocka M, Łusiak-Szelachowska M, Górski A. Bacteriophage Procurement for Therapeutic Purposes. Front Microbiol. 2016 Aug 12;7:1177. doi: 10.3389/fmicb.2016.01177. Erratum in: Front Microbiol. 2016 Nov 09;7:1813. PMID: 27570518; PMCID: PMC4981656.
- Markoishvili K, Tsitlanadze G, Katsarava R, Morris JG Jr, Sulakvelidze A. A novel sustained-release matrix based on biodegradable poly(ester amide)s and impregnated with bacteriophages and an antibiotic shows promise in management of infected venous stasis ulcers and other poorly healing wounds. Int J Dermatol. 2002 Jul;41(7):453-8. doi: 10.1046/j.1365-4362.2002.01451.x. PMID: 12121566.
- Chang RYK, Morales S, Okamoto Y, Chan HK. Topical application of bacteriophages for treatment of wound infections. Transl Res. 2020 Jun;220:153-166. doi: 10.1016/j.trsl.2020.03.010. Epub 2020 Mar 19. PMID: 32268129; PMCID: PMC7293950.
- Hawkins C, Harper D, Burch D, Anggård E, Soothill J. Topical treatment of Pseudomonas aeruginosa otitis of dogs with a bacteriophage mixture: a before/after clinical trial. Vet Microbiol. 2010 Dec 15;146(3-4):309-13. doi: 10.1016/j.vetmic.2010.05.014. Epub 2010 May 12. PMID: 20627620.
- Chadha P, Katare OP, Chhibber S. In vivo efficacy of single phage versus phage cocktail in resolving burn wound infection in BALB/c mice. Microb Pathog. 2016 Oct;99:68-77. doi: 10.1016/j.micpath.2016.08.001. Epub 2016 Aug 4. PMID: 27498362.
- Morozova VV, Vlassov VV, Tikunova NV. Applications of Bacteriophages in the Treatment of Localized Infections in Humans. Front Microbiol. 2018 Aug 2;9:1696. doi: 10.3389/fmicb.2018.01696. PMID: 30116226; PMCID: PMC6083058.
- Goode D, Allen VM, Barrow PA. Reduction of experimental Salmonella and Campylobacter contamination of chicken skin by application of lytic bacteriophages. Appl Environ Microbiol. 2003 Aug;69(8):5032-6. doi: 10.1128/AEM.69.8.5032-5036.2003. PMID: 12902308; PMCID: PMC169133.
- Vieira A, Silva YJ, Cunha A, Gomes NC, Ackermann HW, Almeida A. Phage therapy to control multidrug-resistant Pseudomonas aeruginosa skin infections: in vitro and ex vivo experiments. Eur J Clin Microbiol Infect Dis. 2012 Nov;31(11):3241-9. doi: 10.1007/s10096-012-1691-x. Epub 2012 Jul 10. PMID: 22777594.
- Wills QF, Kerrigan C, Soothill JS. Experimental bacteriophage protection against Staphylococcus aureus abscesses in a rabbit model. Antimicrob Agents Chemother. 2005 Mar;49(3):1220-1. doi: 10.1128/AAC.49.3.1220-1221.2005. PMID: 15728933; PMCID: PMC549253.
- McVay CS, Velásquez M, Fralick JA. Phage therapy of Pseudomonas aeruginosa infection in a mouse burn wound model. Antimicrob Agents Chemother. 2007 Jun;51(6):1934-8. doi: 10.1128/AAC.01028-06. Epub 2007 Mar 26. PMID: 17387151; PMCID: PMC1891379.
- Watanabe R, Matsumoto T, Sano G, Ishii Y, Tateda K, Sumiyama Y, Uchiyama J, Sakurai S, Matsuzaki S, Imai S, Yamaguchi K. Efficacy of bacteriophage therapy against gut-derived sepsis caused by Pseudomonas aeruginosa in mice. Antimicrob Agents Chemother. 2007 Feb;51(2):446-52. doi: 10.1128/AAC.00635-06. Epub 2006 Nov 20. PMID: 17116686; PMCID: PMC1797723.
- Barrow P, Lovell M, Berchieri A Jr. Use of lytic bacteriophage for control of experimental Escherichia coli septicemia and meningitis in chickens and calves. Clin Diagn Lab Immunol. 1998 May;5(3):294-8. doi: 10.1128/CDLI.5.3.294-298.1998. PMID: 9605979; PMCID: PMC104512.
- Biswas B, Adhya S, Washart P, Paul B, Trostel AN, Powell B, Carlton R, Merril CR. Bacteriophage therapy rescues mice bacteremic from a clinical isolate of vancomycin-resistant Enterococcus faecium. Infect Immun. 2002 Jan;70(1):204-10. doi: 10.1128/IAI.70.1.204-210.2002. Erratum in: Infect Immun 2002 Mar;70(3):1664. PMID: 11748184; PMCID: PMC127648.
- Soothill JS. Treatment of experimental infections of mice with bacteriophages. J Med Microbiol. 1992 Oct;37(4):258-61. doi: 10.1099/00222615-37-4-258. PMID: 1404324.
- Ahmad SI. Treatment of post-burns bacterial infections by bacteriophages, specifically ubiquitous Pseudomonas spp. notoriously resistant to antibiotics. Med Hypotheses. 2002 Apr;58(4):327-31. doi: 10.1054/mehy.2001.1522. PMID: 12027527.
- García P, Martínez B, Obeso JM, Rodríguez A. Bacteriophages and their application in food safety. Lett Appl Microbiol. 2008 Dec;47(6):479-85. doi: 10.1111/j.1472-765X.2008.02458.x. PMID: 19120914.
- Dabrowska K, Switała-Jelen K, Opolski A, Weber-Dabrowska B, Gorski A. Bacteriophage penetration in vertebrates. J Appl Microbiol. 2005;98(1):7-13. doi: 10.1111/j.1365-2672.2004.02422.x. PMID: 15610412.
- Clark JR, March JB. Bacterial viruses as human vaccines? Expert Rev Vaccines. 2004 Aug;3(4):463-76. doi: 10.1586/14760584.3.4.463. PMID: 15270651.
- Hermoso JA, García JL, García P. Taking aim on bacterial pathogens: from phage therapy to enzybiotics. Curr Opin Microbiol. 2007 Oct;10(5):461-72. doi: 10.1016/j.mib.2007.08.002. Epub 2007 Sep 27. PMID: 17904412.
- Fischetti VA. Bacteriophage lytic enzymes: novel anti-infectives. Trends Microbiol. 2005 Oct;13(10):491-6. doi: 10.1016/j.tim.2005.08.007. PMID: 16125935.
- Borysowski J, Weber-Dabrowska B, Górski A. Bacteriophage endolysins as a novel class of antibacterial agents. Exp Biol Med (Maywood). 2006 Apr;231(4):366-77. doi: 10.1177/153537020623100402. PMID: 16565432.
- Westwater C, Kasman LM, Schofield DA, Werner PA, Dolan JW, Schmidt MG, Norris JS. Use of genetically engineered phage to deliver antimicrobial agents to bacteria: an alternative therapy for treatment of bacterial infections. Antimicrob Agents Chemother. 2003 Apr;47(4):1301-7. doi: 10.1128/AAC.47.4.1301-1307.2003. PMID: 12654662; PMCID: PMC152521.
- Scharff RL. Economic burden from health losses due to foodborne illness in the United States. J Food Prot. 2012 Jan;75(1):123-31. doi: 10.4315/0362-028X.JFP-11-058. PMID: 22221364.
- US Department of Agriculture. USDA targeting six additional strains of E. coli in raw beef trim. USDA News Release 2012; 0171.12
- Havelaar AH, Kirk MD, Torgerson PR, Gibb HJ, Hald T, Lake RJ, Praet N, Bellinger DC, de Silva NR, Gargouri N, Speybroeck N, Cawthorne A, Mathers C, Stein C, Angulo FJ, Devleesschauwer B; World Health Organization Foodborne Disease Burden Epidemiology Reference Group. World Health Organization Global Estimates and Regional Comparisons of the Burden of Foodborne Disease in 2010. PLoS Med. 2015 Dec 3;12(12):e1001923. doi: 10.1371/journal.pmed.1001923. PMID: 26633896; PMCID: PMC4668832.
- Maukonen J, Mättö J, Wirtanen G, Raaska L, Mattila-Sandholm T, Saarela M. Methodologies for the characterization of microbes in industrial environments: a review. J Ind Microbiol Biotechnol. 2003 Jun;30(6):327-56. doi: 10.1007/s10295-003-0056-y. Epub 2003 May 23. PMID: 12764674.
- Sohaib M, Anjum FM, Arshad MS, Rahman UU. Postharvest intervention technologies for safety enhancement of meat and meat based products; a critical review. J Food Sci Technol. 2016 Jan;53(1):19-30. doi: 10.1007/s13197-015-1985-y. Epub 2015 Sep 25. PMID: 26787929; PMCID: PMC4711421.
- Bajovic B, Bolumar T, Heinz V. Quality considerations with high pressure processing of fresh and value added meat products. Meat Sci. 2012 Nov;92(3):280-9. doi: 10.1016/j.meatsci.2012.04.024. Epub 2012 Apr 24. PMID: 22608831.
- Wolbang C, Fitos JL , Treeby M. The effect of high pressure processing on nutritional value and quality attributes of CucumismeloL. Innov Food Sci Emerg. 2008; 9: 196-200.
- Food and Drug Administration. Irradiation in the Production, Processing and Handling of Food. 1997; 21 CFR 179, 62 FR 64107 64107-64121
- Fatica MK, Schneider K. The use of chlorination and alternative sanitizers in the produce industry. CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources 2009;4: 1-10.
- Moye ZD, Woolston J, Sulakvelidze A. Bacteriophage Applications for Food Production and Processing. Viruses. 2018 Apr 19;10(4):205. doi: 10.3390/v10040205. PMID: 29671810; PMCID: PMC5923499.
- Suklim K, Flick GJ, Vichitphan K. Effects of gamma irradiation on the physical and sensory quality and inactivation of Listeria monocytogenes in blue swimming crab meat (Portunas pelagicus). Radiat Phys Chem. 2014;10: 22-26.
- Roman S, Sanchez-Siles L, Siegrist M. The importance of food naturalness for consumers: results of a systematic review. Trends Food Sci Techno. 2017; 67:44-57
- de Melo AG, Levesque S, Moineau S. Phages as friends and enemies in food processing. Curr Opin Biotechnol. 2018 Feb;49:185-190. doi: 10.1016/j.copbio.2017.09.004. Epub 2017 Oct 5. PMID: 28987913.
- Sulakvelidze A. Safety by nature: potential bacteriophage applications. Microbe 2011; 6:122-126.
- Loc-Carrillo C, Abedon ST. Pros and cons of phage therapy. Bacteriophage. 2011 Mar;1(2):111-114. doi: 10.4161/bact.1.2.14590. PMID: 22334867; PMCID: PMC3278648.
- Endersen L, O'Mahony J, Hill C, Ross RP, McAuliffe O, Coffey A. Phage therapy in the food industry. Annu Rev Food Sci Technol. 2014;5:327-49. doi: 10.1146/annurev-food-030713-092415. Epub 2014 Jan 9. PMID: 24422588.
- Leverentz B, Conway WS, Camp MJ, Janisiewicz WJ, Abuladze T, Yang M, Saftner R, Sulakvelidze A. Biocontrol of Listeria monocytogenes on fresh-cut produce by treatment with lytic bacteriophages and a bacteriocin. Appl Environ Microbiol. 2003 Aug;69(8):4519-26. doi: 10.1128/AEM.69.8.4519-4526.2003. PMID: 12902237; PMCID: PMC169090.
- Martínez B, Obeso JM, Rodríguez A, García P. Nisin-bacteriophage crossresistance in Staphylococcus aureus. Int J Food Microbiol. 2008 Mar 20;122(3):253-8. doi: 10.1016/j.ijfoodmicro.2008.01.011. Epub 2008 Jan 26. PMID: 18281118.
- O'Sullivan L, Bolton D, McAuliffe O, Coffey A. Bacteriophages in Food Applications: From Foe to Friend. Annu Rev Food Sci Technol. 2019 Mar 25;10:151-172. doi: 10.1146/annurev-food-032818-121747. Epub 2019 Jan 11. PMID: 30633564.
- Pirnay JP, Merabishvili M, Van Raemdonck H, De Vos D, Verbeken G. Bacteriophage Production in Compliance with Regulatory Requirements. Methods Mol Biol. 2018;1693:233-252. doi: 10.1007/978-1-4939-7395-8_18. PMID: 29119444.
- Bonilla N, Rojas MI, Netto Flores Cruz G, Hung SH, Rohwer F, Barr JJ. Phage on tap-a quick and efficient protocol for the preparation of bacteriophage laboratory stocks. PeerJ. 2016 Jul 26;4:e2261. doi: 10.7717/peerj.2261. PMID: 27547567; PMCID: PMC4975003.
- Szermer-Olearnik B, Boratyński J. Removal of endotoxins from bacteriophage preparations by extraction with organic solvents. PLoS One. 2015 Mar 26;10(3):e0122672. doi: 10.1371/journal.pone.0122672. PMID: 25811193; PMCID: PMC4374689.
- Vikram A, Woolston J, Sulakvelidze A. Phage Biocontrol Applications in Food Production and Processing. Curr Issues Mol Biol. 2021;40:267-302. doi: 10.21775/cimb.040.267. Epub 2020 Jul 9. PMID: 32644048.
- Lee J, Chung W, Kim G. A mechanically improved virus-based hybrid scaffold for bone tissue regeneration. RSC Adv. 2016; 6:55022–55032.
- Hosseinidoust Z, Olsson AL, Tufenkji N. Going viral: designing bioactive surfaces with bacteriophage. Colloids Surf B Biointerfaces. 2014 Dec 1;124:2-16. doi: 10.1016/j.colsurfb.2014.05.036. Epub 2014 Jun 2. PMID: 24997545.
- Jones HJ, Shield CG, Swift BMC. The Application of Bacteriophage Diagnostics for Bacterial Pathogens in the Agricultural Supply Chain: From Farm-to-Fork. Phage (New Rochelle). 2020 Dec 1;1(4):176-188. doi: 10.1089/phage.2020.0042. Epub 2020 Dec 16. PMID: 36147287; PMCID: PMC9041468.
- Meile S, Kilcher S, Loessner MJ, Dunne M. Reporter Phage-Based Detection of Bacterial Pathogens: Design Guidelines and Recent Developments. Viruses. 2020 Aug 26;12(9):944. doi: 10.3390/v12090944. PMID: 32858938; PMCID: PMC7552063.
- Rees C, Botsaris G. The use of phage for detection, antibiotic sensitivity testing and enumeration. Understanding tuberculosis-global experiences and innovative approaches to the diagnosis Rijeka: InTech. 2012;293–306.
- Mole R, Maskell T. Phage as a diagnostic; the use of phage in TB diagnosis. J Chem Technol Biotechnol. 2001;76:683-688.
- McNerney R, Traoré H. Mycobacteriophage and their application to disease control. J Appl Microbiol. 2005;99(2):223-33. doi: 10.1111/j.1365-2672.2005.02596.x. PMID: 16033452.
- Olsen I. Modification of phage for increased antibacterial effect towards dental biofilm. J Oral Microbiol. 2016 Sep 27;8:33089. doi: 10.3402/jom.v8.33089. PMID: 27680404; PMCID: PMC5040820.
- Smith GP. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science. 1985 Jun 14;228(4705):1315-7. doi: 10.1126/science.4001944. PMID: 4001944.
- Sidhu SS. Phage display in pharmaceutical biotechnology. Curr Opin Biotechnol. 2000 Dec;11(6):610-6. doi: 10.1016/s0958-1669(00)00152-x. PMID: 11102798.
- Benhar I. Biotechnological applications of phage and cell display. Biotechnol Adv. 2001 Feb 1;19(1):1-33. doi: 10.1016/s0734-9750(00)00054-9. PMID: 14538090.
- Willats WG. Phage display: practicalities and prospects. Plant Mol Biol. 2002 Dec;50(6):837-54. doi: 10.1023/a:1021215516430. PMID: 12516857.
- Ebrahimizadeh W, Rajabibazl M. Bacteriophage vehicles for phage display: biology, mechanism, and application. Curr Microbiol. 2014 Aug;69(2):109-20. doi: 10.1007/s00284-014-0557-0. Epub 2014 Mar 18. PMID: 24638925.
- Christensen DJ, Gottlin EB, Benson RE, Hamilton PT. Phage display for target-based antibacterial drug discovery. Drug Discov Today. 2001 Jul 1;6(14):721-727. doi: 10.1016/s1359-6446(01)01853-0. PMID: 11445463.
- Chiang YN, Penadés JR, Chen J. Genetic transduction by phages and chromosomal islands: The new and noncanonical. PLoS Pathog. 2019 Aug 8;15(8):e1007878. doi: 10.1371/journal.ppat.1007878. PMID: 31393945; PMCID: PMC6687093.
- ZINDER ND, LEDERBERG J. Genetic exchange in Salmonella. J Bacteriol. 1952 Nov;64(5):679-99. doi: 10.1128/jb.64.5.679-699.1952. PMID: 12999698; PMCID: PMC169409.
- Neidhardt F, Ingraham J, Low K. Generalized Transduction. In Escherichia coli and SalmonellaTyphimurium: cellular and Molecular Biology; American Society of Microbiology. 1996.
- Morse ML, Lederberg EM, Lederberg J. Transduction in Escherichia Coli K-12. Genetics. 1956 Jan;41(1):142-56. doi: 10.1093/genetics/41.1.142. PMID: 17247607; PMCID: PMC1209761.
- Thierauf A, Perez G, Maloy AS. Generalized transduction. Methods Mol Biol. 2009;501:267-86. doi: 10.1007/978-1-60327-164-6_23. PMID: 19066827.
- Wilhelm S, Suttle C . Viruses and Nutrient Cycles in the Sea. Bioscience1999, 49(10):781.
- Chaturongakul S, Ounjai P. Phage-host interplay: examples from tailed phages and Gram-negative bacterial pathogens. Front Microbiol. 2014 Aug 20;5:442. doi: 10.3389/fmicb.2014.00442. PMID: 25191318; PMCID: PMC4138488.
- Williams HT. Phage-induced diversification improves host evolvability. BMC Evol Biol. 2013 Jan 22;13:17. doi: 10.1186/1471-2148-13-17. PMID: 23339571; PMCID: PMC3605116.
- Yang J, Li Y, Jin S, Xu J, Wang PC, Liang XJ, Zhang X. Engineered biomaterials for development of nucleic acid vaccines. Biomater Res. 2015 Feb 19;19:5. doi: 10.1186/s40824-014-0025-8. PMID: 26331076; PMCID: PMC4552455.
- Moingeon P, de Taisne C, Almond J. Delivery technologies for human vaccines. Br Med Bull. 2002;62(1):29-44. doi: 10.1093/bmb/62.1.29. PMID: 12176848; PMCID: PMC7110014.
- Wang LF, Yu M. Epitope identification and discovery using phage display libraries: applications in vaccine development and diagnostics. Curr Drug Targets. 2004 Jan;5(1):1-15. doi: 10.2174/1389450043490668. PMID: 14738215.
- March JB, Clark JR, Jepson CD. Genetic immunisation against hepatitis B using whole bacteriophage lambda particles. Vaccine. 2004 Apr 16;22(13-14):1666-71. doi: 10.1016/j.vaccine.2003.10.047. PMID: 15068849.
- Bazan J, Całkosiński I, Gamian A. Phage display--a powerful technique for immunotherapy: 2. Vaccine delivery. Hum Vaccin Immunother. 2012 Dec 1;8(12):1829-35. doi: 10.4161/hv.21704. Epub 2012 Aug 21. PMID: 22906938; PMCID: PMC3656072.
- Jansen KU, Knirsch C, Anderson AS. The role of vaccines in preventing bacterial antimicrobial resistance. Nat Med. 2018 Jan 9;24(1):10-19. doi: 10.1038/nm.4465. PMID: 29315295.
- Omidfar K, Daneshpour M. Advances in phage display technology for drug discovery. Expert Opin Drug Discov. 2015 Jun;10(6):651-69. doi: 10.1517/17460441.2015.1037738. Epub 2015 Apr 24. PMID: 25910798.
- Clark JR, March JB. Bacteriophages and biotechnology: vaccines, gene therapy and antibacterials. Trends Biotechnol. 2006 May;24(5):212-8. doi: 10.1016/j.tibtech.2006.03.003. Epub 2006 Mar 29. PMID: 16567009.
- Jepson CD, March JB. Bacteriophage lambda is a highly stable DNA vaccine delivery vehicle. Vaccine. 2004 Jun 23;22(19):2413-9. doi: 10.1016/j.vaccine.2003.11.065. PMID: 15193403.
- Curiel TJ, Morris C, Brumlik M, Landry SJ, Finstad K, Nelson A, Joshi V, Hawkins C, Alarez X, Lackner A, Mohamadzadeh M. Peptides identified through phage display direct immunogenic antigen to dendritic cells. J Immunol. 2004 Jun 15;172(12):7425-31. doi: 10.4049/jimmunol.172.12.7425. PMID: 15187120.
- McGuire MJ, Sykes KF, Samli KN, Timares L, Barry MA, Stemke-Hale K, Tagliaferri F, Logan M, Jansa K, Takashima A, Brown KC, Johnston SA. A library-selected, Langerhans cell-targeting peptide enhances an immune response. DNA Cell Biol. 2004 Nov;23(11):742-52. doi: 10.1089/dna.2004.23.742. PMID: 15585132.
- Barry MA, Dower WJ, Johnston SA. Toward cell-targeting gene therapy vectors: selection of cell-binding peptides from random peptide-presenting phage libraries. Nat Med. 1996 Mar;2(3):299-305. doi: 10.1038/nm0396-299. PMID: 8612228.
- Dunn IS. Mammalian cell binding and transfection mediated by surface-modified bacteriophage lambda. Biochimie. 1996;78(10):856-61. doi: 10.1016/s0300-9084(97)84338-6. PMID: 9116055.
- Larocca D, Witte A, Johnson W, Pierce GF, Baird A. Targeting bacteriophage to mammalian cell surface receptors for gene delivery. Hum Gene Ther. 1998 Nov 1;9(16):2393-9. doi: 10.1089/hum.1998.9.16-2393. PMID: 9829538.
- Larocca D, Kassner PD, Witte A, Ladner RC, Pierce GF, Baird A. Gene transfer to mammalian cells using genetically targeted filamentous bacteriophage. FASEB J. 1999 Apr;13(6):727-34. doi: 10.1096/fasebj.13.6.727. PMID: 10094933.
- Hart SL, Knight AM, Harbottle RP, Mistry A, Hunger HD, Cutler DF, Williamson R, Coutelle C. Cell binding and internalization by filamentous phage displaying a cyclic Arg-Gly-Asp-containing peptide. J Biol Chem. 1994 Apr 29;269(17):12468-74. PMID: 8175653.
- Sperinde JJ, Choi SJ, Szoka FC Jr. Phage display selection of a peptide DNase II inhibitor that enhances gene delivery. J Gene Med. 2001 Mar-Apr;3(2):101-8. doi: 10.1002/jgm.165. PMID: 11318108.
- Piersanti S, Cherubini G, Martina Y, Salone B, Avitabile D, Grosso F, Cundari E, Di Zenzo G, Saggio I. Mammalian cell transduction and internalization properties of lambda phages displaying the full-length adenoviral penton base or its central domain. J Mol Med (Berl). 2004 Jul;82(7):467-76. doi: 10.1007/s00109-004-0543-2. Epub 2004 May 19. PMID: 15150649.
- Nakanishi M, Eguchi A, Akuta T, Nagoshi E, Fujita S, Okabe J, Senda T, Hasegawa M. Basic peptides as functional components of non-viral gene transfer vehicles. Curr Protein Pept Sci. 2003 Apr;4(2):141-50. doi: 10.2174/1389203033487234. PMID: 12678853.
- Rajotte D, Arap W, Hagedorn M, Koivunen E, Pasqualini R, Ruoslahti E. Molecular heterogeneity of the vascular endothelium revealed by in vivo phage display. J Clin Invest. 1998 Jul 15;102(2):430-7. doi: 10.1172/JCI3008. PMID: 9664085; PMCID: PMC508902.
- Ivanenkov VV, Menon AG. Peptide-mediated transcytosis of phage display vectors in MDCK cells. Biochem Biophys Res Commun. 2000 Sep 16;276(1):251-7. doi: 10.1006/bbrc.2000.3358. PMID: 11006114.
- Cisek AA, Dąbrowska I, Gregorczyk KP, Wyżewski Z. Phage Therapy in Bacterial Infections Treatment: One Hundred Years After the Discovery of Bacteriophages. Curr Microbiol. 2017 Feb;74(2):277-283. doi: 10.1007/s00284-016-1166-x. Epub 2016 Nov 28. PMID: 27896482; PMCID: PMC5243869.
- DE VICENTE JORDANA R. Study on adsorption of bacteriophage by filters. Appl Microbiol. 1959 Jul;7(4):239-47. doi: 10.1128/am.7.4.239-247.1959. PMID: 13661869; PMCID: PMC1057512.
- Dini C, Islan GA, de Urraza PJ, Castro GR. Novel biopolymer matrices for microencapsulation of phages: enhanced protection against acidity and protease activity. Macromol Biosci. 2012 Sep;12(9):1200-8. doi: 10.1002/mabi.201200109. Epub 2012 Jul 30. PMID: 22847825.
- Dubos RJ, Straus JH, Pierce C. THE MULTIPLICATION OF BACTERIOPHAGE IN VIVO AND ITS PROTECTIVE EFFECT AGAINST AN EXPERIMENTAL INFECTION WITH SHIGELLA DYSENTERIAE. J Exp Med. 1943 Sep 1;78(3):161-8. doi: 10.1084/jem.78.3.161. PMID: 19871319; PMCID: PMC2135327.
- Düzgüneş N, Sessevmez M, Yildirim M. Bacteriophage Therapy of Bacterial Infections: The Rediscovered Frontier. Pharmaceuticals (Basel). 2021 Jan 5;14(1):34. doi: 10.3390/ph14010034. PMID: 33466546; PMCID: PMC7824886.
- Echeverría-Vega A, Morales-Vicencio P, Saez-Saavedra C, Gordillo-Fuenzalida F, Araya R. A rapid and simple protocol for the isolation of bacteriophages from coastal organisms. MethodsX. 2019 Nov 7;6:2614-2619. doi: 10.1016/j.mex.2019.11.003. PMID: 31763194; PMCID: PMC6864167.
- Gao M, Wang C, Qiang X, Liu H, Li P, Pei G, Zhang X, Mi Z, Huang Y, Tong Y, Bai C. Isolation and Characterization of a Novel Bacteriophage Infecting Carbapenem-Resistant Klebsiella pneumoniae. Curr Microbiol. 2020 May;77(5):722-729. doi: 10.1007/s00284-019-01849-8. Epub 2020 Jan 7. PMID: 31912220.
- García R, Latz S, Romero J, Higuera G, García K, Bastías R. Bacteriophage Production Models: An Overview. Front Microbiol. 2019 Jun 4;10:1187. doi: 10.3389/fmicb.2019.01187. PMID: 31214139; PMCID: PMC6558064.
- Gill JJ, Hyman P. Phage choice, isolation, and preparation for phage therapy. Curr Pharm Biotechnol. 2010 Jan;11(1):2-14. doi: 10.2174/138920110790725311. PMID: 20214604.
- González-Menéndez E, Fernández L, Gutiérrez D, Rodríguez A, Martínez B, García P. Comparative analysis of different preservation techniques for the storage of Staphylococcus phages aimed for the industrial development of phage-based antimicrobial products. PLoS One. 2018 Oct 11;13(10):e0205728. doi: 10.1371/journal.pone.0205728. PMID: 30308048; PMCID: PMC6181408.
- Hatfull GF. The secret lives of mycobacteriophages. Adv Virus Res. 2012;82:179-288. doi: 10.1016/B978-0-12-394621-8.00015-7. PMID: 22420855.
- Jurač K, Nabergoj D, Podgornik A. Bacteriophage production processes. Appl Microbiol Biotechnol. 2019 Jan;103(2):685-694. doi: 10.1007/s00253-018-9527-y. Epub 2018 Nov 24. PMID: 30474729.
- Karthik K, Muneeswaran N, Manjunathachar H, Gopi M, Elamurugan A, Kalaiyarasu S .Bacteriophages: Effective Alternative to Antibiotics. Advances in Animal and Veterinary Sciences 2014;2 (3):1 -7
- Li M, Guo M, Chen L, Zhu C, Xiao Y, Li P, Guo H, Chen L, Zhang W, Du H. Isolation and Characterization of Novel Lytic Bacteriophages Infecting Epidemic Carbapenem-Resistant Klebsiella pneumoniae Strains. Front Microbiol. 2020 Jul 21;11:1554. doi: 10.3389/fmicb.2020.01554. PMID: 32793133; PMCID: PMC7385232.
- Luong T, Salabarria AC, Edwards RA, Roach DR. Standardized bacteriophage purification for personalized phage therapy. Nat Protoc. 2020 Sep;15(9):2867-2890. doi: 10.1038/s41596-020-0346-0. Epub 2020 Jul 24. PMID: 32709990.
- Mancuso F, Shi J, Malik DJ. High Throughput Manufacturing of Bacteriophages Using Continuous Stirred Tank Bioreactors Connected in Series to Ensure Optimum Host Bacteria Physiology for Phage Production. Viruses. 2018 Oct 1;10(10):537. doi: 10.3390/v10100537. PMID: 30275405; PMCID: PMC6213498.
- Manohar P, Ramesh N. Improved lyophilization conditions for long-term storage of bacteriophages. Sci Rep. 2019 Oct 23;9(1):15242. doi: 10.1038/s41598-019-51742-4. PMID: 31645642; PMCID: PMC6811570.
- Mattila S, Ruotsalainen P, Jalasvuori M. On-Demand Isolation of Bacteriophages Against Drug-Resistant Bacteria for Personalized Phage Therapy. Front Microbiol. 2015 Nov 13;6:1271. doi: 10.3389/fmicb.2015.01271. PMID: 26617601; PMCID: PMC4643220.
- Merabishvili M, Pirnay JP, Verbeken G, Chanishvili N, Tediashvili M, Lashkhi N, Glonti T, Krylov V, Mast J, Van Parys L, Lavigne R, Volckaert G, Mattheus W, Verween G, De Corte P, Rose T, Jennes S, Zizi M, De Vos D, Vaneechoutte M. Quality-controlled small-scale production of a well-defined bacteriophage cocktail for use in human clinical trials. PLoS One. 2009;4(3):e4944. doi: 10.1371/journal.pone.0004944. Epub 2009 Mar 20. PMID: 19300511; PMCID: PMC2654153.
- Merabishvili M, Pirnay J, Vogele K, Malik D. Production of phage therapeutics and formulations: Innovative approaches. In Phage Therapy: A Practical Approach; Górski A, Mi˛edzybrodzki R, Borysowski J, Eds: Springer: Cham, Switzerland 2019; 3–41.
- Khan Mirzaei M, Nilsson AS. Isolation of phages for phage therapy: a comparison of spot tests and efficiency of plating analyses for determination of host range and efficacy. PLoS One. 2015 Mar 11;10(3):e0118557. doi: 10.1371/journal.pone.0118557. Erratum in: PLoS One. 2015;10(5):e0127606. PMID: 25761060; PMCID: PMC4356574.
- Mutti M, Corsini L. Robust Approaches for the Production of Active Ingredient and Drug Product for Human Phage Therapy. Front Microbiol. 2019 Oct 8;10:2289. doi: 10.3389/fmicb.2019.02289. PMID: 31649636; PMCID: PMC6791927.
- Jafari N, Abediankenari S. Phage Particles as Vaccine Delivery Vehicles: Concepts, Applications and Prospects. Asian Pac J Cancer Prev. 2015;16(18):8019-29. doi: 10.7314/apjcp.2015.16.18.8019. PMID: 26745034.
- Adesanya O, Oduselu T, Akin-Ajani O, Adewumi OM, Ademowo OG. An exegesis of bacteriophage therapy: An emerging player in the fight against anti-microbial resistance. AIMS Microbiol. 2020 Jul 22;6(3):204-230. doi: 10.3934/microbiol.2020014. PMID: 33134741; PMCID: PMC7595837.
- Paczesny J, Bielec K. Application of Bacteriophages in Nanotechnology. Nanomaterials (Basel). 2020 Sep 29;10(10):1944. doi: 10.3390/nano10101944. PMID: 33003494; PMCID: PMC7601235.
- Pallavali RR, Degati VL, Lomada D, Reddy MC, Durbaka VRP. Isolation and in vitro evaluation of bacteriophages against MDR-bacterial isolates from septic wound infections. PLoS One. 2017 Jul 18;12(7):e0179245. doi: 10.1371/journal.pone.0179245. PMID: 28719657; PMCID: PMC5515400.
- Pirnay JP, Blasdel BG, Bretaudeau L, Buckling A, Chanishvili N, Clark JR, Corte-Real S, Debarbieux L, Dublanchet A, De Vos D, Gabard J, Garcia M, Goderdzishvili M, Górski A, Hardcastle J, Huys I, Kutter E, Lavigne R, Merabishvili M, Olchawa E, Parikka KJ, Patey O, Pouilot F, Resch G, Rohde C, Scheres J, Skurnik M, Vaneechoutte M, Van Parys L, Verbeken G, Zizi M, Van den Eede G. Quality and safety requirements for sustainable phage therapy products. Pharm Res. 2015 Jul;32(7):2173-9. doi: 10.1007/s11095-014-1617-7. Epub 2015 Jan 14. PMID: 25585954; PMCID: PMC4452253.
- Regulski K, Champion-Arnaud P, Gabard J . Bacteriophage manufacturing: From early twentieth-century processes to current GMP. In Bacteriophages; Harper DR, Abedon ST, Burrowes BJ, McConville ML, Eds; Springer: Cham, Switzerland 2018;1–31.
- SCHMIDT JM, STANIER RY. ISOLATION AND CHARACTERIZATION OF BACTERIOPHAGES ACTIVE AGAINST STALKED BACTERIA. J Gen Microbiol. 1965 Apr;39:95-107. doi: 10.1099/00221287-39-1-95. PMID: 14328417.
- Tylenda CA, Calvert C, Kolenbrander PE, Tylenda A. Isolation of Actinomyces bacteriophage from human dental plaque. Infect Immun. 1985 Jul;49(1):1-6. doi: 10.1128/iai.49.1.1-6.1985. PMID: 4008044; PMCID: PMC262048.
- Van Twest R, Kropinski AM. Bacteriophage enrichment from water and soil. Methods Mol Biol. 2009;501:15-21. doi: 10.1007/978-1-60327-164-6_2. PMID: 19066806.
- Yang M, Liang Y, Huang S, Zhang J, Wang J, Chen H, Ye Y, Gao X, Wu Q, Tan Z. Isolation and Characterization of the Novel Phages vB_VpS_BA3 and vB_VpS_CA8 for Lysing Vibrio parahaemolyticus. Front Microbiol. 2020 Feb 21;11:259. doi: 10.3389/fmicb.2020.00259. PMID: 32153543; PMCID: PMC7047879.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
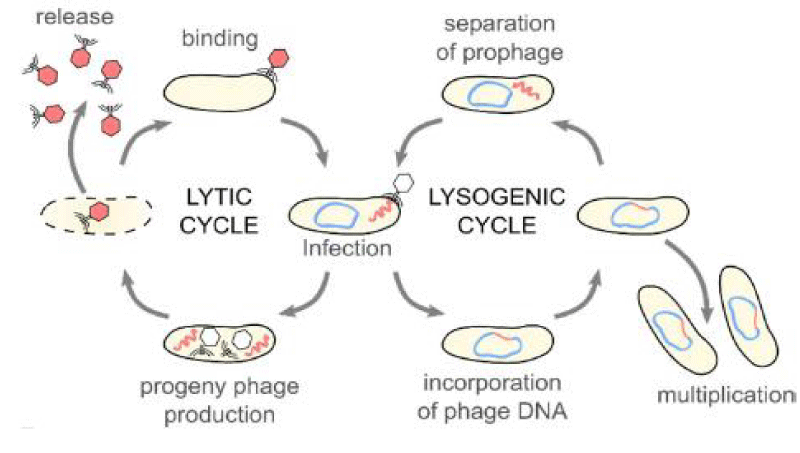
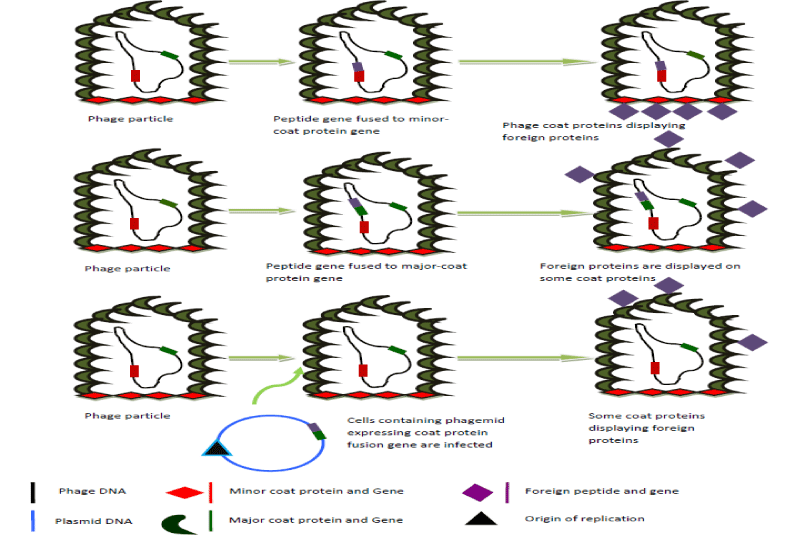
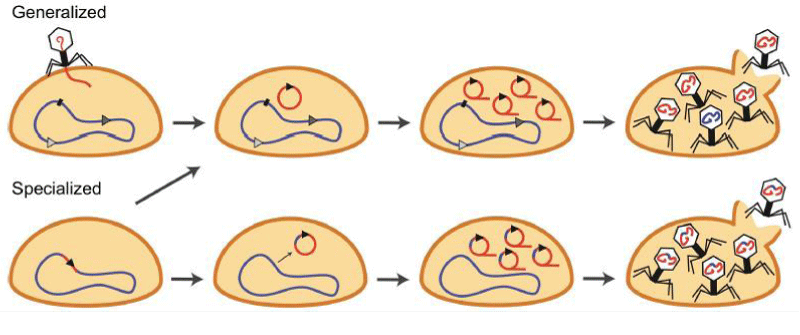


 Save to Mendeley
Save to Mendeley
