International Journal of Veterinary Science and Research
Tuberculosis in goats in Benoue area of North Cameroon: Prevalence, diagnostic performance of intradermal tuberculin skin test and zoonotic risk factors
Julius Awah-Ndukum11,2*, Emmanuel Assana3, Victor Ngu-Ngwa3, Achille Olivier Tchedele3, Mohamed Moctar Mouliom Mouiche3, Jean-Pierre Kilekoung Mingoas3 and André Pagnah Zoli3
2College of Technology, University of Bamenda, Bambili, Cameroon
3School of Veterinary Medicine and Sciences, University of Ngaoundéré, P.O. Box 454, Ngaoundéré, Cameroon
Cite this as
Awah-Ndukum J, Assana E, Ngu-Ngwa V, Tchedele AO, Mouliom Mouiche MM, et al. (2021) Tuberculosis in goats in Benoue area of North Cameroon: Prevalence, diagnostic performance of intradermal tuberculin skin test and zoonotic risk factors. Int J Vet Sci Res 7(2): 095-107. DOI: 10.17352/ijvsr.000087Copyright
© 2021 Awah-Ndukum J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Tuberculosis (TB) due to Mycobacterium bovis is a wasting disease of animals with severe public health significance. Though widely diagnosed in cattle and the performance of Tuberculin Skin Test (TST) at different cut-off points compared in various environmental conditions, there is dearth of information with respect to TB in goats in Cameroon. This study estimated the prevalence of bovine TB in goats in Benuoe area of Cameroon, based on the performance of TST against detection of tuberculous-like lesions and acid-fast bacilli as gold tests. The study detected goat TB based on tuberculous-like lesions (27.87%), acid-fast bacilli (3.29%); and bovine TB positive reactions (12.28%, 95%CI: 9.19–15.95), (8.95%, 95%CI: 6.31–12.23) and (5.37%, 95%CI: 3.36–8.09) at Single intradermal cervical tuberculin (SICT) ≥2.5mm, ≥3mm, and ≥4mm and (2.30%, 95%CI: 1.06–4.32), (1.79%, 95%CI: 0.72–3.65) and (1.02%, 95%CI: 0.28– 2.60) at Single intradermal comparative cervical tuberculin (SICCT) ≥2mm, ≥3mm, and ≥4mm cut-off points, respectively. SICT and SICCT sensitivity (11.76%) against detection of tuberculous-like lesions was significantly lower [p< 0.05] with slight agreements [Kappa=0.161] compared to sensitivity (100%) and perfect agreements [Kappa=1.00] against detection of acid-fast bacilli at these cut-offs. The Bayesian model revealed a goat TB prevalence of 18.41% (95%CI: 11.73–27.00) using SICT and 4.28 (95%CI: 1.26–8.60) using SICCT with the performance characteristic being higher for SICT than SICCT at ≥2mm cut-off. However, two-graph ROC (TG-ROC) analysis revealed that the optimal goat TB diagnosis with SICCT was at ≥2mm cut-off point. Many goat handlers were aware of health hazards of zoonotic TB but ignorant about goat TB and its possible zoonotic transmission to humans. The study reports the first comparative tuberculin skin test of goats in Benoue area of North-Cameroon and confirmed that zoonotic TB is a neglected health and production problem of goats in Cameroon that needs further investigated.
Introduction
Mycobacterium bovis is the causative agent of Tuberculosis (TB) in a wide range of hosts among domestic animals (such as bovine, caprine, ovine), wild animals (such as buffalos) and humans [1]. Mycobacterium tuberculosis has also been isolated from livestock including cattle and goats [2,3]. Endemic zoonotic TB is neglected worldwide and under-investigated in sub-Saharan Africa including Cameroon [4-6]. However, in areas where bovine TB is endemic and not controlled or partially controlled, humans become infected following close contact with infected animals, consumption of contaminated raw meat and unpasteurized dairy products from infected animals, through skin penetration when handling infected carcasses and organs [7,8] and by inhaling cough spray from infected cattle [9-14]. M. bovis in human has been reported in the West and Northwest Regions of Cameroon [15,16].
Though animal TB has severe public health significance and is neglected in Cameroon, widespread TB in cattle (prevalence range 4.67% – 24.98%) has been diagnosed in the country following comparative cervical tuberculin test, detection of tuberculous-like lesions during abattoir slaughter meat inspection, Acid fast staining of bacilli and molecular analysis [4,17,18], no information with respect to other animals are available. Mixed herding of cattle and other livestock particularly sheep and goats are widespread [19] with potentially infected cattle, sheep and goats also living in close proximity with humans [4,20] in pastoral communities in Cameroon. The inadequacies of control measures and poor understanding of the epidemiology of TB in other livestock poses additional risks for humans particularly caretakers of livestock in the country. Keeping other livestock in close contact with cattle could increase the risk of positive tuberculin reactions in cattle [11,21] and Tuberculin Skin Test (TST) positive cattle have been considered and treated as “open” cases of TB and potential transmission sources of the infection to other animals and humans [22]. Therefore, animals and communities in areas where TB is endemic in cattle are at risk of infection with M. bovis. A strong association between typical and atypical mycobacterial prevalence in cattle in Cameroon, which share the same environment as other livestock, has been described earlier [4,19,23, 24] suggesting high risks of exposure and transmission of multiple mycobacteria infections.
Intradermal cervical Tuberculin Skin Test (TST) is the international choice method for field diagnosis of bovine TB in live animals [25,26] and the World Organisation for Animal Health (OIE) recommended difference between the increase in skin thickness for the test to be positive should be at least 4mm after 72 hours [26]. However, the performance of TST is affected by environmental and host factors; and the nature of the tuberculin used [27-32]. A perfect cut-off point in a specific geographic area or country may not be useful in another environment or another country [27,30,33] and the ability of the test to accurately predict true positive disease status depends on its sensitivity, specificity and prevalence of the disease in the population tested [27]. Severe interpretations of TST are used in bovine TB endemic parts of Africa based on reference performance, determination of appropriate localised cut-off values and discretion of the veterinarian for significant reduction of the disease [19,21,28,30,34-36].
In most African regions, large groups of goats and sheep frequently share the same microenvironments (watering points, pasture, markets and housing) with infected cattle [2-4,17,19,25,37-39]. Though tuberculosis due to M. bovis, M. caprae and M. tuberculosis [3,40-46] and tuberculosis-like lesions [47] in small ruminants have been described elsewhere, there is dearth of information with respect to TB in goats in Cameroon. A comprehensive research of the molecular epidemiology, risk factors, reservoir and maintenance hosts’ status and public implications of zoonotic TB in livestock including goats in Cameroon cannot be overemphasized. Accurate diagnosis of TB in goats, at different stages of the disease and under various environmental conditions, is necessary to provide useful epidemiological information and facilitate the development of effective strategies against zoonotic TB in livestock and humans.
The performance of TST at different cut-off points against detection of tuberculous-like lesions and acid fast bacilli as gold tests for maximum diagnosis of TB has been investigated and compared in livestock in various environmental conditions [19,24,27,48-52]. In this context, this study was carried out to estimate the prevalence and assess the diagnostic performance of TST in the diagnosis of bovine TB in goats in Benuoe area of Cameroon.
Materials and methods
Study area and animal population
Goats from local livestock markets (originating principally from Benuoe Administrative Division and neighbouring localities) destined for slaughter at the Garoua Municipal abattoir and goats at the level of farms in Benoue and its environs (8°50’ – 10° N and 13° – 14°50’ E) of North Region Cameroon were sampled for the study (Figure 1). The choice of the study areas was following reports of suspected tuberculous lesions in slaughtered livestock in abattoirs in the regions and the presence of communities with strong cultures of livestock rearing. The ethnic groups in the study area are mostly agro-pastoralists with passionate traditions for livestock rearing. Also, it was common to find mix-livestock husbandry and several domestic species (cattle, sheep, goats, horses, donkeys and fowls) cohabiting within the same farm or being present in the same microenvironment (such as watering points, mineral licks, vaccination posts).
An estimated default prevalence of 50% was used to estimate the number of goats required to detect at least one TST positive reactor with a desired 95% confidence and precision of ≥ 10% as previously described [53].
The selection of goats destined for slaughter in the study was based on haphazard arrival of animals at the abattoir and on random-number generation method of goat owners from the daily abattoir records. However, the goats used for the TST performance study were judged fit for slaughtered and were not slaughtered until at least 72 hours stay at the abattoir.
The selection of goat herds was done by the random-number generation method of goat keeping communities, goat owners and locations of goat farms from records at the Divisional Delegations of the Ministry of Livestock, Fishery and Animal Industries (MINEPIA). Herd sizes ranged from 5 to 50 goats and 50% to 30% of animals at least six months old within the chosen goat farms were sampled depending on herd size, respectively. Goats on free ranch (scavenging) that could not be humanely captured and restrained were excluded for the study.
Overall, 391 goats were included in the study (330 from goat farms and 61 from among those to be destined for slaughter). Information relating to location, husbandry practices, breed, physiological status, sex, age and origin of the animals as provided by the handlers were recorded at TST. The breeds were determined by phenotypic description and were composed of indigenous breeds namely Sahel, Kirdi and Djallonké [54-58]. The age was estimated by examining the incisors [59]. The Body Condition Score (BSC) was estimated on a scale of 1 to 5 [60] and classified as lean (1 to 2), moderate (3 to 4) and fat (5) [61]. The goats in this study were reared traditionally with or without shelter such as free ranch (scavenging) or extensive systems and semi-intensive that depend on natural pasture.
Detection of tuberculosis in goats
The goats sampled in farms were subjected to TST only, while those sampled from among those destined for slaughter at the Garoua Municipal slaughter house were subjected to TST, intensive post-mortem inspection to detect tuberculous-like lesions as well as laboratory analysis (Ziehl-Neelsen stain) of samples following standard procedures. TST in this study was composed the single intradermal cervical tuberculin skin test (SICT) and single intradermal comparative cervical tuberculin skin test (SICCT). SICT and SICCT were performed on 391 (330 goat in farms and 61 goats that were slaughtered at least 72 hours days later) based on previously described standard procedures [26]. Following slaughter, intensive meat inspections were carried out by AOT assisted by the veterinary staff of the abattoir based on the government’s legislation regulating veterinary health inspection and notification of contagious animal diseases [54]. Evidence of pathologies was also supported by post mortem examination of carcasses as earlier described [61-64]. Briefly, the inspection procedure employed visual examination, palpation, incision and careful examination of organs (lungs, liver, spleen, kidneys and udder), lymph nodes of the thoracic and head regions, the mesenteric lymph nodes, and other lymph nodes of the body and various other parts / organs of the carcass. Tissues specimens, with or without tuberculous-like lesions, were collected into sterile plastic containers from each of the slaughtered goats and stored at −20oC for up to two months before analysis.
Grinding of the samples [65] and direct smear microscopy with Ziehl-Neelsen (ZN) staining for confirmation of acid-fast tubercle bacilli were done following standard procedures [26,66,67].
Risk factor analysis
Information on risk factors for TB in goats was obtained by examination of goat environment and questionnaire interview of goat handlers (goat farmers and butchers) in the Benoue area. The questionnaires were semi-structured to collect information on a range of variables including animal management and husbandry practices, lifestyle and demographic information, interactions with the animals; level of knowledge of zoonotic TB and level of the respondent exposure to zoonotic TB.
Ethical consideration
The researchers to avoid hazards to all persons and animals involved in the project performed risk assessments of the project. Ethical clearances were obtained from the required authorities before carrying out the study. The willing goat farmers and butchers who participated in the survey were often disorderly, illiterate and from various cultural communities in Benoue area with distinct vernaculars. Considerable time and patience were needed to obtain their maximum cooperation. The purpose of the study was explained to the targeted participants usually with the assistance of resident veterinarians; and where necessary trusted and knowledgeable intermediaries were engaged. An animal was tested after the owner gave an informed consent. Apart from minor intradermal injections of avian and bovine tuberculins and procedural restraining manipulations for safety purposes, the animals were not subjected to suffering. Slaughtering and dressing of goat carcasses were done as described by the Cameroon veterinary services [54]. All laboratory analyses including ZN staining were carried out in a laboratory equipped with a category II Biosafety cabinet.
Data analysis
The obtained data was entered into Microsoft Excel and then transferred to SPSS 20 software. Frequency distributions of bovine TB in goats were generated for the different diagnostic techniques and TST cutoff points. The Chi-square test was used to evaluate the sensitivity of TST and assess various associations. In addition, the ROC (Receiving Operating Characteristic) and TG-ROC (Two-Graph Receiver Operating Characteristics) analysis was used to evaluate diagnostic characteristics of TST at different cutoff points [53].
A Bayesian ROC model was developed to compute the prevalence of tuberculosis in goats and the sensitivity and the specificity of different tests and run with WinBugs [68]. The Bayesian ROC approach was based on a multinomial model using a Monte Carlo Markov Chain simulation [69]. The model allows the simultaneous estimation of prevalence and test characteristics, combining the prior knowledge values to obtain the posterior distribution for each of the parameters (Supplementary materials). Briefly the results from SICT, SICCT, tuberculous-like lesions and acid fast bacilli over a range of cut-off points were combined together in the Bayesian model and run in Wingbugs 1.4. The prior information on the characteristics of TST was obtained from previous studies on diagnosis of tuberculosis in goats [70]. The validation of the Bayesian model was based on the number of parameters (PD), the Deviance Information Criterion (DIC) values from posterior mean of the multinomial probabilities (DIC_Pr) and from posterior mean of the parameter of the model using parent nodes (DIC_P) and on the Bayesian-p values (Bayesp) as described by Berkvens, et al. [71]. The validation of the Bayesian model was run in Wingbugs 1.4 and R i.386 4 (Supplementary materials).
Results
Prevalence of tuberculosis in goats
SICCT done on 391goats showed 9 (2.30%, 95% CI : 1.06 – 4.32), 7 (1.79%, 95% CI: 0.72 – 3.65) and 4 (1.02%, 95% CI : 0.28 – 2.60) bovine TB positive reactors at ≥ 2 mm, ≥ 3 mm, and ≥ 4 mm cut-off points, respectively (Table 1). While SICT showed 48 (12.28%, 95% CI : 9.19 – 15.95), 35 (8.95 %, 95% CI: 6.31 – 12.23) and 21 (5.37%, 95% CI : 3.36 – 8.09) bovine positive reactors at ≥ 2.5 mm, ≥ 3 mm, and ≥ 4 mm cut-off points, respectively.
Irrespective of the cut-off point no SICCT bovine TB positive reaction was recorded among thin goats and goats that share the same environment with other livestock. Also, endogenous (breed, sex and age) and exogenous (mix-husbandry and sharing the same microenvironments with other livestock including cattle, poultry, sheep and pigs) factors did not (p>0.05) influence the TST results of goats in the present study.
Overall, 17 (27.87%, 95% CI: 17.15 – 40.83) of 61 tested and slaughtered goats at the Garoua abattoir presented tuberculous lesions at post mortem examination. The tuberculous lesions were detected in male (15) and female (2) animals aged ≤ 2 years (14); 2
However, Avian TB Single Intradermal Comparative Cervical Tuberculin (ATB-SICCT) skin results test on the 391 goats showed 39 (9.97%, 95% CI : 7.19 – 13.38), 23 (5.88%, 95% CI: 3.77 – 8.70) and 10 (2.56%, 95% CI : 1.23 – 4.65) positive reactors at ≥ 2 mm, ≥ 3 mm, and ≥ 4 mm cut-off points, respectively. While Avian TB Single intradermal cervical tuberculin (ATB-SICT) skin test showed 80 (20.46%, 95% CI : 16.57 – 24.80), 56 (14.32%, 95% CI: 11.00 – 18.19) and 35 (8.95 %, 95% CI : 6.31 – 12.23) positive reactors at ≥ 2.5 mm, ≥ 3 mm, and ≥ 4 mm cut-off points, respectively. No ATB-SICCT positive reaction was recorded among thin goats and goats that were kept separately from other livestock irrespective of the cut-off point. However, endogenous (breed, sex and age) and exogenous (mix-husbandry and sharing the same microenvironments with other livestock including cattle, poultry, sheep and pigs) factors did not (p>0.05) influence the Avian TB TST results of goats in the present study.
Diagnostic performance of tuberculin skin test to detect bovine tuberculosis in goats
The performances of SICCT technique at various cut-off points to diagnose bovine TB in goat in Benoue area of North Cameroon was based on detection of tuberculous-like lesions and acid fast bacilli as references for defining the disease status revealed that among the tested and slaughtered goats in the study. Tuberculous-like lesions and acid-fast bacilli were detected in 2SICCT bovine TB positive animals at all the cut-off points used in this study (≥ 2mm, ≥ 3 mm and ≥ 4 mm cut-off points). For SICT bovine TB positive reactors, acid-fast bacilli were detected in 2 out of 7, 6 and 3 animals with tuberculous-like lesions at the ≥ 2 mm, ≥ 3 mm, and ≥ 4 mm cut-off points, respectively.
Based on computed sensitivity, specificity and kappa values of SICCT compared to detection of tuberculous-like lesions and acid-fast bacilli, severe interpretations of SICCT tests detected more bovine TB diseases cases. The SICCT bovine TB sensitivity values against detection of tuberculous-like lesions (11.76% (0.00 –27.08)) was significantly lower [P<0.05] against detection of acid fast bacilli (100%) to define disease status at the cut-off points used in this study (≥ 2 mm, ≥ 3 mm and ≥ 4 mm). While 100% SICCT bovine TB specificity was obtained against detection of tuberculous-like lesions and detection of acid fast bacilli to define disease status at all cut-off points used in the study (≥ 2 mm, ≥ 3 mm and ≥ 4 mm). In addition, slight agreements [Kappa = 0.161] were recorded for SICCT bovine TB interpretations for the cutoff points with detection of tuberculous-like lesions compared to perfect agreements [Kappa = 1.00] with detection of acid fast bacilli to define disease status. It is worth mentioning that overall, the performance of all SICCT bovine TB cut-off points against detection of tuberculous-like lesions and acid fast bacilli to define disease status revealed positive predictive values of 100% and negative predictive values of 74.58% (95% CI: 63.47 – 85.69). Interpretations of SICT bovine TB cutoff points detected more reactors than with SICCT bovine TB cutoff points. However, the computed sensitivity, specificity and kappa values of SICT bovine TB according to detection of tuberculous-like lesions [(0.00 – 64.57%), (73.28 – 100) and (≤0.37)] and detection of acid fast bacilli (100), (69.39 – 100) and (≤0.550), respectively, revealed fair to moderate performance of SICT bovine TB cutoff points in goats.
ATB-SICCT positive animals did not show tuberculous-like lesions and Acid-fast bacilli on further examination. However, 7 (≥ 2.5 mm cut-off) and 2 (≥ 3 mm cut-off) tuberculous-like lesions and no Acid-fast bacillus were detected in ATB-SICT positive animals.
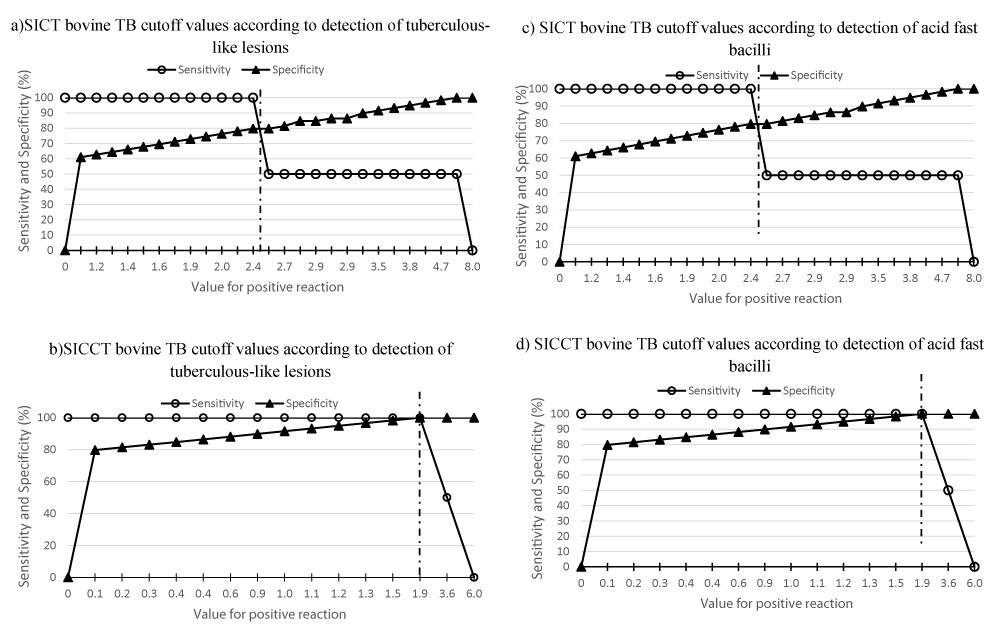
ROC (Receiving Operating Characteristic) analysis showed that the area under the curve according to detection of tuberculous-like lesions and acid fast bacilli approximated to 1.00 for all SICCT bovine TB cut off points, indicating that these cut off values are very informative for the detection of bovine TB in goats. However, the area under the ROC curves according to detection of tuberculous-like lesions and detection of acid fast bacilli for all SICT bovine TB cutoff points were between 0.74 – 1.0 suggesting that these cut off values are only fairly informative for the detection of bovine TB in goats. Further performance analysis conducted with two-graph ROC (TG-ROC) to visualize sensitivity and specificity curves according to ranges of SICT bovine TB and SICCT bovine TB cutoff values according to detection of tuberculous-like lesions and detection of acid fast bacilli to define disease status is shown in figure 1. Based on the detection of tuberculous-like lesions and acid fast bacilli to define disease status, the TG-ROC findings confirmed severe interpretations of TST for bovine TB detection in goats [particularly at cutoff points of ≥ 2 mm for SICCT and at ≥ 2.5 mm for SICT] as for ROC, Kappa, sensitivity and specificity evaluations.
A Bayesian ROC approach using the conditional dependence between SICT bovine TB or SICCT bovine TB and tuberculous-like lesions and acid fast bacilli techniques, revealed a SICT bovine TB true prevalence of 18.41% (18 95%CI: 11.73 – 27.00) and SICCT bovine TB true prevalence of 4.28% (95% CI: 1.26 – 8.60) with the performance of SICT (sensitivities and specificities) being higher than that of the SICCT at ≥ 2mm cut-point (Tables 2). The validation criteria of the Bayesian ROC model areindicated in Table 3, Figure 2.
Awareness of goat handlers of the risk of zoonotic tuberculosis in goat
All goat handlers (43) participated in the present study responded to the questionnaire including goat breeders and traders (32) and slaughtering butchers (11). Overall, the respondents were predominantly males (97.67%), over 25 years old (79.07%), had at least a primary educational status (82.86%) and kept goats for income generation (100%). The level of awareness and potential risk factors associated with exposure of goat handlers to zoonotic TB from goats are summarized in Figure 3.
Although many goat handlers were aware of Zoonotic TB (55.81%) and its occupational hazards (67.44%), most were ignorant of TB in goats (74.42%) and the modes of transmission (79.07%) of Zoonotic TB in animals (including goats (86.05%)) to humans. Most respondents (88.37%) had not been tested for TB nor vaccinated against TB (as recommended by the country’s regulations) and were not aware of farm biosecurity measures (53.13%). The respondents, apart from goats, had regular contacts with other animals (83.72%) as well as encountered goat TB (< 20%) and human TB (< 42%), willingly consumed fresh milk pasteurised and unpasteurised milk (97.67%) and often allowed suspicious goat meats with tuberculous-like lesions for eventual human consumption (>10%).
Discussion
The detection rates of macroscopic tuberculous-like lesions (27.87%, 95% CI: 17.15 – 40.83)in goats in this study are much higher than values which ranged from < 1 to 4.25% reported for cattle in the Littoral and Western highland regions of Cameroon [19,20] but similar to 22.28% in cattle in Maroua area [24] and 23.75% in cattle in Garoua – Benoue [23,72]. However, the prevalence based on detection of tuberculous-like lesions in this study is higher than the values reported in goats in Bauchi abattoir (0.03%) in by detection of tuberculous-like lesions [47] and Bodija abattoir (0.3%) using deletion typing technique [3] in Nigeria, Mdjo abattoir in Ethiopia (4.2%) [25] and slaughter houses in Northern Algeria (6.03%) [39] by detection of tuberculous-like lesions. Goat TB has is distributed in different regions of Ethiopia and incidence rates ranging from 0 to 6.5% have been reported in [25]. The differences in the prevalence rates could be attributed to the duration of the investigation with shorter periods having limited effect on the amount of data to be obtained in determining the true prevalence of the disease; use of more sensitive diagnostic technique (deletion typing) giving more reactors especially in bovine TB endemic areas; difference in exposure to TB-infected animals and occurrence of animal TB in the geographical locations [45,47]. Absence of bovine TB positive goats in endemic agropastroal communities have been attributed to restriction of grazing of the flocks within the communities, housing of goats separately from other livestock at night and separate herding of goats [46].
Post mortem examination of tuberculous-like lesions and demonstration of acid fast bacilli by direct microscopy were used in this study to define disease status of bovine TB in goats, to evaluate the performance of TST as opposed to bacteriological culture that was used elsewhere as reference diagnostic test [26]. However, macroscopic examination of tuberculous-like lesions and demonstration of acid fast bacilli have also been used in cattle by Ameni, et al. [28] in Ethiopia, Ngandolo, et al. [34] in Tchad and Awah-Ndukum, et al. [24]to evaluate the diagnostic performances of TST. In this study optimal detection of bovine TB in goats was obtained at severe interpretations of SICCT bovine TB and SICT bovine TB, particularly at ≥ 2 mm and ≥ 2.5 mm, respectively. However, significantly lower SICCT bovine TB prevalence estimates based on TST at cut off points ≥ 4 mm (1.02%), ≥ 3 mm (1.79%) and ≥ 2 mm (2.30%) were obtained in goats in Benoue area compared to 3.59% – 7.48%, 8.92% – 13.25% and 11.77% – 17.26% recorded in cattle by Awah-Ndukum, et al. [19] in the highland regions. The results of this study are similar to those of Kassa, et al. [61] who reported a SICCT bovine TB prevalence of 0.5% at> 4 mm and 3.8% at ≥ 2 mm in small ruminants in pastoral Afar region Ethiopia.No SICCT bovine TB positive reactor was reported in goats kept in communities with camel and cattle in Eritrea [46] and Ethiopia [41, 42]. However, the SICTbovine TB prevalence obtained in this study (12.28%, 8.95 % and 5.37% at ≥ 2.5 mm, ≥ 3 mm, and ≥ 4 mm cut-off points, respectively) are higher than 3.1% reported by Tafesse, et al. [73] and Ketema, et al .[43] and 1.46% by Silvano, et al. [74] and 0.5% by Mamo, et al. [44] in goat in Ethiopia; and significantly lower than 24.92% in sheep in Spain [45] using SICCT (≥ 4 mm). Though SICCT bovine TB prevalence estimates in the study were not influenced by endogenous (body condition score, breed, sex and age) and exogenous (mix-husbandry and sharing the same microenvironments with other livestock including cattle, poultry, sheep and pigs) factors of goats in the present study, the findings suggest that bovine TB is endemic in goats in the Benoue area of North Cameroon. The findings of the present study are similar to those of Ameni, et al. [28] who reported that improved diagnostic performances of TST in zebu cattle in Ethiopia were obtained at severe interpretations of > 2 mm cut off point. In Chad, Ngandolo, et al. [34] also stated that optimum diagnostic performance of TST in Arab zebus and Bororo zebus was > 2 mm cut off point. The present results agrees with those of Awah-Ndukum, et al. [19, 24] who observed that improved diagnosis of bovine TB in cattle at severe interpretations of TST though at ≥ 3 mm and ≥ 3.5 mm cut off points in the highlands [Adamawa and Northwest] and Maroua areas of Cameroon. The true bovine TB prevalence of 18.41% (95%CI: 11.73-27) of TB observed in the present study for the 61 slaughtered goats computed when the conditional dependence between SICT bovine TB and tuberculous-like lesions and acid fast bacilli was used in a Bayesian model was within the confidence interval of the prevalence (27.87%, 95% CI : 17.15 – 40.83) revealed by macroscopic tuberculous-like lesions on the 330 goats in farms. This finding indicates that the Bayesian model could be validated to estimate the prevalence of TB in goats in Benoué particularly as the Bayesp values were lower than 0.5. However, when using the conditional dependence between SICCT bovine TB and tuberculous-like lesions and acid fast bacilli technique was computed, thetrue prevalence was lower (4.28 %, 95% CI: 1.26 – 8.60). Therefore, interpretations of SICT cutoff points detected more bovine TB reactors than at the SICCT cutoff points.
The ROC analysis and sensitivity evaluations support interpretation at all SICCT bovine TB cutoff points but only fairly informative at SICT bovine TB cutoff points in this study. However, further performance analysis with two-graph ROC (TG-ROC) based on the detection of tuberculous-like lesions and acid fast bacilli to define disease status, also confirmed severe interpretations of TST for bovine TB detection in goats [particularly at cutoff points of ≥ 2 mm for SICCT and at ≥ 2.5 mm for SICT] as for ROC, Kappa, sensitivity and specificity evaluations. Since bovine TB in cattle is high in Benoue area [23,72,75] where mix-husbandry of goats, sheep, cattle and other livestock was observed to be common in the present study, severe interpretations of TST for the diagnosis of bovine TB in goats should be adopted in Cameroon. Awah-Ndukum, et al. [19] had proposed severe interpretations of TST for the diagnosis of bovine TB in Bos indicus cattle in Cameroon, where the prevalence of bovine TB is high and widespread. Bovine TB infection from single individual cases and flock outbreaks have been reported in other parts of the world having epidemiological links with TB-infected cattle herds and sheep have also been considered as potential source of TB [45]. Though sheep and goats are not routinely tested for TB in Cameroon and the prevalence of TB in sheep (traditionally kept in close proximity with goats, cattle and other livestock in agro-pastoral communities in Cameroon) was not determined in the present study, an epidemiological link of TB between TB infected sheep, goats and cattle is most likely in Benoue and the other endemic parts of Cameroon where mix-husbandry of these animals occur. Following continuous close contact and sharing of pastures between small ruminants with potentially infected cattle and very low prevalence of SICCT bovine TB in small ruminants [42], sheep and goats have been associated as a reservoir for a long period of time and transmitted diseases to other susceptible animals [25,45].
The performance of TST has also been affected by environmental factors, host factors, (status of immunity, genetics, etc.), prevalence of the disease in the population tested and the nature of the tuberculin used [25,27-32,45]. A perfect cut-off point in a specific geographic area may not be so useful at another environment [27,30] and the ability of the test to accurately predict the true positive disease status depends on its sensitivity, specificity and prevalence of the disease in the population tested [27]. Excessively high sensitivity of TST will generate false positive reactions during interpretations of test results. However, severe interpretations for improved diagnosis have been done in regions or herds where M. bovis infection had been confirmed based on the discretion of the veterinarian [30].
In this study, the best individual sensitivity [11.76% at ≥ 2 mm, ≥ 3 mm and ≥ 4 mm cut off points] of TST, with detection of tuberculous-like lesions as the reference test, recorded is lower than the median individual sensitivity [80% (52.0–100)] stated by OIE [26] at the recommended > 4 mm cut off point [27]. Also, the best individual sensitivity (100% at ≥ 2 mm, ≥ 3 mm and ≥ 4 mm cut off points] of TST, with detection of acid fast bacilli in tuberculous-like lesions as the reference testis higher than the median individual sensitivity stated by OIE at the recommended cut off point. The OIE proposed value is a median from a very wide dispersion [52.0–100%] compared to very narrower dispersions for best overall values in the present study [(0.00 – 27.08) and 100%]. However, the SICCT bovine TB sensitivities obtained in this study are higher to the values reported in cattle by Ameni, et al. [28] in Ethiopia (68.8% at > 2 mm cut off point) and Delafosse, et al. [36] in Chad (94% at ≥ 4 mm).
Various factors can influence the sensitivity of TST and the hypersensitivity reactions can fluctuate considerably depending on the animal. Delayed hypersensitivity reactions provoked by tuberculin injection can become established 3 to 6 weeks after exposure of the host to bacilli agents while recently infected animals may not react sufficiently to tuberculin injection [76]. The reaction is reduced in young animals [calves] and pregnant females [cow] near term [77]. Anergy has been reported to cause false negative reactions during TST but the reasons are still poorly understood [78]. However, recently infected cattle, cattle under stress due to malnutrition, gastrointestinal parasitoses, other concurrent infections and cattle with generalized TB would be anergic and fail to react to TST [77,78]. This suggest that livestock including goats presenting differential SICCT bovine TB skin thickness of ≤ 4 mm should not be excluded that they are not affected by bovine TB, especially in highly endemic areas and animals sensitized to environmental mycobacteria such as in Cameroon as previous stated by Awah-Ndukum et al., [19,23,24]. These animals could actually be infected but low reacting or not reacting at all because their immune systems may not be sufficiently stimulated for a positive response to occur at the ≥ 4 mm OIE recommended cut-off point [77,78]. Also, conditions such as stress may compromised their immune function [79] and animals may be sensitized to environmental mycobacteria [80]. Furthermore, in late stages or towards the end of the course of the disease, the capacities of the infected hosts may become saturated and the expected hypersensitivity reactions may not be observed [81]. Also, 1 – 5% of some animals may be totally anergic during their entire lifespan [48,82]. These phenomena are responsible for the fluctuating sensitivities of TST according to environments and amongst animal populations.This study revealed that severe interpretation of TST, at cut off values less than the OIE recommended cut off value of > 4 mm, is essential for optimal diagnosis of bovine TB in goats in Benoue of North Cameroon. The interpretations should be done at cut-off points of ≥ 2 mm for SICCT bovine TB and ≥ 2.5 mm for SICT bovine TB given the epidemiological and environmental context of the region.
Assessing the knowledge and perception of goat handlers (breeders and traders and butchers) on TB in the present study revealed high rate of contacts and interactions between humans, goats and other livestock including cattle the potential reservoir host of zoonotic TB. Though no respondent reported consuming raw meat and few had encountered at least one TB-infected goat, many respondents hand encountered at least one human TB-infected patient and most consume raw and pasteurized milk. Most respondents agreed that they would consult the veterinary service upon suspicion of TB in goat while few reported including suspected TB-infected goats in the human food chain. Though many respondents were aware of zoonotic TB and the potential occupational health hazards of zoonotic TB, many were ignorant of TB in goats and the transmission of zoonotic TB from goats to humans such as spread by aerosol, consumption of unpasteurized milk and consumption of raw or insufficiently cooked contaminated meat from infected animals [4,27,79,83-85]. Poor knowledge of biosecurity measures and use of personal protective equipment, unwillingness to be tested for TB (as required by regulation) and lack of health education of the goats handlers in Cameroon including Benoue area about zoonoses such as bovine TB and their public health implications explained the severe lack of knowledge and poor perception observed in this study. Consumption of raw meat and unpasteurized milk has been reported in other parts of Cameroon [4,75]. Inclusion of suspected TB contaminated meat in the human food chain, non-respect of the decision of veterinary inspectors, non-declaration of bovine TB at farm level and false or poor knowledge about zoonotic TB observed in this study have been previously reported by Awah-Ndukum, et al. [4] in the country.
The lack of protective wear, non-respect of standard operating procedures at the abattoirs, unsolicited visitors including consumers of meat to the abattoir and poor sanitation of abattoir and animal farm environment were noted in the study. This finding agrees with Khattak, et al. [86] who stated that animals handlers such as abattoir workers did not use protective equipment and adopt appropriate safe working techniques; and were at high risk of acquiring zoonotic TB. The study further suggests that incubation of various pathogens could be occurring in the Garoua abattoir and livestock farms including TB agents, which are hazardous to animal handlers and consumers of slaughtered animal products. Therefore, zoonotic TB is a serious professional, occupational and accidental hazard to animal breeders and handlers, meat handlers and abattoir workers and visitors of animal structures in the study area. Suitable pre-employment screening programs for animal handlers [85], the use of protective wears, equipment and standard working procedures [62-64] and not allowing unsolicited visitors should be established at the abattoir and goat farms of Benoue of North Cameroon.
There is an operational and functional “One Health” National Strategy as well as a National Program for the prevention and control of emerging and re-emerging zoonoses in Cameroon. The “One Health” National Strategy evolved from the combined efforts of sectors of animal health, human health and environmental health working jointly in a trans-sectoral and synergic manner for the management of health security of animal and human population [87]. In 2014, the National Program for the prevention and control of emerging and re-emerging zoonoses was enacted in Cameroon. Five priority zoonotic diseases were identified from a list of relevant zoonoses for Cameroon including rabies, anthrax, highly pathogenic avian Influenza, Ebola and Marburg Virus disease, and bovine tuberculosis [88]. However, poor implementation of essential control measures of zoonoses including animal tuberculosis (e.g., restricting movement of infected animals, reporting disease to the veterinary services, testing of animals) has been reported in Cameroon [4]. Tuberculosis is an important notifiable disease worldwide and bovine tuberculosis is endemic in Cameroon. However, there is dearth of information on the epidemiological situation of tuberculosis in small ruminants which is commonly raised together with cattle in the same micro-environments in livestock producing areas of the country including the Northern regions. There are little or no concerted veterinary and medical efforts to maximize zoonotic tuberculosis detection rates. Active involvement of populations at risk and good health systems are lacking such that appropriate preventive measures and planning for effective control programs of zoonoses in animals and humans cannot be achieved [4,89]. Bovine tuberculosis is widely endemic in cattle in Cameroon [4,17,18]. However, determining the prevalence and risk factors of tuberculosis in all livestock according to their origin are essential to improve the epidemiology the disease in Cameroon. There are also concerns about tuberculosis in other farm animals such as sheep, horses, donkeys and pigs since the occurrence and epidemiology of the disease in these animals is poorly understood. Furthermore, the zoonotic potential and status of tuberculosis in human communities as well as the relation between the burden and associated risk factors of tuberculosis in livestock and livestock professionals in major livestock producing zones in the country are poorly understood.
Conclusion
The study reports the first comparative TST in goats in Benoue area of North Cameroon and confirmed that bovine TB is an existing livestock health and production problem in Cameroon. The study showed relatively high prevalence of tuberculosis-like lesions in slaughtered goats but relatively lower prevalence of bovine TST positive reactors in live goats in Benoue and its environs where ignorance about zoonotic TB is high, consumption of unpasteurized milk is common and a potential TB in goats slaughtered at Garoua municipal abattoir. Although the estimated prevalence rate was low, the risk and public health importance of potential zoonotic TB in goats should not be overlooked. Bacteriological and molecular studies would be necessary to determine the circulating strains in the Benoue area and beyond as well as establish the prevalence and risk factors of caprine tuberculosis in the country. Public awareness campaigns and health education especially among small ruminant professional and in agropastoral communities should be highlighted to disseminate knowledge, associated risk factors and control measures of tuberculosis. The need for intensification of the integrated “One Health” approach and involving sectoral policies including interdisciplinary strategies between animal and human health experts, concerned target stakeholders and affected communities about the need for detailed information on animal and human tuberculosis for effective management in the country cannot be overemphasized.
Data availability
The data used to support the findings of this study are included in the manuscript.
The authors are grateful to the butchers of Garoua abattoir, staff of MINEPIA of Benoue Division and Veterinary Research Laboratory IRAD Wakwa – Ngaoundéré for allowing the collection and analysis of samples. The authors also express their gratitude to the goat farmers used in the study for their generous cooperation.
- Neill SD, Pollock JM, Bryson DB, Hanna J (1994) Pathogenesis of Mycobacterium bovis infection in cattle. Vet Microbiol 40: 41-52. Link: https://bit.ly/3AxbFwx
- Cadmus S, Palmer S, Okker M, Dale J, Gover K, et al. (2006) Molecular Analysis of Human and Bovine Tubercle Bacilli from a Local Setting in Nigeria. Journal of Clinical Microbiology 44: 29-34. Link: https://bit.ly/3CjH2e9
- Cadmus SI, Adesokan HK, Jenkins AO, van Soolingen D (2009) Mycobacterium bovis and M. tuberculosis in goats, Nigeria. Emerg Infect Dis 15: 2066-2067.
- Awah-Ndukum J, Kudi AC, Bah GS, Bradley G, Ngu-Ngwa V, et al. (2014) Risk factors analysis and implications for public health of bovine tuberculosis in the highlands of Cameroon. Bulletin of Animal Health and Production in Africa 62: 353-376. Link: https://bit.ly/2XAZVL5
- Boukary AR, Thys E, Mamadou S, Rigouts L, Matthys F, et al. (2011) La tuberculose à Mycobacterium bovis en Afrique subsaharienne. Annales de Médecine Vétérinaire 155: 23-37.https://bit.ly/3AlSUM6
- Stop TB Partnership (2006) The Global Plan to Stop TB, 2006-2015. Actions for life: towards a world free of tuberculosis. Int J Tuberc Lung Dis 10: 240-241. Link: https://bit.ly/3kooy6t
- Kaneene JB, Miller R, De Kantor IN, Thoen CO (2010) Tuberculosis in wild animals. Int J Tuberc Lung Dis 14: 1508-1512. Link: https://bit.ly/2Xwo6tW
- Belchior I, Seabra B, Duarte R (2011) Primary inoculation skin tuberculosis by accidental needle stick. BMJ Case Reports 2011: bcr1120103496. Link: https://bit.ly/2XlveJk
- Collins CH, Grange JM (1987) Zoonotic implication of Mycobacterium bovis infection. Irish Veterinary Journal 41: 363-366.
- Cosivi O, Grange JM, Daborn CJ, Raviglione MC, Fujikura T, et al. (1998) Zoonotic Tuberculosis due to Mycobacterium bovis in Developing Countries. Emerg Infect Dis 4: 59-70. Link: https://bit.ly/3EpVmUu
- Etter E, Donado P, Jori F, Caron A, Goutard F, et al. (2006) Risk Analysis and Bovine Tuberculosis, a re-emerging Zoonosis. Ann N Y Acad Sci 1081: 61-73. Link: https://bit.ly/2YV0wHF
- Moda G, Daborn CJ, Grange JM, Cosivi O (1996) The zoonotic importance of Mycobacterium bovis. Tuber Lung Dis 77 : 103-108. Link: https://bit.ly/3AeZZ14
- Thoen C, Lobue P, de Kantor I (2006) The importance of Mycobacterium bovis as a zoonosis. Vet Microbiol 112: 339-345. Link: https://bit.ly/2YWK6P0
- Shitaye JE, Tsegaye W, Pavlik I (2007) Bovine tuberculosis infection in animal and human populations in Ethiopia: a review. Veterinarni Medicina 52: 317-332. Link: https://bit.ly/3hJ3IwO
- Niobe-Eyangoh SN, Kuaban C, Sorlin P, Cunin P, Thonnon J, et al. (2003) Genetic biodiversity of Mycobacterium tuberculosis complex strains from patients with pulmonary tuberculosis in Cameroon. J Clin Microbiol 41: 2547-2553. Link: https://bit.ly/3Er9HA1
- Nkongho F, Kelly R, Ndip L, Sander M, Ngandolo R, et al. (2014) Molecular Epidemiology of Bovine Tuberculosis in Cameroon. In: 6th International M bovis conference. Cardiff, United Kingdom.
- Awah-Ndukum J, Kudi AC, Bradley G, Smith NH, Ane-Anyangwe I, et al. (2013) VPK: Molecular genotyping of Mycobacterium bovis isolated from cattle tissues in the North West region of Cameroon. Trop Anim Health Prod 45: 829-836. Link: https://bit.ly/3hGVgOv
- Koro-Koro F, Bouba, Foko E, Ngatchou AF, Eyangoh S, et al. (2013) First insight into the current prevalence of bovine tuberculosis in cattle slaughtered in Cameroon: the case of main abattoirs of Yaoundé and Douala. British Microbiology Research Journal 3: 272-279. Link: https://bit.ly/3ErYJtR
- Awah-Ndukum J, Kudi AC, Bah GS, Bradley G, Tebug SF, et al. (2012) Bovine Tuberculosis in Cattle in the Highlands of Cameroon: Seroprevalence Estimates and Rates of Tuberculin Skin Test Reactors at Modified Cut-Offs. Vet Med Int 2012: 798502. Link: https://bit.ly/3nOLjSO
- Awah-Ndukum J, Kudi AC, Bradley G, Ane-Anyangwe IN, Fon-Tebug S, et al. (2010) Prevalence of bovine tuberculosis in abattoirs of the Littoral and Western highland regions of Cameroon: a cause for public health concern. Veterinary Medicine International. Link: https://bit.ly/3lv9BPf
- Tschopp R, Schelling E, Hattendorf J, Aseffa A, Zinsstag J (2009) Risk factors of bovine tuberculosis in cattle in rural livestock production systems of Ethiopia. Prev Vet Med 89: 205-211. Link: https://bit.ly/2VMju20
- O'Reilly LM, Daborn CJ (1995) The epidemiology of Mycobacterium bovis infections in animals and man: A review. Tuber Lung Dis 76: 1-46. Link: https://bit.ly/3lwwthk
- Awah-Ndukum J, Nkongho FE, Ngu-Ngwa V (2019) The Status of Bovine Tuberculosis in Cameroon. In: Tuberculosis in Animals: An African Perspective. edn. Edited by Asseged BD, Nicolaas PJK, Thoen CO. Switzerland AG. Gewerbestrasse 11, 6330 Cham, Switzerland: Springer Nature 283 - 304.
- Awah-Ndukum J, Temwa J, Ngwa VN, Mouiche MM, Iyawa D, et al. (2016) Interpretation criteria for comparative intradermal tuberculin test for diagnosis of bovine tuberculosis in cattle in Maroua area Cameroon. Vet Med Int 2016: 4834851. Link: https://bit.ly/3nHG5Zo
- Dibessa ZA (2020) Review on Epidemiology and Public Health Importance of Goat Tuberculosis in Ethiopia. Vet Med Int 2020: 7. Link: https://bit.ly/3zgOZ24
- OIE (2012) Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. Paris, France: World Organisation for Animal Health 7.
- de la Rua-Domenech R, Goodchild T, Vordermeier M, Clifton-Hadley R (2006) Ante mortem diagnosis of Bovine Tuberculosis: the significance of unconfirmed test reactors. Government Veterinary Journal 16: 65-71. Link: https://bit.ly/3zdcAkd
- Ameni G, Hewinson G, Aseffa A, Young D, Vordermeier M (2008) Appraisal of Interpretation Criteria for the Comparative Intradermal Tuberculin Test for Diagnosis of Tuberculosis in Cattle in Central Ethiopia. Clin Vaccine Immunol 15: 1272-1276. Link: https://bit.ly/2XAS547
- Francis J, Choi CL, Frost AJ (1973) The diagnosis of tuberculosis in cattle with special reference to bovine PPD tuberculin. Aust Vet J 49: 246-251. Link: https://bit.ly/39bfmvL
- Monaghan ML, Doherty ML, Collins JD, Kazda JF, Quinn PJ (1994) The tuberculin test. Vet Microbiol 40: 111-124. Link: https://bit.ly/3hZUUCX
- Marcotty T, Matthys F, Godfroid J, Rigouts L, Ameni G, et al. (2009) Zoonotic tuberculosis and brucellosis in Africa: neglected zoonoses or minor public-health issues? The outcomes of a multi-disciplinary workshop. Ann Trop Med Parasitol 103: 401-411. Link: https://bit.ly/3tPovmU
- Dürr S, Müller B, Alonso S, Hattendorf J, Laisse CJM, et al. (2013) Differences in Primary Sites of Infection between Zoonotic and Human Tuberculosis: Results from a Worldwide Systematic Review. PLoS Negl Trop Dis 7: e2399. Link: https://bit.ly/3tKoIIg
- Humblet MF, Gilbert M, Govaerts M, Fauville-Dufaux M, Walravens K, et al. (2010) New assessment of bovine tuberculosis risk factors in Belgium based on nationwide molecular epidemiology. Journal of Clinical Mirobiology 48: 2802-2808. Link: https://bit.ly/2XCzhRT
- Ngandolo BN, Diguimbaye-Djaibé C, Müller B, Didi L, Hilty M, et al. (2009) Diagnostics ante et post mortem de la tuberculose bovine au sud du Tchad : cas des bovins destinés à l’abattage. Revue d'élevage et de médecine vétérinaire des pays tropicaux 62: 5-12. Link: https://bit.ly/3lzNh6R
- Kazwala RR, Kambarage DM, Daborn CJ, Nyange J, Jiwa SFH, et al. (2001) Risk Factors Associated with the Occurrence of Bovine Tuberculosis in Cattle in the Southern Highlands of Tanzania. Vet Res Commun 25: 609-614. Link: https://bit.ly/3kgzEtS
- Delafosse A, Goutard F, Thébaud E (2002) Epidémiologie de la tuberculose et de la brucellose des bovins en zone périurbaine d’Abéché, Tchad. Revue d'élevage et de médecine vétérinaire des pays tropicaux 55: 5-13. Link: https://bit.ly/39dke3c
- Cadmus S, Agada C, Onoja I, Salisu I (2010) Risk factors associated with bovine tuberculosis in some selected herds in Nigeria. Trop Anim Health Prod 42: 547-549. Link: https://bit.ly/2YWhJAw
- Akinseye VO, Adesokan HK, Ogugua AJ, Adedoyin FJ, Otu PI, et al. (2016) Sero-epidemiological survey and risk factors associated with bovine brucellosis among slaughtered cattle in Nigeria. Onderstepoort J Vet Res 83: a1002. Link: https://bit.ly/2XyTGHB
- Naima S, Borna MR, Bakir M, Djamel Y, Fadila B, et al. (2011) Tuberculosis in cattle and goat of North Algeria. Vet Res 4: 100-103. Link: https://bit.ly/3AkN3qD
- Crawshaw T, Daniel R, Clifton-Hadley R, Clark J, Evans H, et al. (2008) TB in goats caused by Mycobacterium bovis. Vet Rec 163: 127. Link: https://bit.ly/3khKTSX
- Tschopp R, Aseffa A, Schelling E, Berg S, Hailu E, et al. (2010) Bovine tuberculosis at the wildlife-livestock-human Interface in Hamer Woreda, south Omo, southern Ethiopia. PLoS One 5: e12205. Link: https://bit.ly/2Z5VwjH
- Tschopp R, Bobosha K, Aseffa A, Schelling E, Habtamu M, et al. (2011) Bovine tuberculosis at a cattle-small ruminant-human interface in Meskan, Gurage region, Central Ethiopia. BMC Infect Dis 11: 318-322. Link: https://bit.ly/3lP67r5
- Ketema T, Fufa D, Teshale S, Ameni G (2010) Prevalence of tuberculosis in goats Mid Rift Valley area of Oromia, Ethiopia. African Journal of Microbiology Research 5: 1473-1478. Link: https://bit.ly/3nDDRdm
- Mamo GK, Abebe F, Worku Y, Legesse M, Medhin G, et al. (2012) Tuberculosis in goats and sheep in afar pastoral region of Ethiopia and isolation of mycobacterium tuberculosis from goat. Veterinary Medicine International 2012: 869146. Link: https://bit.ly/3nDu1Ix
- Munoz-Mendoza M, Romero B, Cerro Ad, Gortazar C, Garcıa-Marın JF, et al. (2015) Sheep as a Potential Source of Bovine TB: Epidemiology, Pathology and Evaluation of Diagnostic Techniques. Transbound Emerg Dis 63: 635-646. Link: https://bit.ly/3AiVyCl
- Ghebremariam MK, Michel AL, Vernooij JCM, Nielen M, Rutten VPMG (2018) Prevalence of bovine tuberculosis in cattle, goats, and camels of traditional livestock raising communities in Eritrea. BMC Veterinary Research 14: 73. Link: https://bit.ly/3khKobx
- Danbirni S, Abubakar HU, Allam L, Pewan SB, Barde IJ, et al. (2016) Prevalence of tuberculosis-like lesions in goats slaughtered at Bauchi central abattoir, Bauchi State. Sokoto Journal of Veterinary Sciences 14: 45-48. Link: https://bit.ly/2ZarNpX
- de la Rua-Domenech R, Goodchild AT, Vordermeier HM, Hewinson RG, Christiansen KH, et al. (2006) Ante mortem diagnosis of tuberculosis in cattle: A review of the tuberculin tests, [gamma]-interferon assay and other ancillary diagnostic techniques. Res Vet Sci 81: 190-210. Link: https://bit.ly/3hGknRy
- Pollock JM, McNair J, Bassett H, Cassidy JP, Costello E, et al. (2003) Specific Delayed-Type Hypersensitivity Responses to ESAT-6 Identify Tuberculosis-Infected Cattle. J Clin Microbiol 41: 1856-1860. Link: https://bit.ly/39bAqSI
- Amadori M, Tameni S, Scaccaglia P, Cavirani S, Archetti IL, et al. (1998) Antibody Tests for Identification of Mycobacterium bovis - Infected Bovine Herds. J Clin Microbiol 36: 566-568. Link: https://bit.ly/3EjBzG6
- Buddle BM, Livingstone PG, de Lisle GW (2009) Advances in ante-mortem diagnosis of tuberculosis in cattle. N Z Vet J 57: 173-180. Link: https://bit.ly/3hFMmB0
- Thom ML, Hope JC, McAulay M, Villarreal-Ramos B, Coffey TJ, et al. (2006) The effect of tuberculin testing on the development of cell-mediated immune responses during Mycobacterium bovis infection. Vet Immunol Immunopathol 114: 25-36. Link: https://bit.ly/3hIBy53
- Thrusfield M (2007) Veterinary epidemiology, 3rd edn. Oxford, UK: Blackwell Science Ltd, a Blackwell publishing company. Link: https://bit.ly/3nDx2Zn
- MINEPIA (2002) La stratégie sectoriel de l’élevage, des peches et industries animales. In: Cabinet Management 2000 MINEPIA. In. Edited by Doufissa A. Yaounde, Cameroon: Ministry of Livestock, Fisheries and Animal Industries, Yaounde.
- Dumas R (1980) Contribution à l’étude des petits ruminants du Tchad. Revue D’élevage et de Médecine Vétérinaire Des Pays Tropicaux 33: 215–233. Link: https://bit.ly/39f62qw
- Gueye A (1997) Moutons et Chèvres du Sénégal : Caractérisation morpho-biométrique et typage sanguin. In. Univerté Cheikh Anta Diop, Senegal. Link: https://bit.ly/3hG2qCF
- Oudanang KM (2009) Sub-sector in Chad : a key-study of raw milk commodity chain in N’Djamena. In. Institut des Sciences et Industries du Vivant et de l’Environnement (Agro Paris Tech).
- Ngere LO (1987) Principles for Indigenous Animal Improvement in the Tropics - African Experiences with Sheep and Goats. In: FAO Animal Production and Health Paper. Volume 66, Animal genetic resources: Strategies for improved use and conservation (with Proceedings of the EAAP/PSAS Symposium on Small Populations of Domestic Animals) edn. Edited by Hodges J. Rome, Italy: Food and Agriculture Organization of the United Nations and of the United Nations Environment Programme 394. Link: https://bit.ly/3kicbZj
- Turton J (1999) How to estimate the age of cattle Onderspoort, South Africa: National Department of Agriculture, ARC- Onderspoort Veterinary Institute.
- Villaquiran M, Gipson TA, Merkel RC, Goetsch AL, Sahlu T (2004) Body Condition Scores in Goats. In. American Institute for Goat Research 1–8. Link: https://bit.ly/3AlO1Tg
- Kassa GM, Abebe F, Worku Y, Legesse M, Medhin G, et al. (2012) Tuberculosis in goats and sheep in pastoral Afar Region of Ethiopia and isolation of Mycobacterium tuberculosis from goat. Veterinary Medicine International 869146. Link: https://bit.ly/3lxyeL3
- Gracey JF, Collins DS (1992) Meat hygiene, 9 edn. London, UK: Bailliére Tindall 832.
- Grist A (2008) Bovine Meat Inspection - Anatomy, Physiology and Disease Conditions, 2 edn. Nottingham, UK: Nottingham University Press 296.
- FAO (1994) Manual on meat inspection for developing countries. In: FAO Animal Production and Health Paper 119. Edited by Herenda D, Chambers PG, Ettriqui A, Seneviratna P, Silva TJPd. Rome, Italy: Food and Agriculture Organization of the United Nations 388.
- Diguimbaye C, Hilty M, Ngandolo R, Mahamat HH, Pfyffer GE, et al. (2006) Molecular Characterization and Drug Resistance Testing of Mycobacterium tuberculosis Isolates from Chad. J Clin Microbiol 44: 1575-1577. Link: https://bit.ly/3lvE0g9
- Strong BE, Kubica GP (1985) Isolation and Identification of Mycobacterium tuberculosis - A Guide for the Level II Laboratory In. Atlanta, Georgia, USA: Department of Health and Human Services, Public Health Service, Laboratory Improvement Program Office, Division of Laboratory Training and Consultation 154.
- WHO (1998) Laboratory services in tuberculosis control Part II : Microscopy. In., vol. WHO/TB/98.258. Geneva, Switzerland: World Health Organization 63. Link: https://bit.ly/39dOVp1
- Spiegelhalter DJ, Thomas A, Best NG (2003) Lum: WinBUGS Version 1.4. User Manual. In. MRC Biostatistics Unit, Cambridge 4.
- Gamerman D (1997) Markov Chain Monte Carlo Stochachastic Simulation for Bayesian inference. In. Chapman`and Hall Edition London.
- Sanson RL (1998) Tuberculosis in goats. Surveillance 15: 7-8. Link: https://bit.ly/3tNUoMG
- Berkvens D, Speybroeck N, Praet N, Adel A, Lesaffre E (2006) Estimating disease prevalence in a Bayesian framework using probabilistic constraints. Epidemiology 17: 145-153. Link: https://bit.ly/3nVpJwn
- Nkongho FE, Muwonge A, Ndip L, Keyy RF, Sander M, et al. (2016) Abattoir-based estimates of mycobacterial infections in Cameroon. Science Report 6: 24320. Link: https://go.nature.com/39c82zJ
- Tafesse K, Dawo F, Sori T, Ameni G (2011) Prevalence of caprine tuberculosis in Mid- Rift valley area of Oromia, Ethiopia. African Journal of Microbiology Research 5: 1473-1478. Link: https://bit.ly/3EpQT4a
- Silvano SSH, Pinheiro SR, de Souza CCD, do Rosario PAM, Alves CJ, et al. (2011) Mycobacterium bovis infection in goats from the northeast region of Brazil. Braz J Microbiol 42: 1437-1439. Link: https://bit.ly/3lv4PkN
- Kelly RF, Hamman SM, Morgan KL, Egbe NF, Ngwa VN, et al. (2016) Knowledge of bovine tuberculosis, cattle husbandry and dairy practices amongst pastoralists and small-scale dairy farmers in Cameroon. PLoS One 11: e0146538. Link: https://bit.ly/2VOD8KV
- Francis J (1971) Susceptibility to tuberculosis and the route of infection. Australian Veterinary Journal 47: 414-414.
- Ameni G, Medhin G (2000) Effect of gastrointestinal parasitosis on tuberculin test for the diagnosis of bovine tuberculosis. Journal of Applied Animal Research 18: 221-224. Link: https://bit.ly/2VTKsVG
- Inangolet F, Demelash B, Oloya J, Opuda-Asibo J, Skjerve E (2008) A cross-sectional study of bovine tuberculosis in the transhumant and agro-pastoral cattle herds in the border areas of Katakwi and Moroto districts, Uganda. Trop Anim Health Prod 40: 501-508. Link: https://bit.ly/2XAVWOD
- Thoen CO, LoBue PA, Enarson DA, Kaneene JB, de Kantor IN (2009) Tuberculosis: a re-emerging disease of animals and humans. Vet Ital 45: 135-181. Link: https://bit.ly/3AiBwba
- Davies PDO, Pai M (2008) The diagnosis and misdiagnosis of tuberculosis. Int J Tuberc Lung Dis 12: 1226-1234. Link: https://bit.ly/3ziKQKM
- Lepper AW, Pearson CW, Corner LA (1977) Anergy to tuberculin in beef cattle. Australian Veterinary Journal 53: 214-216. Link: https://bit.ly/3tP4Jbj
- Pollock JM, Neill SD (2002) Mycobacterium bovis Infection and Tuberculosis in Cattle. Vet J 163: 115-127. Link: https://bit.ly/3AkSBkX
- Biet F, Boschiroli ML, Thorel MF, Guilloteau LA (2005) Zoonotic aspects of Mycobacterium bovis and Mycobacterium avium-intracellulare complex (MAC). Vet Res 36: 411-436. Link: https://bit.ly/3hEuNRu
- Ibrahim S, Cadmus SIB, Umoh JU, Ajogi I, Farouk UM, et al. (2012) Tuberculosis in Humans and Cattle in Jigawa State, Nigeria: Risk Factors Analysis. Vet Med Int 2012: 865924. Link: https://bit.ly/2YWoimE
- Robinson P, Morris D, Antic R (1988) Mycobacterium bovis as an occupational hazard in abattoir workers. Aust N Z J Med 18: 701-703. Link: https://bit.ly/2XB0Xqr
- Khattak I, Mushtaq MH, Ahmad MUD, Khan MS, Haider J (2016) Zoonotic tuberculosis in occupationally exposed groups in Pakistan. Occup Med (Lond) 66: 371-376. Link: https://bit.ly/3kek9To
- CamOHNS (2012) Cameroon "One Health" National Strategy. In.: The chain of Health as defined in the organization chart of the Ministry of Livestock, Fisheries and Animal Industries, the Ministry of Environment and Nature Protection, the Ministry of Forests and Wildlife, the Ministry of Public Health, Yaounde Cameroon 15.
- CamOHNS (2016) Zoonotic diseases considered for prioritization in Cameroon. In.: Zoonotic Disease Prioritization for Inter-sectoral Engagement in Cameroon. Preparedness and Response; One Health in action. Inter-sectoral prioritization workshop held in Yaoundé, Cameroon 2016: 32.
- Awah-Ndukum J, Mouiche MMM, Bayang HN, Ngu-Ngwa V, Assana E, et al. (2018) Seroprevalence and Associated Risk Factors of Brucellosis among Indigenous Cattle in the Adamawa and North Regions of Cameroon. Vet Med Int 2018: 10. Link: https://bit.ly/3hJefYC
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
