International Journal of Veterinary Science and Research
Effect of weaning age on morphological and histological changes that occur in a rat’s testis
Yonatan Crispel1,2*, Yonatan Danin2, Geula Klorin2,3 and Ze’ev Hochberg2
2Rappaport Family Faculty of Medicine, Technion, Israel Institute of Technology, Haifa, Israel
3Department of Pathology, Rambam Health Care Campus, Haifa, Israel
Cite this as
Crispel Y, Danin Y, Klorin G, Hochberg Z (2021) Effect of weaning age on morphological and histological changes that occur in a rat’s testis. Int J Vet Sci Res 7(1): 024-028. DOI: 10.17352/ijvsr.000076Background: Weaning of mammalian progeny is associated with a change in body shape and physical characteristics. It programs growth, body composition, and the tempo of physiological development and maturation, as well as litter size and parity and, thereby, reproductive strategy.
Methods: To evaluate the effects of weaning age on morphological and histological changes that occur in a rats’ testis, we assessed testis size weekly and histomorphometry in 30 days old pups, and 90 days old mature rats that had been weaned early (d16), normally (d21), or late (d26).
Results: Early weaning resulted in large testes at age 30 days and beyond, a wide seminiferous tubule wall, and the ratio of blood vessel to the stroma was bigger than in late weaned animals. At age 90 days, and the next generation the litter size of early-weaned rats was bigger than those weaned on days 26 or 21.
Conclusions: Early weaning significantly enhances the testes’ size, vasculature, and seminiferous tubular walls, and enhances the liter size compared to late weaning.
Introduction
Mammalian life history starts with a stage of infancy, defined mostly by lactation, and subsequently a gradual weaning in humans towards childhood, adolescence, and adult diet [1]. Weaning from lactation is responsive to sex, stress and other environmental cues that are presumed to inform the developing organism about risks and opportunities in its current and future environment [2].
The weaning age defines life history transition from infancy to childhood in humans and to juvenility in the rat [3] and this transition phase is highly important in shaping (programming for) future growth and maturation trajectories [4].
We have previously shown that in humans a delayed infancy-childhood transition has a lifelong impact [3,4]. Correspondingly, in the rat, the age at weaning from lactation determines a rat’s growth trajectory, but also body composition and maturational tempo [5].
We showed that early-weaned rats developed faster than normal, and at adulthood early weaned rats were leaner and longer than late-weaned animals, which were heavier and shorter [5]. Early-weaned progeny matured more rapidly, females showed earlier vaginal opening and estrous and males showed earlier onset of testicular growth [5]. These effects intensified in future generations who were also weaned according to the same schedule.
Surprisingly, early weaned rats and their offspring had bigger testes at maturity [5]; an observation which triggered the present study. Here, we examined the impact of weaning age on adult morphology and histology of rat’s testes and how these traits change over three-generations.
Note: This experiment is part of a large study examining the effect of weaning from lactation on growth, sex, and stress, for more detail, you can see Crispel, et al. 2013.
Materials and methods
Animals
The animals that we used for the present report were previously described in details [5]. In short, gestating outbred Sprague-Dawley mother rats from timed pregnant, colonies were housed at the Animal Facility of the Technion Faculty of Medicine. All animal procedures have been approved by the Technion Animal Use and Care Committee and were performed under the supervision of an animal veterinarian surgeon. Normally rats are weaned at day 21 (d21) of life; such rats in all groups served as control. The number of female pregnancies used for replicates in each weaning group was three, to provide 12 males and 12 female pups per group.
Animals were grown uninterrupted other than for weekly measurements until weaning on the designated d16 (early weaning) d21 (control) or d26 (late weaning). On weaning day, mothers were removed, and non-lactating cross-foster mothers that had weaned litters successfully in the recent month were introduced [6]. Upon weaning on d16, both chow cubes and chow powder were introduced to the cages to ascertain ad libitum feeding by young pups. On d21 and d30, both groups were sacrificed, and from each rat in first generation, testes for histology and morphology were excised.
Short-term effects
To assess the short-term effects and to provide insights about how early weaning alters normal reproductive strategy, we measured testicular size weekly for 12 weeks. Nine males of the early-weaned group (16-day breastfeeding after birth) and nine males of the late-weaned group (26 day) were sacrificed. In future generation, the rat testes were excised on day 30.
To evaluate the long-term consequences of early and late weaning and the effect on testicular size, testicular size was determined daily from day 30 once a week until day 90 using a self-built orchidometer based on the human Prader orchidometer with mock-ups ranging from 0.5 to 5ml [7]. Testes measuring more than 1 ml were considered early pubertal, in first generation second group of rats that had been weaned by cross-foster mothers on d16, or d26 and were sacrificed on d90 for testicular histomorphometry.
Histomorphometry
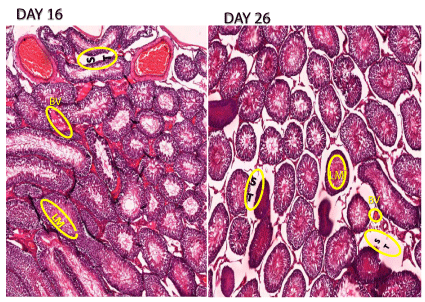
Histologic sections were prepared from 5-6 sites along the testicles, fixed in 0.05 mmol/L saline phosphate buffer containing 4% formaldehyde. Paraffin embedded specimens were longitudinally sectioned (6 μm), stained with hematoxylin and eosin for quantitative evaluation of lumen, seminiferous tubule wall, stroma and blood vessels, Figure 1.
An Image analyzer (Trichip RGB video camera; Sony, Tokyo, Japan) installed on a light microscope (Zeiss, Jena, Germany) and attached to a computer equipped with a frame grabber was used to analyze the extension of positively stained tissue, as previously described [8,9]. Images were captured, digitized, and displayed on a high-resolution colored monitor. The ten most intensely stained fields were analyzed at a power lens of 20×10. Images were loaded on screen buffers having a resolution of 760×570 pixels and measured in standardized frames (62993 μm2). Image Pro Plus 4 software (Media Cybernetics, Baltimore, MD) was used to assess the extension of positively stained cells/tissue after segmentation and thresholding.
Statistical analysis
Physiological data were analyzed using SAS 6.12, utilizing two-way ANOVA. Histomorphometric data were analyzed using SPSS 6.0 (Chicago, IL). Positively stained cases in two groups were compared by the Mann-Whitney U test. Comparison between cases used the nonparametric Kruskal-Wallis ANOVA followed by a corrected Mann-Whitney U test for multiple comparisons. Differences with 2-tailed P values < 0.05 were considered significant.
Results
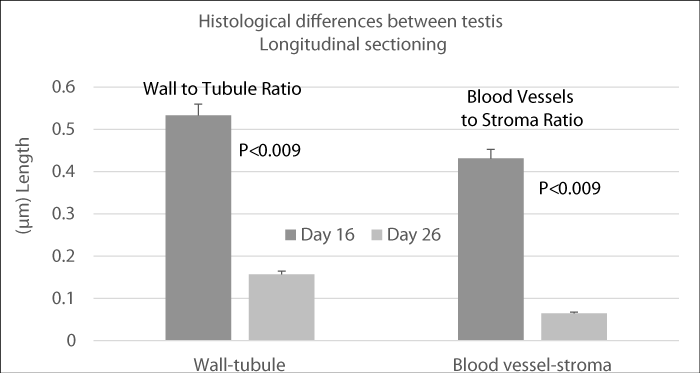
The early-weaned animals’ day 16 had bigger testes than late-weaned animals’ day 26. (4.14±0.24 ml vs 3.29±0.4 ml, p<0.0001). This was mainly due to greater seminiferous tubular lumen and greater testicular vasculature. Histomorphometry showed that the seminiferous tubule / wall ratio was significantly greater in the early-weaned group 0.533±0.03µm vs 0.431±0.04 µm (p<0.009) compare to late-weaned animals. Blood vessel to stroma ratio was significantly higher in the early-weaned group 0.156±0.08 µm vs 0.064±0.009 µm (p<0.0094) compared to the late-weaned group respectively, (Figure 2 longitudinal section).
The ratio between wall and seminiferous tubular lumen was significantly smaller in the early-weaned group 0.994±0.05 µm vs 1.435±0.23 µm (P<0.01) compared to late- weaned rats, (wall was smaller in early weaned). The stroma to seminiferous tubule ratio was significantly smaller in the early-weaned group 0.212±0.02 µm vs 0.385±0.08 µm p<0.05 compared to the late-weaned group respectively,
Seminiferous tubule areas and seminiferous tubule perimeter were comparable in early- and late-weaned. (Figure 3 longitudinal section).
Wall to seminiferous tubule ratio was greater in the early-weaned group 0.566±0.04 µm vs 0.442±0.05 µm (p<0.05) compare to the late-weaned rats. Blood vessel to stroma ratio was greater in the early-weaned group 0.128±0.03 µm vs 0.024±0.004 µm (p<0.007) compared to the late-weaned animals (Figure 1), the ratio between lumen and wall was lower in the early-weaned group 0.770±0.217 µm vs 1.3381±0.278 µm (P<0.05) compared to the late-weaned rats (wall was smaller in early weaned). The stroma to seminiferous tubule ratio was comparable (Table 1, Horizontal section).
To reinforce the claim that large testicles produce more offspring we attach numbers describing volume testis of three generations on days 16 and 26 (Table 2). In Measurements we made to the size of the testicles, we found that the volume of the testicles on day 16 was larger than day 26 significantly in three generation: 4.35ml±0.21 vs 3.98ml±0.13, p<0.0006 4.14ml±0.24 vs 3.29ml±0.399 p<0.0001, 4.15ml±0.24 vs 3.88ml±0.12 p<0.0004, respectively (Figure 4).
Liter size in early-weaned, rats were larger significantly than late weaned animals over four generations: second-generation p<0.02, third generation p<0.001 and fourth generation p<0.009 (Table 2). Day 21 (control not mentioned) was the same like late weaning Day 26.
Discussion
This study is part of a previous study examining the effect of weaning from lactation on rat growth, in main study we found that early-weaned rat have a large testis significantly compared late weaning. To explore the reason of this diversity, Histologic and morphologic examine were taken to find differences between testicles of early and late weaned rats. Two central differences between the testicles of the different groups at histological level found: many blood vessels with a thinner wall and a larger lumen compared to animals weaned later. Recent data suggests that Sertoli cells play a key role with vasculature network formation [10], the vascular density of a tissue is influenced by diverse factors, both pathologic and physiologic. For example, it is well established that angiogenesis is a part of pathologic malignant processes [11,12] increased angiogenesis, blood vessel concentration can be a favorable process in which growth, and development is achieved in breastfeeding, wound healing [13] Or infant organ development, this finding influence metabolic states and we assume that this result derive from different Sertoli concentration with different breastfeeding duration, Which can explain the expression of the large testis volume and explain larger litter in early-weaned group as expected from the results.
The age at sexual maturation plays a central role in reproductive strategy. We thus predicted and found that early-weaned females showed earlier vaginal opening and estrous, and early-weaned males showed earlier onset of testicular growth and attainment of greater testicular volume compared to late-weaned rats [5]. We previously showed that early-weaned progeny is more productive than the progeny of later-weaned rats.
It was previously shown that the critical period for testicular development in the rat is from day 13 of gestation to the 21st postnatal day, and that this stage determines the tissue future structure and function [14]. Here, we focused on a developmental milestone at day 21 – weaning from lactation and its effect on the testes and reproduction.
In the wild, weaning from lactation is responsive to stress and other environmental cues that are presumed to program the developing organism for risks and opportunities in its current and future environment [2]. In the animals facility, the milestone of weaning is determined by the facility’s routine, and it is customary to separate mothers from offspring on day 21, and this day is defined as the life history transition from infancy to juvenility in the rat [3]. This transition is important in programming for future growth and maturation trajectories [4].
The findings presented here on rats lend support to the proposition that the duration of infancy, as indexed by weaning age, predicts and perhaps programs testicular growth, litter size and parity, and, thereby, reproductive strategy.
We have previously shown that short infancy (early weaning) is associated with characteristic fast reproduction long body, underweight, glucose tolerance and insulin sensitivity, but also in large testes and large liter [7]. Among the smallest mammals, testes size is associated with high copulatory frequency and sperm production [15], as observed also in the rats of the present study.
Here, we show the testicles’ structures in early-weaned animals that support greater fertility. These structural components include greater seminiferous tubular volumes, seminiferous tubular wall thickness (as a surrogate for Sertoli cells) and more vasculature in the stroma.
In another rodent - the golden hamster, an active testicular tissue is comprised of 92.5% seminiferous tubules and 1.4% of Leydig cell stroma [16].
In humans the key determinant of a testis’ size is the number of Sertoli cells, which proliferate in fetal and early postnatal life, and Sertoli cells number is a determinant of sperm count in adulthood [17]. Here, Sertoli cells are estimated by seminiferous tubular wall thickness.
Conclusion
Seminiferous tubular wall thickness / Sertoli cells support enhanced reproductive capacity in animal that were weaned early. We propose that this phenomenon is an evolutionary strategy to enhance reproduction in animal with a fast life history. Despite substantial heritability in testicular development, much variation remains to be explained, leaving room for the influence of environmental factors to adaptively adjust the phenotype in the service of fitness goals.
Authors’ contributions
YC and ZH designed and YC, YD, GK and ZH performed the study. YC and ZH contributed to writing of the manuscript.
- Shaoul R, Tiosano D, Hochberg Z (2015) Evo-Devo of Child Growth: The Role of Weaning in the Transition from Infancy to Childhood. Crit Rev Food Sci Nutr 56: 887-895. Link: https://bit.ly/3cP6c9v
- Cook CJ (1999) Patterns of weaning and adult response to stress. Physiol Behav 67: 803-808 . Link: http://bit.ly/38UrXUj
- Hochberg Z (2009) Evo-devo of child growth II: human life history and transition between its phases. Eur J Endocrinol 160: 135-141 . Link: http://bit.ly/3lwmFDm
- Hochberg Z, Albertsson-Wikland K (2008) Evo-devo of infantile and childhood growth. Pediatr Res 64: 2-7 . Link: http://bit.ly/2P55tc5
- Crispel Y, Katz O, Ben-Yosef D, Hochberg Z (2013) Effects of breastfeeding on body composition and maturational tempo in the rat. BMC Med 11: 114 . Link: http://bit.ly/2QiJPBD
- Pérez-Laso C, Ortega E, Martín J, Pérez-Izquierdo MA, Gómez F, et al. (2013) Maternal care interacts with prenatal stress in altering sexual dimorphism in male rats. Horm Behav 64: 624-633 . Link: http://bit.ly/3eV0yW4
- Crispel Y, Shaoul R, Khamaise R, Sabo E, Hochberg ZE (2019) Effect of weaning age on the small intestine mucosa of rats. Appl Physiol Nutr Metab 44: 985-989 . Link: http://bit.ly/3s4clVD
- Naveh E, Werman MJ, Sabo E, Neeman I (2002) Defatted avocado pulp reduces body weight and total hepatic fat but increases plasma cholesterol in male rats fed diets with cholesterol. J Nutr 132: 2015-2018 . Link: http://bit.ly/3vJxSFC
- Klein A, Mazor Y, Karban A, Ben-Itzhak O, Chowers Y, et al. (2016) Early histological findings may predict the clinical phenotype in Crohn’s colitis. United European Gastroenterol J 2016: 2050640616676435 . Link: http://bit.ly/2Pbhfl6
- Rebourcet D, Wu J, Cruickshanks L, Smith SE, Milne L, et al. (2016) Sertoli Cells Modulate Testicular Vascular Network Development, Structure, and Function to Influence Circulating Testosterone Concentrations in Adult Male Mice. Endocrinology 157: 2479-2488. Link: http://bit.ly/3s4cRmx
- Nishida N, Yano H, Nishida T, Kamura T, Kojiro M (2006) Angiogenesis in cancer. Vasc Health Risk Manag 2: 213-219.
- Olivarez D, Ulbright T, DeRiese W, Foster R, Reister T, et al. (19994) Neovascularization in clinical stage A testicular germ cell tumor: Prediction of metastatic disease. Cancer Res 54: 2800-2802. Link: http://bit.ly/3c4XpRE
- Crispel Y, Ghanem S, Attias J, Kogan I, Brenner B, et al. (2017) Involvement of heparanase procoagulant domain in bleeding and wound healing. Thrombosis and Haemostasis. J Thromb Haemost 15: 1463-1472. Link: http://bit.ly/2Qd8eZl
- Orth JM (1982) Proliferation of Sertoli cells in fetal and postnatal rats: a quantitative autoradiographic study. Anat Rec 203: 485-492 . Link : http://bit.ly/3r6bEdf
- Kenagy G, Trombulak SC (1986) Size and function of mammalian testes in relation to body size. Journal of Mammalogy 67: 1-22 . Link : https://bit.ly/38ZJP02
- Sinha Hikim AP , Bartke A, Russell LD (1988) Morphometric Studies on Hamster Testes in Gonadally Active and Inactive States: Light Microscope Findings1. Biology of Reproduction 39: 1225-1237 . Link: http://bit.ly/2P8xOhR
- Sharpe RM, McKinnell C, Kivlin C, Fisher JS (2003) Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction 125: 769-784 . Link: http://bit.ly/3vLTLDW
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
