International Journal of Veterinary Science and Research
Prevalence of bovine fasciolosis in and around Bedelle
Yikeber Walle1* and Mullusew Gashaw2
Cite this as
Shafi W (2021) Prevalence of bovine fasciolosis in and around Bedelle. Int J Vet Sci Res 7(1): 013-023. DOI: 10.17352/ijvsr.000075The study was carried out, in Illubaboral zone of Oromia regional at western part of Ethiopia in Bedelle municipal abattoir from November 2010 to the end of March 2011, in order to determine the prevalence of liver fluke, the species of liver fluke and to compare the diagnostic efficiency of fecal and post mortem examination. Out of 384 livers and fecal samples examined 93(24.21%) and 74(19.27%) were positive of fasciolosis respectively. The most common liver fluke species affecting the cattle was Fasciola gigantica 52(13.54%) cattle were infected with Fasciola gigantica while, Fasciola hepatica were present in 31(8.07%) cattle and 10(2.6%) were mixed infection. There was a strong relationship between fecal examination and post mortem examination of liver lesions, but under local condition post mortem examination was considered a better diagnostic tool for fasciolosis. This study showed that there is no significant association (p>0.05) between the prevalence of age, body condition and origin of animal, but there is a significance difference (p<0.05) in prevalence of fasciola hepatica, between origin of animals.
Abbreviation
AAU: Addis Ababa University; FAO: Food and Agricultural Organization; CO: Degree Centi Grade; Km: Kilo meter; m: meter; mm: milli meter; cm: centi meter; masl: meters Above the sea level; rpm: round per minute; FVM: Faculty of Veterinary Medicine; F.hepatica: Fasciola hepatica; F.gigantica: Fasciola gigantica; GIS: Geographical Information System
Introduction
Bovine fasciolosis is an economicaly important parasite disaese of cattle caused by Fasciolidae, which are trematode of the genus Fasciola. The two most important species of the genus are Fasciola hepatica and Fasciola gigantica. Ethiopia is one of the nation with the highest population of livestock ,more than 31 million cattle [1]. But the productivity is far less than the potential due to several constaints. Like disease, malnutrition and traditional management.The rich potential from the livestock sector is not efficiantily exploited [2].
Fasciolosis caused by Fasciola hepatica and Fasciola gigantica, is one of the most prevalent helminths infection of ruminant in different part of the world including Ethiopia. The presence of fasciolaosis due to F. hepatica and F. gigantica in Ethiopia has long been known and its prevalence and economic significance has been reported by several workers [3-7]. F.hepatica and F. gigantica occurs relatively cooler semi highland and highland and lowland respectively, where the intermidiate are abudentily available during the wet season [8]. Fasciolosis occurs commonly as achronic disease in cattle and the severity often depends on , the nutritional status of host [9]. It is responsible for a wide spread morbidity and mortality espacialy in cattle and sheep characterised by weight loss,anemia, hypho proteinemia [10]. The effect due fasciolosis can also expresed in terms of mortality ,morbidity, reduced growth rate,liver condemnation at slaughter house, reduction in traction power,less weight gain at birth , increased susceptibility tosecondary infection and the expense of control measures [7,10,11].
Diagnosis is based premarily on the clinical sign and seasonal occurance in endemic areas but previos examination, hematological test and examination of feaces for fluke egg are usefull. Coprological analysis is still commonly employed to diagnose bovine Fasciolosis despite the fact that egg cannot be detected untill after the lettent period of infection , when much of the liver damage has already occured [12]. This study was intended to determine the prevalence of fasciolosis in cattle slaughtered at Bedelle municipal abattor, to compare the diagnostic efficiency of fecal examination and post mortem examination and to determine the most prevalent species of liver fluke.
The objective of this study were:
- To estimate the prevalence of liver fluke and most prevalent species of livefluke in cattles slaughtered at Bedelle municipal abattor.
- To compare the diagnostic efficiency of fecal examination and post morte examination in cattles slaughtered at Bedelle municipal abattor
Literature review
The parasite: The taxonomic classification of the organism that fasciolosis is presented as follows [8].
Phylum: platyhelminths
Class: trematode
Subclass: Digenia
Family Fasciolidae
Genus: Fasciola
Species: Fascicla hepatica and Fascicla gigantica
Morphology
The addult parasite F. hepatica has a fiat leaf-like body and measures 20-30 mm long by 8-15 wide [13]. It has an anterior along action (a cephalic cone) on which the oral and ventral suckers, which are approximate of equal size are located. The vitellaria are highly diffuse and branched in the lateral and posterior region of the body. F.gigantica is a parasite very similar to F. hepatica, its length may vary 25-75mm long by 15mm wide (Soulsby, 1982) [14]. In addition, the cephalic cone is proportionally shorter than that of F. hepatica and it’s body even more of like in shape [14]. The egg of F. hepatica is oval, opera vulate, yellow and large (150mmxmm), and about times the size of a fright strongly age [8]. The egg of F.gigantica longer in size and measures (200nmx100hm) (Dunn,1998).
Life cycle
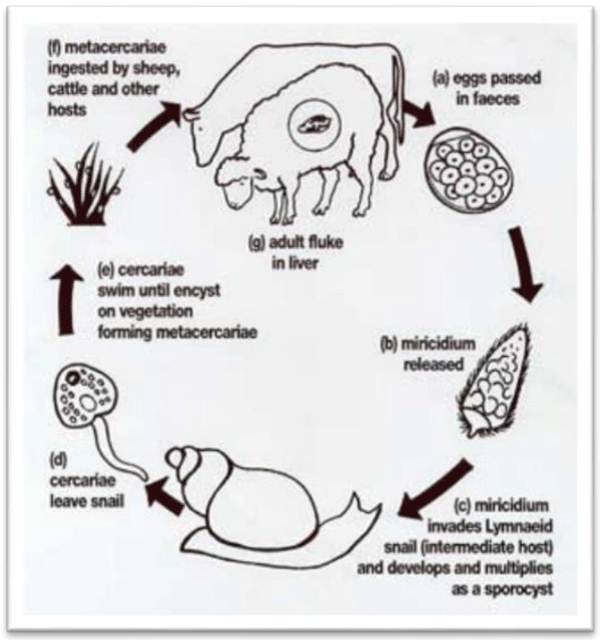
The life cycle of Fascicla species is a typical of disgenic treated. Egg laid by the addult parasite in the bile ducts of their hosts pass into the duodenum with bile. The egg then leave the host through the feaces. At this stage, egg are still not embryonated, further development to maturation taking approximately two weeks. The egg hatch to release the motile miracidium, which will locate and penetrates the intermediate snail host. The need to find the suitable most to penetrate is an urgent one, for those miracidia a failing to do so generally die with in 24 hours. After penetration of the snail, the miracidia loses it’s cilia and become sporocist [15] The sporocistt dividing and forming radial (have sucker and primitive gut), and a full mature radia showing radia and circaria stage. The circaria of Facsiola species have a rounded body measuring between 0.25. 0.35mm long, with a long thin unbranded tail measuring approximately 0.5mm long. The motile circaria snail generally leaves the shall 4-7 weeks after infection by migrating through the tissue of snail. This is during moist condition when a critical temperature of 10oc is exceeded [15].
On emerging from the snail the circadian attaches to submerged blades of grass or other vegetation like watercress, the tail fall away and the circadian body secretes a four-layered cyst covering from cytogeneses gland located on the lateral regions of the body. The formation of the wall may take up to two days. The metacercariae (eneysted, resistant circarian) is the infective form of the definitive host. Generally, Meta circarian are infective to ruminants such as cattle and sheep, but also to other mammals including human being- one meracium hatching from a fluke egg can produce up to4,ooo infective cyst (metacercarial) due to the vegetative multiplication at the sporocyst and radia . The metacercarial cyst is only moderately resistance, not being able to survive dry conditions. If however, they are maintainted in condition of high humidity and cool temperature, they may survive for up to a year [13,14,16]. Infection in non endemic areas. The meta cercarial cyst, when ingested alone with the contaminated vegetation by the definitive host enters in to the small intestine. Releasing the young parasite, which penetrate the gut wall, entering the peritoneal cavity.
From there it migrates directly to the liver over a period of approximately seven days. The Juvenile fluke (also refered adeloscaria) penetrate the liver tissue; through which it migrates, feeding mainly on blood, for about 6. Weeks After this periods, the fake enters the bile ducts, maturing in to a fully a duct parasite after about three months from initial infection. Egg production then commences and completing the life cycle [13] Figure 1.
Adult fakes can survive for many years in the liver of infected hosts and lay between 20,000 and 50,000 eggs per day the rate of egg production is responsible for the degree of pasture contamination and thus greatly influences the epidemology of the disease and also influenced by the grazing habit o the animals. Animals grazing in wet marshy areas favored by intermiediate host are more likely to become infected. Typically, long and wet seasons are associated with a higher rate of infection [16].
Host range
Intermediate host: Snails of the genus lymnae are the inter mediate host for genus Faciola. The epidemiology of Physiology is dependent on the ecology of the snail intermediate host. Lymnae speaces, most important in the transmission of F. hepatica. includes lymnaea trancatula, in North America. L. tormentors in Australia other species, which have been incriminated in the transmission of Fascicla hepatica, includes, L.viator andL. Diaphena (South America ), L. columinela (USA, Australia, Central America and New Zeland ) and L.humilus (North America ) [13,14].
L. truncate is most common intermediate host for F. Lepatica in different part of the world [11] and in Ethiopia (Grabber, 1974). It is amphibious and although they spend hours in shallow water, they periodically emerge on to surrounding mud. They are capable of with standing summer draught or winter freezing for several months by the respectively aestivating or hibernating deep in the mud [8]. It prefers moist temperature conditions (15-22oc) through it appear that various found us the tropics have adaptations to higher temperature mostly in the low land areas and can be breed and servive at the 26 oc with sufficient moisture.
The most important intermediate host of the F.gigantatica is the L.natakensis and L.auricuaria [8,13,14]. L.auricuaria which is the also the important spices in the soothern USA the middle east and pacific islands L.natalensis is recognized intermediate hosts for the F.gigantatica [6]. and other species serving as secondary hosts to this species are L.referense and L.acuminate (Indian and Pakistan)and L.rubiginosa (malaysia) [8].
L.natalensis is strictly aquatic snail aquatic snail often found in African it serves as intermediate hosts F. gigantica, requires, requires well-oxygenated and non-pollute water bodies, and can eastivate during dry periods. Optimal temperature requirement for the completion of the parasite development stage with in the snail is 22-26c. However, in irrigates areas snail breeding is less circumscribed and will be continue all year round, except for period of extreme temperature level [8].
Final host
Final host is responsible for the maturation and laying of a huge number of eggs. Host of F.hepatica is era most mammals, cattle and sheep being most important. F. gigantica affects a wide range of domestic animals and is found in lowland areas replacing F.hepatica. In the unusual host such as moons the fluke maybe found in aberrant site such as the lungs [8].
Epidemiology
Fasciolosis is disease caused by liver fluke cause Fascicle hepatica and Fasciola gigantica. These species of parasite are widely distributed in areas where climatic condition are potentialy wet throughout the year n which the existence of parasites are largely dependent on these factor, and also the possible occurrence of their snail interrelate host. Fasciola gigantica predominate since Fasciola hepatica is more localized species. Fasciola gigantica is found where ever ecological conditions are favorable to the intermediate host such as borders of lakes, flood. prone areas, low lying marshes and drainage ditches. It is absent from temporarily pools and water courses that disappear in the dry season [13].
The distribution and type of intermediate host (Lymnea) also vary depending on localities, for instances, lymnae truncatula is highly distributed in Europe Asia, much of Africa and Northern America, while, while L. natalensis is considered to be African snail host [17].
Factors affecting the production of metacercaria
Availability of satiable snail habitat: The most important intermediate host of Fascicle are limner trance flue and L.nalalensis. The wet mud to free water and the adages of small pond. Hoof marks, wheels ruts or rain ponds may provide following heath rainfall or flooding, temporary hanitats. Fields with clumps o rushes are often suspected sites. Though a slight acid PH environment is optimal PH for L.trncantulla, excessive acid PH levels are determinants sach as occur in peat bags and areas of sphagnum moss [18].
Temperature: Temperature is an important factor affecting the rate of development of snails and of the stage of the paralite out side o the final host. A men day or night temperature of 10c or above is necessary both for snails and all activities cease at 50c. This us also minimum range for the development and hatching of F.hepatica eggs. However, it is only when temperature rise to 15c and it maintantied above that level a significance multiplication of snails and fluke larval stage ensures [8].
Moisture: The ideal moisture condition for snail breeding and the development of fascicla hepaticas with in snail are provided when rainfall exceeds transpiration and field saturations attained. Such condition are also essential for the developmental fluke eggs for mirabilis searching for snails and for the dispersal of cicatrices being shed form the snails [8].
PH: Field with clumps o rushes are common size or they have slight PH eggs incubated at 27c will develop and hatch with in a PH range of 4.2 to 9 but above ph 8.0 development is prolong (Rowcclif and ollerenshaw,1960).
Clinical signs
The clinical features of fasciolosis can have acute, sub acate and chronic forms. Acute fasciolosis occurs as disease out break following a massive, but relatively short term intake. Of metacercarial [8]. The high fluke intake is often the result of certain seasonal and fluke control measures. It typically occurs when stocks are forced to graze in druglt. Aminal suffering from acute fasciolos is especially sheep and goat. May display non clinical sign. Prior to death. While some may display no finical sign prior to death while some may display abdominals pain and discomfort and may develop jaundice [13] and fluid may lick in to the perfumed cavity censing death dve to peritonitis may lick in to the poisoned cavity causing death due to peritonitis. More commonly, on ingestion of finer metacercaries, fever and eosinophicliais seen. Death usually results form blood loss due to hemorrhage and fissile destruction caused by migratory juvenile fluked [13].
Sub-acute Fasciolosis is caused by ingestion of a moderate number of metacercariae and is characterized by anemia, jaundice and ill thrift. The migrating fluke causes extensive tissue damages haemorrhage huge and in particular liver damage. The result is severe animas liver failure and death in 89-10 week’s [19].
Chronic Fasciolosis is the most common animal syndrome in cattle. It occurs when the parasite reaches the hepatic bile duct. The principal effects are bile duct obstruction, destruction of liver fishnet, hepatic fibrosis and anemia. The on set clinical sign is slow, animal become gradually anemic and anorexia as the adult flukes become active with I bile duct and sign may include dependent edema or swelling under the jaw(bottle jaw). Affected animals are reluctant to travel. Death eventually occurs when anima becomes severe. Cattle are typically present with sign of weight loss, anemia and chronic diarrhea (Mitchell, 2001).
In addition to these, a condition known as” black disease” is a complication, which usually is fatal. Here, as secondary infection due to the bacterium clostridium novyi type B, proliferating a necrotic lesion for the fatal outcome [15].
Pathogens and pathophysiology
Fanciless varies according to the parasitic development phase. The two developmental phalli are parenchyma and biliary phases. The parenchyma phase occurs doings the emigrational of fluke through the liver parenchyma an is associated with liver damage and hemorrhage. The brilliancy phase coincides with parasite residence in the bile acts and from hematophagic activity of the adult flukes and from the damage to the bile duct mucosa by their cuticles spines [19].
Presence of flukes in the biliary passage elicities considerable tissue reaction, leading to cholangio hepatitis. The wall of the ducts become infiltrated with eosiophilia, lymphocytes, and macrophage, and eventually become significantly and macrophage. And eventually become significantly thickened form fibrons proliferation and calcification (Jones et al., 1996). The reduction of migration and activity of juvenile flukes through the liter parenechyma is also cited with hepatic fibrosis, which in habit intra-parenchyma maturation, and calcified cholangitis. Which deters flukes in their hepatic price activates both of the selection associated phenomena help cattle to resist chronic Fasciolosis (Fraser, et al. 1991) Besides, the fact that liver possesses considerable functions reserve and degenerating capacity help animals to survive without any significant improvement of hepatic functions even until two there of the organ is damaged [20].
Fasciolosis has major effect on blood components (plasma proteins) Hyphoalbumminemia and hyphoglobuinamia commonly occur in liver fluke infection in all host species. During the parenchyma stage of the infections, live damage caused by the migration flukes compromise liver functions, which in sheep and calves is reflected in a decline of plasma Albumin concentration attributed partly to reduce rate o synthesis and partly to an expansion of the plasma volume[19].
Presence of flukes in the biliary passage elicits considerable fissure reaction, leading to cholangiohepatitis. The wall of the ducts become infiltrated with eosin Phillips, lymphocytes, and macrophage, and even tally become significantly thickened from fibrous proliferation and calcification [21]. The reduction of migration and activity of juvenile flukes through the liver parenchyma is associated with hepatic fibrosis, through the liver parenchyma is associated with hepatic fibrosis, which in habit intra-parenchymal migration, and calcified cholangitis, which defers flukes in their hepatophagic activities both of these lesion associated phenomena help cattle to resist chronic fasciolosis (Fraser, et al. 1991). Besides, the fact that liver possesses considerable functional reserve and regenerating capacity help animals to survive without any significant improvement of hepatic functions even until two third of the organ is damaged [20].
Nevertheless, during biliary stage of the infection loss of blood from hematophagia and into intestine is so extensive, causing severe anemia that synthetic capacity of the liver is insufficient to replace the loss of albumin small molecular size) that oozes through the Hyperplastic bile duct (cholangitis). Thus, a progressive loss of plasma albumin occurs in all infection host species, starting from around the time the fluke commences blood feeding. This result in disturbance in intravascular and extravascular encotic pressure leaching to development of [8].
Diagnostic approaches to fasciolosis
Diagnosis of fascicles is both in animal and man may involve considerations of various as pacts such as history, clinical finding and general epidemiology of the disease confirmation in all cases can be made either by faucal examination or recovery of worms at post-mortem examination. Currently serological and molecular techniques are developed by various researchers. Analysis of the enzyme and hematological profiles are also known to give important due as to the presence of Fasciolosis in animals [22].
History and clinical manifestation
Infection with Fasciola hepatica is usually associated with herds and flocks prizing wet, marshy land. F.gigantica uses a water snail as its intermediate host. Therefore infection with this species is associated with livestock drinking from snail infected mattering places well as with grating wet land which may be seasonally in undated [22]. In acute cases of Fasciolosis, sudden death and severe anemia occurs due to the migrating young fluke choughs the inverse, however no fluke eggs are passed in the feces. In sub-acute cases, sign of rapid loss of condition, severe anemia, high fluke egg count, death occurs 12-20 weeks after infection and in chronic Fasciolosis gradual wasting, severe anemia with as cites, bottle jaw edema and very high fluke agog counts may lead to death more than 20 weeks after infestation [13,17,23].
Post mortem examination
The most direct and reliable technique for the diagnosis of fanciless is liver examination at slaughter or necropsy.
In acute Fasciolosis, there may be peritonitis, particularly on the visceral surface of the hepatic capsule. The migration of the fluke in the liver leave dark. Hemorrhagic streaks and foci. The migration of the fluke in the liver dark hemorrhagic streaks and foci. The liver is swollen. Friable and has capsular perforations marked by hemorrhagic [24].
Calcification of the bile ducts and enlargement of the gimbaled are characteristics lesions overfeed in chronic cases of Acidosis. Progressive billiard cirrhosis which ultimately produces a hard fibro tic liver in which the bile duct are prominent, thickened fibrous and in cattle, often calcified. Histological, the fibrosis is produced by repair to the migratory tract and a cholangitis, the bile ducts walls are markedly thickened and the bile duct are dilated containing fluke and numerous eggs [13].
Faeceal examination
Two point needs to be kept in mind while inter prating faecal examinations result for F.hepatica a the pre-patent penned fo fascicle hepatica is 2-3 mantes. As a result, fluke egg cannot be demonstrated early in the infection. A group of cattle could be caring a nigh burden of young, fluke, but no fluke eggs would show up in their manure b the quantitative volume of fluke egg counts is questionable. Fluke egg pool in the gall bladder and intermittently pass in to the feces the fluke egg count on any given day of ten has little relationship to the number of fluke in the liner; an animal with a negative fecal could be parasitized, where as a high fecal fluke egg count could just be a high number of egg leaving the gallbladder that day, rather than a target fluke burden (Briskey, 1998).
Sedimentation procedure concentrates both feces and eggs at the bottom of a liquid medium usually water, and detect most parasite eggs or cysts that have. too high a specific gravity mainly trematode (fluke) egg (Hendrix 1998). Fecal examinations for fluke egg require use of fecal sedimentation, formalin ether or floatation techniques. Fecal examinations for fluke egg requires use feacal sedimentation, formalin- ether, or floatation technique [25-27].
Commonly used floatation procedures open the operculum and sink the fluke egg rather than floating it for surface detection. Fluke egg are comparatively heavier than strangles eggs and a result the egg do not float in routinely used floatation medium even as saturated salt solution . However floatation fluid of higher specific graviity and the saturated zink sollution, magnesium sulphate and potassium is do mercurate has been used to float eggs of Fasciola. Due to the high specific gravity and the saturation of these solution , damage to observed eggs is very high. Several investigators have tried various types of floatation technical but with inconsistent result because of the collapsibility of fluke egg is very high. Several investigators have fried various types o floatation technique but with inconsistent result because of the collapsibility of fluke egg in solutions of high specific gravity. However a modification of sheathes. Sugar floatation technique with a higher specific gravity has been used to demonstrate fluke with little distortion to the egg [28].
Treatment
The order drugs such as carbontetrachloride hexachoorophenc and hexachhorothene are still used in some countries. One of the choice drugs is tridabendazole which remove all developing stages over one week old. Other drugs are rapoxanide, closantel and nitroxynil, which will remove flukes over four weeks old [8]. Outbreak of chronic fasciolosis can be successfully reared with a single dose of any of arrange of dims (rafoxanide, nitroxuynis, borotianide, closeted oxyclozanid and friclaben dazoles). Albendazole one nateobinian are also effective organist adult fmies albeit at increased dosage rate [29]. In castrating comes. Where the milk is used for human consumption the above drug are either banned or have extended with dreamily period in most countries and has milk-with holding time of up to 3 days (Urquhart 1996). Efficacy of triclabendazole is between go and 100% against immature and mature lakes (merck’s, 2000).
Control and prevention of fasciolosis application of strategic treatment
Prophylaxes by duchies consists of elimination flukes by regular treatment. since local climatic conditions influenced infections, they should be considered when determining the time o treatkent it is evident that the control strategies for liver flukes infection vang according to the region and management practices.
Two treatment are recommended per year for the sahel region. The first is given at end of vaing seeson coctobr-NAOVEBER) to eliminate the aduct paralic to so that the animal pas the dryseason in good condition and to avoid contamination from the end o the dry season (march, April or mey rarely later). When the mature flukes migrate through the hepatic paranenyna. For the second treatment only drugs that are active against immature flnkes should be used [30].
Control of snail
Chemical method: The use of mollucscicides for the control of snail cnter mediate hosts is a potential tool for the control o fluke infections. Before considering chemical control of snail, it should be noted that many habitats are topographical unsuitable for the use of mulwcscides and it is often very difficult to apply them effectively. The are toxic to the environment, cooperation between neighboring properties is refuire for effective cover., and regular (at least early) application is required because rapid repopulation of snails may occur [31].
Biological method: Report from several parts of the world indicates that a number of plates have mulluscidal properties. Planting of these tees and shrubs along streams and irrigation channels can reduces the number of snail in a population. The efficacy of this method of snail in a population the efficacy of this method of snail in a population. The efficacy of this method for control of flukes has not yet been assed. The induction of large numbers of ducks in to rise fields after harvest has of ducks in to rise fields after harvest has been used to reduced the snail proportion [31].
Management of snail habitat: Good drainage and the building o dams at appropriate site in marshy and low-eying area may reduce the snail problem. Water hole should be managed wherever possible to prevent both focusing of the water with excrement from infected animals and development of L.natalens. For this purposes all pools or back waters should be filled in and replaced by well or tanks. This is only possible in will units such as ranches or breeding farms. Trough’s near well should be raised and kept clean to keep livestock away from pasture contamination with metacercaria .This may only be possible when the nomber o animal involved is small. Establish proper watering facilities to prevent animals drinking from lakes, ponds and streams [31].
Forecasting the occurrence of the disease: The Geographical Information System (GlS) can be used to define the epidemiology and distribution of Fasciolosis based on climate, geographic and soil hydrology data. The life cycle o cover fluke and the prevalence of Fasciolosis are domination in climate [13].
For casting the occurrence of Faceless by using a Geographic Information System (GIS) for cast model based on moisture and thermal regime was developed to ashes the risk of Fasciolosis Make it and amenable to effective use of GIS control model in several aspects. Such data indeed to develop predictive models, geographic information system and future expert or knowledge based system. This system would we used to advice farmers and small holders on the most appropriate control strategy for protecting their animals [32].
Bovine fascilosis in Ethiopia
Epidemiology: In Ethiopia, Fasciola hepatica is wide spread in areas with altitude o 1200 to 2560 meters above the sea level while, F.gigantica appears to the most common species in areas below 1800 meter above sea level. Both Fasciola species c0-exist in areas with attitude ranging between 1200 to 1800 meter. Above the sea level [6]. Ethiopia is one of the counties smith suitable climatic conditions or the existence of Fasciolosis. With suitable demotic conations or the existence o fascilosis. The disease cause serious problems in civic stock population of the county. Bothe the F. hepatica and F.gigantia are founding Ethiopia an transmitted by the snail called lymnae truncatula and lymnae natalesis respectively. Their pathogenic significance depends on the favorability of environment they live [2].
Public health importance of fasciolosis
A human case of fasciolosis is emerging as an important disease throughout the world. The cases are associated primarily with the eating o watercress’s contaminated with metacercaria. A person must injects the metacercariae in order to become infected (Marsden, and warren, 1984). The global estimate prevalence of is between 2.4 and 17 million human infections, and further, 180 million at risk of infection (Kendal, 1954). The degree of pathogen city of fascicle hepatica to man depends up on many factors, particularly the number of worms present and the organism infected, for example, the presence of Fasciola hepatica in the bile duct of man cause a variety of symptoms like mialaise, intermittent fever, weight loss and anemia Adult facile hepatica can also be found in aberrant sites such as in the lung and subcutaneously. Here, the parasite is found in the system containing bromines purulent materials, they may be removed surgically [16]. the sporadic human infection was also reported in Ethiopia (Yilma, 1985).
Material and methodology
Study area
This study was carrried out at Bedele woreda which is located in 1llu Abba Boral Zone, western oromiya regional stage and it is about 483km west Adiss Abeba on the main road to Gambela. Geographically Bedele town falls between 80 to 26o 800 N latitudes and 360 to 200 970 E longitudes the total land area. Cover 1140.57 square kilometer with an altitude of 1500 to 2300 m.a.s.c. The annual mean tempraturse ranges from 12.5c to 27.sc and the area receives annual rain fall greater than 14oomm, which is biomal (November to March and May to September). The livestock populations of the woreda were estimated to be 59,233 cattle, 40,543 sheep, 9.786 goat, 38,364 poultry and 1,878 equine. The farming system of the area is mixed farming and 87%of the total population is engaged in agriculture livestock population occupies a significant place in the farm Economy. The most important crop. That grow is the area are tef, tiger millet, maize, sorghum, wheat and seaseme. The woreda have 41 kebeles of which 811.,14%. And15% accounts wainadega, kola and dega respectively. The woreda have 8.87% grazing land, 42.8% farm land, 7.2% forest, 0.9% swamp yare a, and 40% hill [33].
Study population
The study included 384 cat the presented to the abettor for slaughter from various place of in and around Bedelle. A cross- Sectional approach was taken and the study conducted during routine meat inspection on arbitrarily selected cattle slaughtered at the abattoir.
Study design
The cross- sectional type of study was designed to be used or he research with the assumption that it could help to get an understanding of the prevalence of bovine Fasciolosis at Bedele maniacal Abattoir from November 2010 up to up to end of march 2011.
Sample size and sampling method
The total number of cattle required for the study will be calculated based on the formula given by Thrusfield [34] simple random sampling method. By rule of thumb where there is no information for an area. It is possible to take 205 or 50% prevalence. In chins study we will take 50% prevalence to calculate the sample size using the following formula.
n= 1.962(pex)(1-pexp) / d2
where n= sample size
p= expected prevalence
d= desired level of precision (5%)
Therefore n= 1.96x20.5 (1.05)= 384 cattle 0.0025
Study methodology
Carpological examination: Before sampling; an identification number was given to each cattle that were randomly selected in the abattoir. Then fecal samples were collected directly from the rectum of each cattle, using disposable plastic gloves and placed in clean universal bottle and each sample was labelled with cattle identification number, age, sex, BCS, date and origin.
Then the samples were preserved with 10% formalin solution. The samples were collected at night, nine hour before slaughtering the animals and the sample taken to Bedelle regional veterinary laboratory; then coproscopic examinations were performed to detect Fasciola eggs using standard sedimentation technique, as described by [35]. Morphological identification of eggs of Fasciola sp was conducted according to (Urquhart, et al. 1996).
Postmortem examinations: Animals, whose samples taken and examined during the ante mortem examination, were further supervised for their livers and bile duct. Careful examination by visualization and palpation of the entire organ, followed by incision along the bile ducts of the lobes, was done. Liver parenchyma and major bile ducts were examined for the presence of immature and adult Fasciola parasites, respectively. Species are identified based on size and morphological characteristics according to Soulsby [13].
Data management and analysis
The data were recorded on specially designed forms and preliminary analysis’ was done in Microsoft EXCEL (2002). The outcome variables were the care of Fasciolosis detected during routing postmortem inspection and feacal examination of Fasciola spp eggs, SPSS software used for analysis.
Results
Out of 384 cattle slaughtered at Bedele municipal abattoir and examined for fasciolosis 24.21%(n=93)were found to be positive for fasciolosis .And out of 93 liver found to contain fluke infection during post mortem inspection ,52(13.54%) harbored F.gigantica, 31(8.85%) F.hepatica and 10(2.6%) has mixed infection. Of the 384 feacal sample collected from the study animals, 74(19.27%) were positive for fasciola egg Table 1.
Prevalence of coprological examination of bovine fasciolosis in and around Bedelle
The higher prevalence of coprological examination between origin of animal revealed in Dabana 20(24.69%) and lower prevalence revealed in Cawaqa 7(15.9%). Among 101examined young cattle 15(14.87%) were positive of fasciola egg and among 283 examined adult cattle 59(20.84%) were positive of fasciola egg. And among 88 examined poor body condition cettle 13(14.77%) and from 296 examined good body condition cettle 61(20.6%) cattle were positive of fasciola egg. The statistical analysis shows that there is no significance difference (p>0.05) in prevalence between age, body condition and origin of animals (Table 2).
Prevalence post mortem liver examination of bovine fasciolosis in and around Bedelle
The higher prevalence of post mortem liver examination revealed in Dabana 23(28.39%) and lower prevalence revealed in Cawaka 9(20.45%). And among 101 examined young cattle 20(19.8%) cattle were positive of fasciolosis and among 283 examined adult year cattle 73(25.79%) cattle were positive of fasciolosis. Among 88 examined poor body condition cattle 20(22.72%) and from 296 examined good body condition cattle 73(24.66%) were positive of fasciolosis. The statistical analysis shows that there is no significance difference (p>0.05) in prevalence between age, body condition and origin of animals (Table 3).
Prevalence of Fasciola hepatica on examined liver in and around Bedelle
The higher prevalence of fasciola hepatica revealed in Dabana 11(13.58%) and lower prevalence revealed in Cawaka 0(0%). And among 101 examined young cattle 7(6.90%) cattle were positive of fasciola hepatica and among 283 examined adult cattle 24(8.4%) cattle were positive of fasciola hepatica. Among 88 examined poor body condition cattle 4(4.54%) and from 296 examined good body condition cattle 27(9.12%) were positive of fasciola hepatica. The statistical analysis shows that there is no significance difference (p>0.05) in prevalence between age and body condition, but there is significance difference(p<0.05) in prevalence between origin of animals (Table 4).
Prevalence of Fasciola gigantica on examined liver in and around Bedelle
The higher prevalence of Fasciola gigantica revealed in Cawaka 9(20.45%) and lower prevalence revealed in Digicha 5(10.86%). And among 101 examined young cattle 13(12.87%) cattle were positive of fasciola gigantic and among 283 examined adult cattle 39(13.78%) cattle were positive of Fasciola gigantica. Among 88 examined poor body condition cattle 12(13.63%) and from 296 examined good body condition cattle 40(13.51%) were positive of Fasciola gigantica. The statistical analysis shows that there is no significance difference (p>0.05) in prevalence between age, body condition and origin of animals (Table 5).
Prevalence of mixed infection on examined liver in and around Bedelle
The higher prevalence of mixed infection revealed in Dabana 3(3.7%) and lower prevalence revealed in Cawaka 0(0%). And among 101 examined young cattle cattle there is no mixed infection and among 283 examined adult cattle 10(3.53%) cattle were positive of mixed infection. Among 88 examined poor body condition cattle 4(4.54%) and from 296 examined good body condition cattle 6(2.02%) were positive of mixed infection The statistical analysis shows that there is no significance difference (p>0.05) in prevalence between age, body condition and origin of animals (Table 6).
Taking the post mortem examination as a gold standard technique for diagnosing Fasciola species infection. The sensitivity and specifity of faecal examination was found to bee 79% and 100% respectively. This study showed that there is no significant association (p>0.05) between the prevalence of age ,body condition and origin of animal, but there is a significance difference(p<0.05) in prevalence of fasciola hepatica, between origin of animals.
Discussion
Fasciolosis is a wide spread health problems and causes significant losses to the livestock industry in Ethiopia The prevalence indicated by faecal examination in present study of Bedelle municipal abattoir (19.27%) is lower than 81.6% recorded for Ambo [10], 34% recorded for Wolliso [7] and 83.38% recorded for Gonder [36] and higher than 4.9% recorded for Soddo [4] and 15.77% recorded for Wollo [37]. The 19.27% prevalence indicated by faecal examination in Bedelle municipal abattor found in this study is comparable with 18.99% recoreded for Nekemt [38].
Similarly the prevalence of post mortem examination of liver in Bedelle municipal abattor (24.21%) reaveled lower prevalence of bovine fasciolosis when compared to the 46.58% recorded for Jimma municipal abattor [39] and 62.2% recorded for Bahir Dar [37] and reaveled higher prevalence when compared to the 14.0% recorded for Soddo municipal abattor [4] and 14.8% recorded for Dire Dawa [40]. The 24.21% prevalence of fasciolosis found by post mortem liver examination in this study is comparable with the 26% recorded for Mekele [5]. The difference in prevalence of fasciolosis may be related with difference in ecological factors available for the snail intermediate host. The occurrence of fasciolosis in area is influenced by a multi factorial system, which comprise host, parasite, and environmental effect. In the natural foci of fascolosis the fasciola, their intermediate host and final hosts for an association posing a potential epidemiological threat [41]. One of the most important factors that influence the occurrence of fasciolosis in area is the availability of a suitable habitat for the vectors [8].
The prevalence and species involved vary significantly with locality. This might be attributed mainly to variation in the climatic and ecological condition such as altitude, rainfall, temperature and livestock management system [6]. In this study the high prevalence rate of Fasciola gigantica may be associated with the existence of favourable ecological biotopes for lymnae natalensis at the origin of animals brought to the abattor for slaughter. The majority of animals brought to the abattor for slaughter were from lowland. In lowland equatorial regions, therefore, aquatic habitats should be safe to graze about 2 months after death of snails but this period will be extended in cooler habitats for up to 6 months. Similarly, metacercariae which become dry on aquatic vegetation as a result of receding water levels or on hay are likely to be no longer infectious after about 5 weeks in lowland tropical areas but may survive up to about 4 months in cooler climates vegetation[16]. Infection of snails in irrigated land with F. gigantica is promoted by the common practice in many tropical countries of using animal faeces as fertilizer. F. gigantica are tropical aquatic snails which thrive in clear stagnant or slow-moving water with high oxygen content and abundant aquatic vegetation [18].
Relatively small proportion of cattle were found to be infected with fasciola hepatica alone or mixed infection with both species. This may be explained by cattle coming for slaughter from lowland and middle altitude zone of the country which are flood prone areas, drainage ditches are not favourable habitat to lymnae trancatula [8]. Lymnaea spp. snails involved in the transmission of F. hepatica are mud-living and amphibious, living in an environmental nichewhich is subject to flooding and desiccation [42]. They are more likely to be found in habitats that are intermittently wet (flush habitat) than in permanently wet sites and in water that is generally slightly acid [43-55].
Significantly high prevalence of fasciola hepatica between origin of animal (p<0.0.5) was analyzed from the data recorded. This may be attributed the existence of permanent suitable ecological condition for snail intermediate host. Areas like slow flowing rivers, streams and low lying marshy area may contribute to persistent but relatively low grade infection during the dry season (p>0.05). The relatively high prevalence observed due to the presence of short rainy period in the area.
The faecal examination shows low prevalence when compared to post mortem examination and this indicates that the less sensitivity of the test in detecting the actual presence of fasciolosis. A longer period from 8-15 weeks after infection is needed for the appearance of fasciola egg in the faeces so most pathological lesion had already occurred. Furthermore, detection of fasciola egg in some cases is difficult during the patent period because egg is expelled intermittently depending on the evacuation of the gallbladder [17].
Conclusion and recommendations
In general it can be concluded that fasciolosis is one of the major problems for livestock development in Ethiopia by inflicting remarkable direct and indirect losses at different part of the country , where its occurrence is closely linked to the presence of biotopes suitable for the development of the snail intermediate host. The presence finding indicates that coprological examination for the parasite egg has significant limitation in detecting exactly the presence or absence of fasciolosis in animals. Although clinical disease can occur as early as 3 weeks post infection, faecal examination by coproscopy can only confirm the diagnosis after several weeks. The present study indicates that bovine fasciolosis is widely distributed disease with low prevalence rate in Bedele town and its surrounding, while Fasciola gigantica was the prevalent fasciola species in the study area.
Based on the above consideration the following recommendations are made:
➢ Coprological examination should be repeated and supported by other diagnostic methods for better diagnostic technique due to intermittent expulsion of fasciola egg and difficulty of detecting early infection.
➢ Avoiding congregation of animals around permanent water source during the period, since this may lead to infection with Fasciola gigantica.
➢ Marshy area should be drained.
➢ The farmer of the area should be well oriented about the hazards of disease to their livestock so that they can actively participate in control program.
➢ Strategic use of antihelminths should be performed to reduce pasture contamination with fluke eggs. Here proper year round study should be conducted so as to elaborate time of the year beneficial to apply antihelminths.
➢ Further information on epidemiology of the disease, ecology and biology of intermediate host snail should be gathered.
- FAO (1993) Agrostat data statistics division, Rome, Italy. Fasciolosis.
- Graber M (1978) Helminth and helminthiasis of domestic and wild animal in Ethiopia. Rev elleven Med Vet paysTrop 1: 13-95.
- Argaw K (1998) Epidemology of bovine fasciolosis in Galama Awraja(Arsi). In: Ethiopia Veteterinary Association Preceding of the 12th Conference,11-12 June, Addis Ababa. 35-42.
- Fufa A, Loma A, Bekele M, Alemayehu R (2009) Bovine fasciolosis: Coprplogical, abattor surveys and its economic impact due to liver condemnation at Soddo municipal abattor, Southern Ethiopia. Trop Anim Health Prod 42: 289-292. Link: http://bit.ly/2MJF82w
- Takele A (1995) Bovine fasciolosis: Prevalence and economic impact at Mekele municipal abattor D.V.M. Thesis, F.V.M., A,A,U Debrezeit. Ethiopia.
- Yilma JM, Malone JB (1998) A geographic information system fore cast model for strategic control of fasciolosis in Ethiopia. Vet Parasitol 78: 103-127. Link: http://bit.ly/2NOINwF
- Rahmato A (1992) Fasciolosis: Clinical occurance, coprological, abattor and snail surveyin and around Walliso. DVM Thesis. FVM, Debrezeit Ethiopia.
- Urquhart GM, Armour JL, Duncan L, Dunn AM, Jening FW (1996) Veterinary parasitology. 2nd ed. Blackwell, London. 103-113.
- Graber M (1975) Mollusques vector dermatodeses humanis et animal en Ethiopia. Review Med Vet Pays Trop 27: 307-322.
- Yadata B (1994) Epidemiology of bovine and ovine fasciolosis and distribution its snail intermediate host inWestern Shoa. DVM Thesis. FVM, AAU Debrezeit, Ethiopia.
- Njau BC, scholtens RG (1991) The role of traditional harvested hay in transmission of bovine fasciolosis in Ethiopia highland. Vet Res Com 15: 369-372. Link: https://bit.ly/3e5mk8X
- Rokni MB, Massoud J, Hanilo A (2003) Comparison of adult somatic and cystine proteinase antigen of fasciola gigantica in enzyme linked Immunosorbent Assay for diagnosing of bovine fasciolosis. Acta Trop 88: 69-75. Link: http://bit.ly/3e8f1xp
- Dunn AM (1978) Veterinary Helminthology.2nd ed .Bulter and Tanner, Ltd. London.
- Soulsby EJL (1982) Helminth, arthropods and protozoa of domesticated animals, seventh edition, Baliare Tindall, London, UK. 40-52. Link: http://bit.ly/2OgMb0r
- Radostits OM, Blood DC, Gay CC (1994) Atext book of disease ocattle, sheep, goat, pigs and horse. Vet Med 8th ed, 1015-1026.
- Andrews SJ (1999) The life cycle of fasciola hepatica. In: Fasciolosis (Dalton,J.P.ed). CABI Publishing, walling ford, UK. Link: http://bit.ly/3bcN0Tm
- Troncy PM (1989) Helminth of and poultry in tropical Africa. In: Fisher (1989) Manual of tropical veterinary parasitology. CAB international UK. 63-73.
- Kendal S (1954) Fasciolosis in Pakistan. Ann Trop Med Parasit 48: 307-313. Link: https://bit.ly/3q72RHD
- Urquhart GM, Armour JD, Duncan JL, Jening FW (1989) Veterinary parasitology. Low priced ed.English language book societ longman, Black well 286.
- Carlton WW, McGavin MD (1995) Spacial veterinary pathology, second edition. University Graphics, Mosby year book. Inc 81-109.
- JonesTC, Hunt RD, King NW (1996) Veterinary pathology, 6th ed,Santa FC, New Mexico Southborough, Massachusetts.
- Payne WJA (1990) An introduction to animal husbandary in tropics, fourth edition. Black Well Science. Oxford. London 47-74. Link: http://bit.ly/386pDJj
- Reineke RK (1983) Veterinary helminthology. Butter works, Durban professional puplishers, PTY. Ltd. 250-258.
- VEIN (2004) Sheep health and production.Post graduate foundation in veterinary science of University of Sydney 508.
- Kaufman J (1996) Parasitic infection of domestic animals, a diagnostic manual. Berlin Germany, Ciba-Geigy 6-8. Link: http://bit.ly/3bXIpDL
- William FJ (1997) Veterinary parasitology reference manual. 4ed. Washington State 5-7.
- Antonia M, Conceicao P, Rute M, Costa IH, da Costa JM (2002) EvolutIon of a simple sedimentation method (modified maccmaster) for diagnosing of bovIne fasciolosis. Veterinary Parasitol 105: 337-343. Link: http://bit.ly/3bbaAAb
- Sloss MW, Kempt RR, Anne MZ (1978) Veterinary clinical parasitology. 5ed 20-21.
- Drug Administration and Control Authority of Ethiopia (2006) Standerd Tretment Guidelines for Veterinary Practice 47-49.
- Fischer MS, Raspsay R (1989) Manual on tropical veterinary parasitology. The technical center of agricultural and rular cooperation (ACT) C.A.B. International 473. Link: https://bit.ly/3rdYonA
- Jorgen H, Brain P (1994) The epidemiology, diagnosis and control of helminth parasite of ruminants. A hand book.Rome: Food and agricultural organization of the United Nation 72.
- Smyth D (1994) Introduction animal parasitology, 3rd ed .Cambrdge University Press. UK. 203-212. Link: http://bit.ly/3qeLrsq
- Bedelle Woreda Agricultural Bureau (2006).
- Briskey DW (1998) Diagnosis of liver fluke infection in cattle. Veterinary Bulletin 68: 1-4.
- Thrushfield M (1995) Veterinary epidemology second edition, University of Edinbergh, Blackwell Science 180-188. Link: https://bit.ly/3bfCtXX
- Hansen J, Perry B (1994) The Epidemiology, Diagnosis, and Control of Helminth
Parasites of Ruminants, International Laboratory for Research on Animal Disease,
Nairobi, 4th edition. Link: http://bit.ly/37D7twT - Mulualem E (1996) Epidemology of bovine fasciolosis in woreda of south Gonder administrative zone Bording Lake Tana. M.Sc.thesis. Faculty of science, AAU, Adis Ababa, Ethiopia.
- Fekadu R (1988) Preliminary survey on bovine fasciolosis around Bahir Dar D.V.M. thesis, Adiss Ababa University, F.V.M, Debrezeit, Ethiopia.
- Wassie M (1995) Prevalence of bovine and ovine fasciolosis:Apreliminary survey in Nekemt and its surrounding area.D.V.M.Thesis. A.A.U. Debrezeit, Ethiopia.
- Tadele T, Worku T (2007) The prevalence and economic significance of bovine fasciolosis ai Jimma abattor, Ethiopia. Internet journal of veterinary medicine 3: 13.
- Daniel F (1995) Economic importance of organs condemination due to Fasciolsis and hydatidosis in cattle and sheep slaughter at Dire Dawa abaattor D.V.M.Thesis,F.V.M. A.A.U. Debrezeit, Ethiopia.
- Maqbool A, Hayat CS, Alchrar T, Hashmi A (2002) Epidimology of fasiolosis in under diffeent managemental condition. Vet Arhiv 72: 221-228. Link: https://bit.ly/2NX2xOv
- Kendall SB, Ollerenshaw CB (1963) The effect of nutrition on the growth of Fasciola hepatica in its snail host. Proc Nutr Soc 22: 41-46. Link: http://bit.ly/3bYralQ
- Ollerenshaw CB (1971) Some observations on the epidemiology of fascioliasis inrelation to the timing of molluscicide applications in the control of the disease. Veterinary Record 88: 152-164. Link: https://bit.ly/3rbMYkr
- Chandler AC (1949) Presice de parasitology. 5th ed. Paris, mason et. cie. 139: 1085.
- Fraser CM, Bergeon JA, Mays A, Aeillo SE (1990) Anemia and fluke infection in ruminants. The merck veterinary manual, seventh edition. Merck and co. Inc. Raahways,N. J., U.S.A 1832.
- Girmay W (1998) Prevalence of fasciolosis in Kallu province D.V.M.Thesis, Adiss Ababa UnIversity, F.V.M, Debrezeit Ethiopia.
- Graber M (1975) Helminth and helminthiasis of domestic and wild animal in Ethiopia. Bulletin of Animal Health Production in Africa 1: 77-91. Link: http://bit.ly/3uS30Sy
- Hendrix CM (1991) Diagnostic veterinary parasitology. 2nd edition. Auburn University Alabama Mosby 45-46.
- Malone JB, Gomes R, Hansen,Yilma JM, Sligenberg J, et al. (1998) A geographic information system on potential distribution and abundance of fasciola hepatica and fasciola gigantica in east Africa based on food and agricultural organization database. Ve parasitol 78: 87-101. Link: http://bit.ly/306XdKX
- Markos T (2002) Effect of Triclabendazole(Fascinex) on acute fasciolosios in sheep in central highland of Ethiopia. Bull Anim Hlth Prod Afr 48: 87-92.
- Mitchel GBB (2003) Treatment and control of liver fluke in sheep and cattle. Technical notes West main road, Edinburgh.
- Rowcliff SA, Ollenshaw CB (1996) Observation on the bionomics of the egg of fasciola hepatica. Ann Trop Med Parasite 54: 172-181. Link: http://bit.ly/384QTI5
- Yilma J (1983) Economic significance of bovine fasciolosis:An assesement trial at Debrezeit governmental abattor 2nd student scientific journal. F.V.M.,A.A.U. Debrezeit, Ethiopia 47.
- Yilma JM, Malone JB (1995) Study on ovine fasciolosis and other helminth parasite at Holleta. F.V.M.,A.A.U. Debrezeit Ethiopia.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
