International Journal of Veterinary Science and Research
Prevalence of camel trypanosomosis and associated risk factors in Arero district, Borena Zone, Southern Ethiopia
Aden Giro and Kula Jilo*
Cite this as
Giro A, Jilo K (2020) Prevalence of camel trypanosomosis and associated risk factors in Arero district, Borena Zone, Southern Ethiopia. Int J Vet Sci Res 6(1): 014-022. DOI: 10.17352/ijvsr.000048A cross-sectional study was conducted to determine the prevalence of camel trypanosomosis and assesses its associated risk factors in Arero district, Borena Zone, Oromia region, southern Ethiopia from November 2015 to March 2016. Blood samples were collected from randomly selected 385 camels. Giemsa-stained blood smears and Buffy coat technique were used for the detection of Trypanosomes infection. Out of 385 examined camels, 41 (10.65%) were positive for Trypanosoma evansi. There was statistically significant difference between age groups and trypanosome infection (P<0.05). Higher prevalence of the infection was recorded in Age group of >4 years (18.12%) followed by 3-4 years (6.98%) and <3 years old camels (4.67%) respectively. Higher infection was found in males (11.69%) as compared to female (10.39%) camels. However, there was no significant (P>0.05) difference in prevalence between sex and area of studies. The prevalence was varied among study localities within the district revealing the highest prevalence in Oroto (15.24%) and the lowest (5.66%) in Didole. A questionnaire survey was done to 50 camel owners to assess knowledge of the community about camel trypanosomosis. All respondents were familiar with the disease, its typical clinical signs and season of occurrence. The result of the current study revealed that camel trypanosmosis was relatively low prevalent in the study area. Thus, there is need of further study with the use of more sensitive diagnostic tests in order to establish effective control measures.
Introduction
Camel (Camelus dromedarius) is one of important livestock which enormously well adapted to hot and arid environments prominently due to its unique anatomical, physiological and behavioral characteristics. It plays an important role in the arid and semi-arid lowlands of eastern and southeastern Ethiopia where nomadic and semi-nomadic pastoral and agro-pastoral production systems predominate [1]. The camels are of great importance socially and culturally as well as economically and thus cornerstone in the social organization of many of the camel-keeping societies [2].
World Camel population is estimated to be around 25.89million spread across 47 countries. About 85% of the camel population inhabits mainly Eastern and Northern Africa and rest in Indian subcontinent and Middle East countries. Somalia has the highest population of 7million followed by Sudan 4.25million [3]. Ethiopian camel population is around 1.16million, out of which, 434,291 inhabits in Afar region, 353,124 in Somali region and 239,357 in Oromia region. The camels kept in Oromia region found in arid and semi-arid lowlands of Borana, West and East Hararge, Bale and Guji zone [4].
Borena pastoralist probably started camel production in early 1560 in the gada period of Abbay Horro Dullacha (14th Abba Gada Borana). The main driving forces behind the increased camel production in the Borena pastoralist have been ecological changes, social conditions (religion, marriage linkage, conflict) and extensive seasonal migration [5]. The camel populations of Borana pastoralist area are 77147 [4]. The dominance of other livestock species over camels perhaps might have masked the potential contributions of these animals to the national and household economy. As a result, the camels have been neglected, or at least their importance underestimated, by the society as such [6].
Currently, due to population growth, increased frequencies of drought recurrence, shrinkage and deterioration of the rangeland by bush encroachment (grazing land for their cattle) together with increasing aridity are the major governing factor for the expansion of camel production. Thus camels have been indispensable alternative to cope up with the escalating rangeland ecological challenges [7]. In spite of these facts, its development is hampered by different constraints. The most important constraints to camel productions are a diseases, poor veterinary service and lack of attention from government [8]. In Borana area the major health problems besides camel trypanosomosis reported in camel are camel pox, pneumonia, contagious skin necrosis and parasitism [9].
Parasitism is one of the major problems in camel production that affect the productivity of livestock [10-12]. Of these parasitic diseases, camel trypanosomosis is the main disease prevalent in most areas where camels are found [13]. In Borena, the disease is well known to the breeders by the local name “Dhukana” and is given the first priority in its order of importance among camel diseases [14].
Camel trypanosomosis is an infectious disease caused by the hemoflagellate protozoan parasite caused by Trypanosoma evansi. It was discovered by Griffith Evans, in 1880 from infected camels and horses in India. The local Indians had a local name for the disease called Surra, meaning emaciated [15]. T. evansi is spread mechanically by Haematophagus flies such as horseflies (Tabanus) and stable flies (Stomoxys) which are endemic in Africa, Asia and South America. Although in America the vampire bat also acts as a vector as well as reservoir hosts [16].
An essential factor in mechanical transmission of trypanosome is interrupted feeding; the flies move quickly from one host to the other to transmit the parasite within a short period before the parasite dies. Trypanosomes do not survive for more than 10-15minutes in the proboscis of the fly. The tsetse fly (Glossina spp.), like other blood sucking flies, can act as a mechanical vector for T. evansi in the areas where they co-exist. However, since camels are usually kept in non-tsetse endemic areas, the role of the tsetse fly in transmission of surra is insignificant [17].
Surra has numerous negative impacts on productivity. It causes anorexia that leads to weakness, emaciation, drop in milk yield and decrease in meat yield, poor traction power, increased abortion and death. This disease is major causes of economic losses in camel rearing areas, causing morbidity of up to 30.0% and mortality of around 30% [13]. Trypanosomosis has a wide host spectrum, the main host species varies with the geographical region. In Africa, camels are the most important host, whilst in Central and South America the horse is principally affected. The disease is most severe in horse, donkey, mules, camels, dogs and cats [18].
In Ethiopia, the prevalence of camel trypanosomosis and its vectors have not yet been fully documented in most parts of the country. A study conducted in southern Ethiopia indicates that trypanosomosis is one of the leading health problems [19] and a prevalence of 21% has been reported in eastern Ethiopia [20]. However, there is no documented information about the prevalence of camel trypanosomosis and associated risk factors in the study area. The prevalence of the disease and associated risk factors is crucial to establish effective control strategy. Therefore the objectives of the present study were:
➢ To determine the prevalence of camel trypanosomosis in the study area
➢ To identify the associated risk factors with disease occurrence in the study areas.
Study methodology
Study area
This study was conducted from November 2015 to March 2016 in Arero district in some selected pastoral association such as Horoto, Bobella, Silala, Didole and Tille. The district of Arero is geographically located 4o45’0’N and 38o49’0’E at a distance of 650km south of Addis Ababa. The area is bordered on the southwest by Dire, on the west by Yabello, on the north by Bule Hora, on the northeast by the Guji Zone, on the east by the Somali Region, and on the south by Moyale. The Dawa River is the only river in this woreda that separates Arero from Odo Shakiso and Liben. Capital city of Arero woreda is Meta Gefersa [21]. The altitude of this woreda ranges from 750 to 1700 m.a.s.l. A survey of the land in this woreda shows that 20% is arable (1.7% was under cultivation), 40.3% pasture, 1.6% forest, and the remaining 38.1% is considered swampy, mountainous or otherwise unusable. The forested area includes Arero State Forest, which covers about 10.65 square kilometers and is the most southerly of the high forests of Ethiopia, one of the few places in the Borena Zone with well-grown trees of Juniperus procera [22].
Arero district was characterized by arid and semi-arid lowlands with some mid-altitude areas. Pastoral and agro-pastoral population whose livelihood is mainly dependent on range livestock production predominantly occupies the semi-arid lowlands. The area rain pattern is bimodal type with the main rainy season (ganna) extending from mid-March to May and the small rainy season (hagayya) from mid-September to mid-November. The other two seasons are the cool dry season (adolessa) extending from June to August and the major dry season (bona) extending from December to February [23]. The climate of Arero is generally tropical climate, with little rainfall throughout the year with the annual rainfall was estimated at 400-700mm. The temperature of area is an average of 18°C and reaching as high as 25°C [22].
Study population
The study population consisted of camels of all age groups and both sex residing in Arero district that were managed under pastoral production systems. All study animals were randomly selected from the population at temporary livestock camps (“Fora”). A total of 5 pastoral Associations (PAs), mainly Oroto, Bobella, Silala, Didole and Tille were included in the study. The PAs were identified on the basis of accessibility to villages by vehicle or proximity to road and camel population and then selected randomly.
Study design
A cross-sectional study design was carried out between November 2015 to March 2016 to determine the prevalence of camel trypanasomosis and assess associated risk factors in the study area. Community Animal Health Workers were used as channel to reach camel owner and camel population in the PAs. Herds were visited and samples were collected early in the morning before camels were released to the field. The collected samples were immediately kept in ice box and transported immediately to the Yabello Regional Veterinary parasitology laboratory (Y.R.V.L) for examination. Camels were selected for blood sample by using simple random sampling method. Also questionnaire was prepared and displayed for randomly selected animal owner from five peasant associations to assess the knowledge, attitudes and practices of camel owners concerning camel trypanosomosis.
Sample size determination
The number of animals to sample was calculated according to Thrusfield [24], considering a minimum expected prevalence of 50%, an accepted error of 5% and a confidence level of 95%, since there was no previous survey conducted in the study area. Therefore, the sample size for this study was determined using the standard formula indicated below
Where; N=Sample size, Pexp=expected prevalence, d=desired absolute precision. Hence, 384 camels of different ages and both sex were randomly selected from herds in this study. The age of the animals were recorded based on information from the owners during sampling. Camels below 3 years of age were considered as calves (ogore), those between 3-4years as young (lamacha) animals, while those above 4 years of age were considered as adults according to the owner of camel.
Study methodology
Blood sample collection: Animals were restrained by the owner and samples were taken early in the morning before the herds were released for grazing. The whole blood samples (5ml) were collected from jugular vein into heparinized vacutainer tubes that contain EDTA. After proper disinfection of the site with 70%, alcohol samples were labeled based on species, identification number, sex, age and village and immediately kept in cooler box and transported immediately to the Y.R.V.L. for examination.
Examination of blood sample: Parasitological examination of blood samples was conducted using Giemsa stained thin blood smears and MHCT (Buffy coat technique) according to Murray, et al., [25].
I. Giemsa stained thin blood smear
The blood smears were achieved directly after blood collection from heparinized vacutainer tube taking the blood by heparinized capillary tube into clean slides after labeling of the slide. Thin smear was made as per method described by [25].
The air-dried smears were fixed in absolute methanol for 3minutes to avoid all possible deteriorations. The fixed blood smears were immersed in upright position in Giemsa stain solution for 30minutes. The stain was then poured off; the slide washed thoroughly in distilled water and allowed to drip-dry in an upright position before microscopic examination. After air drying, the slides were examined under oil immersion objective lens (100x) for detection and identification of trypanosome species based on their morphological characteristics depending on their size, the shape of the posterior end, the size and position of kinetoplast and absence or presence of flagellum according to Murray, et al., [25].
II. Microheamatocrit centrifugation techniques (Buffy coat technique)
The blood was taken from heparinized vacutainer tube into heparinized capillary tube. Then, the tube was sealed and heparinized capillary tube containing blood was centrifuged for 5minutes at 12,000 revolutions per minute. Then the capillary tube was taken from centrifuge. Trypanosomes were usually found in or just above Buffy coat layer. Therefore, capillary tube was cut using a diamond tipped pen 1mm below the Buffy coat to include the upper most layers of the red blood cells and 3mm above to include the plasma. Contents of the capillary tube was then poured onto a clean slide, and mixed and covered with a 22×22mm cover slip. The preparation was then examined using a bright field microscope with the condenser top out and the diaphragm closed under 40×objective and 10×eyepieces. The species were identified based on the characteristic of the morphology of trypanosomes according to Paris, et al., [26].
Questionnaire survey: To study herd prevalence of camel trypanosomosis data collected should contain all rainy season of the year in the study area, since occurrence of disease is epidemiologically linked with rainy season. However, our study period only come across short rainy season of the area called ‘Hagayya’ extending from mid-September to mid-November. To fill this gap of data accessibility a year back designed semi-structured questionnaire were prepared for 50 camel owners and asked to obtain information on the herd size, seasonal occurrence of the disease in the herds and associated risk factors. Camel diseases of importance, trypanosomosis situation and trypanosomosis control measures were also asked.
Data collection and analysis
Questionnaire data were collected from camel herders at the wells and dwelling area of pastoral association and temporary livestock camps (“Fora”). The data collected during sampling and laboratory results were entered in Microsoft Excel spread sheet. Descriptive statistic was used to estimate the prevalence for camel trypanosomosis in the study area. Risk factors such as age, sex and study areas were considered and their difference with infection was analyzed by chi-square. The statistical software SPSS version 20 was used for data analysis.
Results
Overall prevalence
Of the total 385 blood samples collected and examined, 41(10.65%) samples were positive for T. evansi. Of the total camels examined 9(11.69%) males and 32(10.39%) females were positive for camel trypanosomosis, Sex-wise analysis reveals that there was statistically non-significant varietion (P>0.05) (Table 1).
There was significant difference in prevalence of parasite with age (P<0.05) of camels observed. Highest trypanosome infection was recorded in age group of more than 4 years (18.12%) followed by 6.98% and 4.67% in 3-4years and less than 3years of age group respectively (Table 2).
There was no statistically significant variation (P>0.05) in trypanosomosis prevalence among the five PAs of Arero district (Table 3). In this study, T. evansi infection was found in all the five examined PAs of the district. The prevalence was found to be different among camels from different PAs, The highest prevalence 16(15.24%) of the disease was recorded in Oroto followed by Bobella 10(12.05%), silala 7(9.46%) and Tille 5(7.14%). The lowest prevalence 3(5.66%) was at Didole village (Table 3).
Questionnaires survey results
Demographic characteristics of the respondents: Among demographic characteristic sex and number of family member were collected with structured, questionnaire survey during field work. Camel rearing practice was dominated by male (92.0%). Among respondents, 22.0% were having family size of 1-5 persons, 46.0% were having family size of 6-10 persons while 32.0% were having family size of 11 or more persons (Table 4).
Animal production management: Majority of the respondents (56.0%) were having number of camel between 21-40 while, 34.0% of respondents were having camel 41 and above. Only 10.0% of respondents were having camel ‘between’ 1-20 (Table 5).
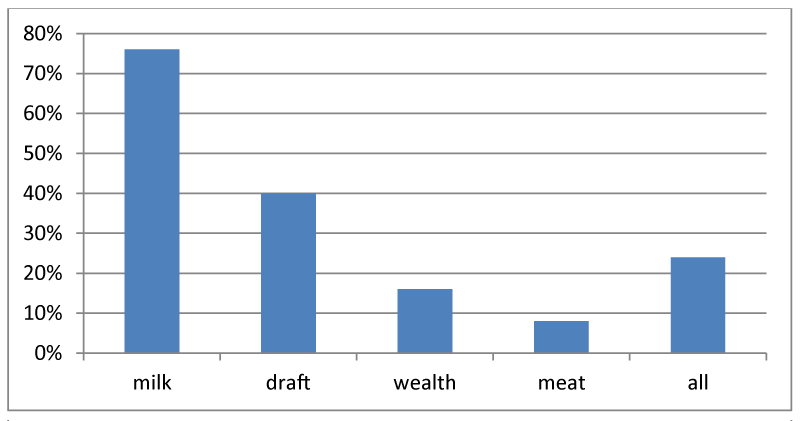
Primary purpose of camel production in study area was mentioned by respondent for Milk production (76.0%) which followed by draft purpose (40.0%), as wealth indicator (16.0%), and meat production (8.0%) while the other (24%) for all purpose ((Figure 1).
Feed and water shortage was reported as a problem in camel production particularly in the dry season (80.4%), Widespread of diseases(81.32%), poor veterinary service and conflict (2.2%) are the major constraint to camel production in the area.
Householders awareness on camel trypanosomosis: A 100% of respondents mentioned that they knew camel trypanosomosis, which they call “Dhukkaana” in their vernacular name. They mentioned that camel contract the disease when moved to other place for long distance and get mixed with camels from neighboring herds. The herder added that congregation of camel herds around water and in pasture into close proximity facilitated efficient transmission of the disease by the biting flies. Further, on the assessment of the route of migration, it claimed that it depend on season as the camel prefers the dry ecology instead of wet ecology. So, their movement depends on dry season (“Bona and Adolessa”) and wet seasons (“Hagayya and Ganna”).
Majority of respondent (70.0%) said that camel more than 4 years of age were affected while 36.0% said camel below 3 years of age and 32.0% respondents said camel between 3-4years of age were affected. Clinical signs were also responded as emaciation (80.0%), hair loss (78.0%), abortion (42.0%), milk reduction (26.0%), inapentance (24.0%) urine odour (10.0%) weakness (8.0%) and dullness (6.0%) (Table 6).
The majority (80%) of respondent stated that the disease occurs at onset of major rainy season (“Ganna”) extending from March to May some (46%) respondents said this disease may occur in short rainy season (hagayya). The responder added that they relate between seasonal outbreaks of camel trypanosomosis and increase in number of insect flies responsible for disease transmission during the rainy season. However, 20% argued as the disease happen mostly at the onset of warmer dry season (“Bona”) from December to February of the year. These respondents mention the reason for the occurrence of the disease during the dry season, because camels are usually in stress suffer from low plane of nutrition and trek for long distances in search of water and pasture (Table 7).
(12.0%) of respondents mentioned that not having access to drugs in the study area to control the occurrence of the disease in their herds. majority (86%) of them they buy drug from market and administer by themselves. (22.2%) of the herder use the traditional remedy, application of a concoction of herbs, mixed with soup and the branding of edematous areas on sick animals using hot metal. (30%) of herders mentioned that veterinary health post was very far away from their kebele and this situation forced them to look for other options like illegal drug trade (contraband) (Figure 2).
Discussion
The overall prevalence of camel trypanosomosis in the study area was found to be 10.65% using parasitological examination. This might be associated with the season of the study period and sensitivity of the diagnostic techniques used. The overall prevalence of 10.65% recorded in the current study interestingly, in agreement with the investigations made by Getahun [9], Megarsa, B. [27], Tekle and Abebe [14], who recorded 10.2%, 10.5% and 10.9% of prevalence of camel trypanosomosis in, Liban district, Borena low land, Borana southern Ethiopia respectively. This might be due to the same agro-ecological condition of the study area. The current finding was in consistent with that reported by Hagos, et al., [28], who obtained 12.1% prevalence of camel Trypanosomosis by using parasitological examination in dry and wet areas of Bale Zone. From abroad country of Pakistan and Saudi Arabia the current results are parallel with the investigations made by Bhutto, et al., [29], Shah, et al., [30] and Hussain, et al., [31], who reported 11.25%, 10.9% and 13.2% prevalence of camel trypanosomosis respectively.
The present result was higher compared with the investigations made by Kassa, et al., [32], Tadesse, et al., [33], Eshetu, et al., [34] and Tayib, et al., [35], who reported 4.4%, 3.5%, 6.5% and 8.1%, prevalence in Fentale district South East Shoa Zone, Jijiga Administrative Zone of the Ethiopian Somali Region, Jijiga Administrative Zone of the Ethiopian Somali Region and in Babile district, eastern Hararghe zone, Oromia regional state, eastern Ethiopia respectively. This may be due to type of test used, appropriate technique used during sampling and appropriate procedure used. This result was higher compared with report in the Punjab region of Pakistan in camels [36], who reported the prevalence of 3.3% and 4% from parasitological and serological examinations, respectively.
However, the present finding was lower than the previous study of Abera, et al., [37] and Bogale, et al., [18], who reported 17.9% and 18.22% prevalence at Sawena district and Delo Mena District of Bale Zone, Oromia Region, Southwest Ethiopia, respectively. The prevalence in the current study was also lower than the findings by previous workers who reported a prevalence of 21% in eastern Ethiopia [20], 20% in Dire Dawa, southeastern Ethiopia [37], 28% in Kenya [38], 29% in Niger [39] and 33% in Sudan [40]. The possible explanation for the lower prevalence rate detected in this study might be related to distribution, challenge and density of parasite vector is low. The current study was also conducted during the short rainy season where the distribution of biting flies is very low in this season as their reproduction is high during major rainy season.
In general this variation in prevalence of camel trypanosomosis might be due to difference in agro-ecology of the study areas, management system, production system, population density, different sensitivity of different test methods used and new techniques such as PCR. In addition, seasons of the year when the studies were conducted which have a direct effect on the distribution of biting flies responsible for the mechanical transmission of T. evansi also cause variation in prevalence [32].
In this study, a significantly (P<0.05) higher prevalence of camel trypanosomosis was noted in adult camels (18.12%), followed by young ones (6.98%) and calf less than 3 years old (4.67%). This result agrees with a previous report from Jijiga, Somali Region, Ethiopian, who reported that in adult camels above 4 years old had a significantly higher prevalence of infection as compared to the young ones below 4years old [34]. The current result is in line with that of Abera, et al., [41], in bale zone in which higher infection rate was recorded in camel above 4 years. Similar suggestion was also given in the study in Saudi Arabia who reported that the younger were less susceptible to infection than adults [42].
The higher prevalence in old camels might be due to their long distance movement in search of water and pasture as a result they were more exposed to biting flies and many stress impose them more susceptible to the infection. This idea in line with Bogale, et al., [18], who reported the higher prevalence in old camels might be due to; heavy stress through their use for transportation of goods from one place to another and poor management.
This result in close agreement with the report of Atarhouch, et al., [43], who showed that the infection rate of camel trypanosomes is increased with age up to maximum in the 7-10years old age. However, this result is reverse to the observations of Lemecha, et al., [44], who reported a higher prevalence in young compared to adults by using standard parasitological techniques. The present study was contradicted with that of Pathak and Khanna [45], who reported that all camels were equally susceptible to trypanosome infection regardless of breed and age.
The present study shows that calves were less infected than the other age group. The possible reason why calves were less infected than other age groups might be due to; Owners keep them in the residence area and they do not go to distant areas in searching of water and pasture where the fly burden is high. Young animals are also bitten less frequently than older ones due to the greater defensive behavior they exhibit making it hard for biting flies to feed readily on the former [46]. A previous report from Fantale district southeast Showa zone, Ethiopia is also in agreement with the current finding that young calves below one year of age were less infected than other age groups or in some case free of T. evansi infection [32]. One study have demonstrated a greater sensitivity to infection in calves and once infected, poor capacity to control subsequent parasitaemia leading to their rapid mortality hence removing them from the pool of positive animals used in the calculation of prevalence rates [47,48]. This phenomenon might also explain the low prevalence observed in this age category.
In the present study, there was no statistically significant difference observed between sex and the disease (P>0.05). This might be due to all camels were equally susceptible to trypanosome infection regardless of breed and sex [45]. However, the highest prevalence of the disease was recorded in male (11.69%) than female (10.39%). This might be due to the fact that male camel were used for work all the time and travel from one place to another place to provide transportation service more than female camels, so that they have a higher probability of acquiring an infection. Frequent travel could also compromise their immune response to infection due to the stress of fatigue.
The present study is in agreement with the finding of Abera, et al., [41], who recorded higher infection rate in male (20.3%) than female (17.3%). Similarly, Bogale, et al., [18], in Delo Mena District, Bale zone also reported on sex related differences in prevalence of camel trypanosomes, that a higher infection was found in males (20.25%) as compared to females (17.72%). However, other studies in Asia have reported sex related differences in prevalence in camels [30], where females (15.68%) were observed to be more susceptible to the disease than males (11.76%) counterparts. The current study was also contradicted with report from the same continent, Pakistan who reported a higher infection was found in females (15.79%) as compared to males (9.84%). This record might be due to stress during pregnancy and lactation, which could decrease resistance in female camel and render them more susceptible to infection [29].
In the present study, there was no statistically significant difference observed between the prevalence of camel trypanosomes infection among the PAs of district (P>0.05). The prevalence was found to be different among different sites of district; the highest prevalence of the disease was observed in Oroto 16(15.24%), followed by Bobella 10(12.05%) and Silala 7(9.46%) whereas the lowest was recorded in Tille 5(7.14%) and Didole 3(5.66%) during the study period. This might be due to the difference in management system, vector density, poor veterinary services, ecological difference, and lack of awareness of the animal owners about the disease.
The majority of Arero district householder possesses 30.5 camels with range of 21-40heads of camels. The average herd size recorded in this studyis equivalent to Getahun and Kassa that reported from Shinile 22.7 and Jijiga 35.2 in eastern Ethiopia. Recent starting of camel production may create gap in the herd and health management. In line with this, Megarsa, et al., [49], stated that late comers into camel business, such as Borana and Guji have less experience with dromedaries and acquired less adequate traditional knowledge and difference in camel herding strategies that has already been demonstrated to influence some production parameters may also result in variations in disease occurrence.
Milk production was mentioned by respondent to be the primary purpose of camel production in the area followed by draft purpose, as wealth indicator and meat production. Magarsa, B. [27], also reported Pastoral communities of Borena lowland produce camels primarily for milk production followed by transport, cash income by sale and meat production. Similarly, Mehari, et al., [50], have described the potentials camel production in Eastern Ethiopia including provision of food, transportation and draught power.
It was evident from this study that all of the herders are aware of camel trypanosomosis which they call ‘Dhukkana’ in their vernacular. They described the disease accurately and ranked it as a disease of first priority in camels. This shows the camel trypanosomosis is very important disease in the area from the past to nowadays and continues to pose a significant impact on the livelihood of pastoral communities. All of the herder deeply explain the possible further transmission factors of the disease. All of the interviewed herders knew the role of insects (biting flies) as mechanical transmitters of trypanosomes which are abundant during rainy seasons. In addition, the informants were able to link increased cases of infection to a build-up of the biting fly population. It is known that biting flies play an important role in the transmission mechanisms of camel trypanosomosis [51,52].
The herders mentioned that camel contract the disease when travel to other place for long distance and get mixed with camels from neighboring herds by the biting flies. Van den Bossche and Vale [53], support this idea who reported that management factor and movement patterns of animals may lead to increased risk of developing infection. The herders added that congregation of camel herds around water and in pasture into close proximity facilitated efficient transmission of the disease by the biting fly. This idea is supported by Luckins, [54], who reported that local epidemics of infection occur where conditions exist for the spread of infection with T. evansi, such as when many animals are stabled together or closes herded and particularly when the biting fly population is abundant during the wet season. During this study, all herders were able to mention two or more of the typical clinical signs and symptoms of camel trypanosomosis. The herders recognized camel trypanosomosis through signs of emaciation (80%), abortion (42%), milk reduction (26%), innapetance (24%), urine odour (10%), weakness (8%) and dullness (6.0%). Bogale, et al., [18], who reported that camel trypanosomosis causes anorexia, weakness and emaciation that lead to low milk and meat yield, poor traction power, increased abortion and death, gave similar suggestion. In another study by Catley, et al., [55], report loss of tail hair; was also mentioned by herders (78%) as a sign of camel trypanosomosis.
With regard to their routes of movement, they mentioned that the majority of migrations of owners and their animals are seasonally to southern ward and northern ward of the district. They move to Southern ward of district during dry seasons (“Bonna and Adolessa”) and Northern ward during wet seasons (“Haggaya and Ganna”). The reasons for that might be lack of browsing, water and biting insects in certain season and area according to the owners of camels. This movement put their animal on extra risk of contracting the disease from another district. This idea is in line with report of Magarsa, B. [27], Seasonal herd mobility was observed particularly during the dry season for foraging and watering purposes, and to some extent during wet season to avoid disease occurrences. Trypanosomosis was frequently diagnosis in herds that has been moved to other locations. Macpherson, et al., [56], also reported the transhumance by seasonal movement of livestock has profound effect on the epidemiology and spread of this disease in Africa.
With regard to the temporal occurrence of the disease, majority of the respondents (80%) mentioned that the disease mainly occur in major rainy season (“Ganna”) this idea in line with report by Gruvels and Beil [57,58], who reported seasonal incidence of disease and Outbreak in chad usually occur during rainy season. The herder added that they relate between seasonal outbreaks of infection and increase in number of insect flies responsible for disease transmission during the rainy season. This in line to Surveys in various tropical areas have shown a definite correlation between seasonal outbreaks of T. evansi infection and increase in number of flies responsible for disease transmission during the rainy season [13]. A small number of the herders reported occurrence of disease during dry season (“Bona”) of the area. According to this pastoralists, occurrence of the disease during the dry season was due to camels are usually in a low plane of nutrition and trek for long distances in search of water and pasture. This is likely to stress the animals and subsequently trigger clinical signs of trypanosomes in animals. Evans, et al., [50], have also reported a high prevalence of camel trypanosomosis occurring in the dry season in semi-arid rangelands in Kenya.
Majority (86%) of respondent mentioned they buy drug from market and administer by themselves. (12.0%) of respondents mentioned that not having access to drugs in the study area to control the occurrence of the disease in their herds. (22.2%) of the herder use the traditional remedy, application of a concoction of herbs, mixed with soup and the branding of edematous areas on sick animals using hot metal. (30%) of herders mentioned that veterinary health post was very far away from their PAs and this situation forced them to look for other options like illegal drug trade (contraband). Magarsa, B. [27], also reported in Borana pastoralist area Health care is mainly practiced by herders (self-treatment) using drugs from open market and private veterinary drug shops. They utilize relatively limited veterinary service or consult nearby experienced traditional healers.
Conclusion and recommendation
The present study results seem to indicate that T. evansi infection was prevalent in the study area. The disease causes a significant impact on the camel production in the study area by affecting health and productivity of camels. In Ethiopia, camel husbandry is the main source of living for millions of pastoralists in the arid and semi-arid zones of Ethiopia. Camel owners of the study area were familiar with the disease, associated clinical signs, season of occurrence and effect on production due to disease. The most important concern to camel production is widespread diseases, poor veterinary service and lack of availability of drug. In line with the above conclusion, the following recommendations are forwarded:
➢ Effective prevention and control measures should be designed against the parasite and their vectors to minimize the disease.
➢ Attempt should be made to expand government veterinary services to serve the community in the study areas.
➢ Further studies should be conducted involving different seasons of the year along with the use of more sensitive diagnostic tests in order to establish effective control measures.
- Getahun T, Belay K (2002) Camel husbandry practices in eastern Ethiopia: The case of Jijiga and Shinile zones. Nomadic Peoples 6: 158-179. Link: http://bit.ly/3b8uytg
- Wako DD, Younan M, Bauman MPO, Gluecks IV, Tessema TS (2012) Sociocultural importance of camels among the pastoralists of Northern Kenya. Abstract; Proc. 3rd Int. Conf. Isocard, 29th. 2012. Muscat (Sultanate of Oman) 182-184.
- Food and Agriculture Organization (2011) FAOSTAT 2011. Link: http://bit.ly/3bbxrJB
- Central Statistical Agency (CSA, 2014/15) Agricultural sample survey 2014/15(2007 E.C) Volume II report on livestock and livestock characteristics (private peasant holdings). Addis Ababa statistical bulletin statistical bulletin 578. Link: http://bit.ly/2OrpE0D
- Biffa D, Chaka H (2002) Camel and the changing system of Borena pastoral production. In: Proceeding of the Annual Conference of the Ethiopian Veterinary Association (EVA). June 2002, Addis Ababa, Ethiopia. 72-87. Link: http://bit.ly/2UnouqF
- Ministry of Information (MOI) (2005) Export products of Ethiopia. Press release of Ministry of Information, Department of press and audiovisual. Addis Ababa, Ethiopia 1-5.
- Ministry of Agriculture (MOA) (2013) Draft document on the establishment of Disease-free zones, Ministry of Agriculture, Ethiopia, 23.
- Dirie MF, Wallbank KR, Adem AA, Bronstein S, Ibrahim MD (2003) Camel Trypanosomiasis and it vectors in Somalia. Veterinary Parasitology 32: 285-291. Link: http://bit.ly/2SfU7zT
- Getahun D (1998) The prevalence of camel trypanosomosis and factors associated with the disease occurrence in Leben district Borena zone Oromiya Region, Ethiopia. MSc Thesis, Addis Ababa University Faculty of Veterinary Medicine and Freie Universitat Berlin Faculty of Veterinary Medicine.
- Dabasa G, Zewdei W, Shanko T, Jilo K, Gurmesa G, et al. (2017) Composition prevalence and abundance of Ixodid cattle ticks at Ethio-Kenyan Border, Dillo district of Borana Zone, Southern Ethiopia. J Vet Med Anim Health 9: 204-212. Link: http://bit.ly/36Tdh40
- Dabasa G, Shanko T, Zewdei W, Jilo K, Gurmesa G, et al. (2017) Prevalence of small ruminant gastrointestinal parasites infections and associated risk factors in selected districts of Bale zone, south eastern Ethiopia. J Parasitol Vector Biol 9: 81-88. Link: http://bit.ly/36YCHNs
- Mata W, Galgalo W, Jilo K (2018) Prevalence of the major ectoparasites of poultry in extensive and intensive farms in Jimma, southwestern Ethiopia. J Parasitol Vector Biol 10: 87-96. Link: http://bit.ly/2OkHDpB
- Njiru ZK, Bett O, Ole-Mapeny IM, Githiori J, Ndung’u JM (2002) Trypanosomosis and helminthiasis in camels: comparison of ranch and traditional camel management systems in Kenya. J Camel Res Prac 55: 67-71. Link: http://bit.ly/2H4fz5X
- Tekle T, Abebe G (2001) Trypanosomosis and Helminthiasis: Major Health Problems of Camels (Camelus dromedaries) in the Southern Rangelands of Borena, Ethiopia. J Camel Prac Res 8: 39-42. Link: http://bit.ly/31pFKNO
- Al-Rawashdeh O, Sharif LA, Al-Qudah KM, Al-Ani FK (2000) Trypanosoma evansi infection in camels in Jordan. Rev Elev Med Vet Pays Trop 20: 233. Link: http://bit.ly/31nrrcp
- Felicia NCE, Anthony KBS (2005) Camel trypanosomosis - a review. Veterinarski Arhive 75: 439-452. Link: http://bit.ly/2Or0NKl
- Brun R, Hecker H, Lun ZR (1998) Trypanosoma evansi and Trypanosoma equiperdum distribution, biology, treatment and phylogenetic relationship (a review). Vet Parasitology 79: 95-107. Link: http://bit.ly/2Scir5I
- Bogale B, Kelemework F, Chanie M (2012) Trypanosmosis in Camel (Camelus dromedaries) in Delo-Mena District, Bale Zone, Oromia Region, Southwest Ethiopia. Acta Parasitologica Globalis 3: 12-15. Link: http://bit.ly/2Sh8hAU
- Tefera M, Gebreab F (2004) A study on the productivity and diseases of camels in Eastern Ethiopia. Trop Anim Health Prod 33: 265-274. Link: http://bit.ly/2GOqnVB
- Zeleke M, Bekele T (2001) Effect of season on the productivity of camels (Camelus dromedarius) and the prevalence of their major parasites in eastern Ethiopia. Trop Anim Health Prod 2: 321-329. Link: http://bit.ly/2GTYKuk
- Central Statistical Agency (CSA) 1994 Population and Housing Census of Ethiopia: Results for Oromia Region 1.
- Central Statistical Agency (CSA) 2007 Population and Housing Census of Ethiopia: Results for Oromia Region 1.
- Coppock DL (1994) The Borana Plateau of Southern Ethiopia: Synthesis of Pastoral Research, Development and Change, Addis Ababa, Ethiopia 1980-1991. Link: http://bit.ly/3bbzJZd
- Thrusfield M (2005) Veterinary Epidemiology. 3rd ed. Black well Science, Oxford 233.
- Murray M, Morrison WI, Murray PK, Clifford DJ, Trail JCM (1979) Trypanotolerance a review. World Animal Review 31: 2-12. Link: http://bit.ly/2OpDGzW
- Paris J, Murray M, Mcodimba F (1982) A comparative evaluation of the parasitological techniques currently available for the diagnosis of African trypanosomiasis in cattle. Acta trop 39: 307-317. Link: http://bit.ly/2RWrlpa
- Megersa B (2010) An epidemiological study of major camel diseases in the Borana lowland, Southern Ethiopia. DCG Report No 58. Link: http://bit.ly/2S9zOny
- Hagos AA, Yilkal A, Esayass T, Alemu T, Fikru GR. et al. (2009) Parasitological and serological survey on trypanosomosis (surra) in Camels in dry and wet areas of Bale Zone, Oromyia Region Ethiopia. Revue Méd Vét 160: 569-573. Link: http://bit.ly/396wtws
- Bhutto B, Gadahi JA, Shahl G, Dewani P, Arijo AG (2010) Field investigation on the prevalence of trypanosomiasis in camels in relation to sex, Age, Breed and Herd size. Pakistan Vet J 30: 175-177. Link: http://bit.ly/36UAQtg
- Shah S, Phulan M, Memon M, Rind R, Bhatti W (2004) Trypanosome infection in Camels. Pakistan Vet J 24: 209-210. Link: http://bit.ly/2RUsgWU
- Hussein H, Al-Asgah N, Al-Khalifa M, Diab F (1991) The blood parasites of indigenous livestock in Saudi Arabia. Arab-Gulf Journal Science Research 9: 143-160. Link: http://bit.ly/31lyp1F
- Kassa T, Eguale T, Chaka H (2011) Prevalence of camel trypanosomosis and its vectors in Fentale district. South East Shoa Zone. Ethiopia Vet Arhive 81: 611-621. Link: http://bit.ly/37Xrs9u
- Tadesse A, Omar A, Aragaw K, Mekbib B, Sheferaw D (2012) A Study on Camel Trypanosomosis in Jijiga Zone. J Vet Advanc 2: 216-219. Link: http://bit.ly/370lmDL
- Eshetu Z, Desta B, Amare L (2013) Prevalence of Trypanosoma evansi Infection in the One-Humped Camel (Camelus dromedarius) in Jijiga Administrative Zone of the Ethiopian Somali Region Global Veterinaria 10: 233-238. Link: http://bit.ly/2OqqACG
- Tayib M, Mezene W, Tadesse B, Adem H (2015) Cross Sectional Study of Camel Trypanosomosis in Babile District, Eastern Hararghe Zone, Oromia Regional State, Eastern Ethiopia. Academic J Animal Dis 4: 112-117. Link: http://bit.ly/2RVBFNV
- Hassan M, Muhammad G, Gutiérrez C, Iqbal Z, Shakoor A (2006) Prevalence of T. evansi infection in equines and camels in the Punjab Region, Pakistan. Ann. New York Acad Sci 1081: 322-324. Link: http://bit.ly/370kC1r
- Mohammed R (1999) Camel trypanosomosis prevalence and drug sensitivity test in Dire-Dawa administrative region, Southern Ethiopia. DVM thesis, Addis Ababa University Faculty of Veterinary Medicine, Debre-Zeit, Ethiopia.
- Njiru Z, Ole-Mapeny I, Ouma J, Ndung’u J, Olaho-Mukani W (2001) Prevalence of trypanosomosis in camel calves: a pilot study in Laikipia District of Kenya. Rev Elev Med Vet PaysTrop 34: 183-186.
- Pacholek X, Gamatic D, Franek SG, Tibayrene R (2001) Prevalence of T. evansi trypanosomosis in young camels in west Niger. Revue D'elevage et de Medecine Veterinaire des Pays Tropicaux 44: 177-182.
- Elamin EA, El-Bashir MOA, Saheed EMA (1999) Prevalence and infectionpattern of T. evansi in camels in mid-eastern Sudan. Trop Anim Hlth Prod 30: 107-114. Link: http://bit.ly/37VCjR7
- Abera D, Birhanu T, Tajudin B (2014) Prevalence of Camel Trypanosomosis at Selected Districts of Bale Zone Southern Ethiopia. Sci Technol Arts Res J 3: 103-106. Link: http://bit.ly/2SkwA0E
- Mohamed E, Bernard F (2013) Surveillance of Camel Trypanosomosis in Al-Jouf region, Saudi Arabia. Camel Int J Vet Sci 1: 65-74. Link: http://bit.ly/31vFkW8
- Atarhouch T, Rami M, Bendahman M, Dakkak A (2003) Camel trypanosomiasis in Morocco1: Result of a first epidemiological survey. Vet Parasitol 111: 277-286. Link: http://bit.ly/2ShpNoE
- Lemecha H, Lidetu D, Hussein I (2008) Prevalence and distribution of camel Trypanosomosis in the semi-arid and arid Awash Valley. J Anim Prod 8: 1-9.
- Pathak KML, Khanna ND (1995) Trypanosomiasis in camel (Camelus dromedarius) with particular reference to Indian sub-continent A Review. Int J Animal Sci 10: 157-162. Link: http://bit.ly/37Urz5K
- Kelly DW (2001) Why are some people bitten more than others? Trends Parasitol 17: 578-581. Link: http://bit.ly/2Uj2yNA
- Pathak K, Arora K, Kapoor M (1993) Camel Trypanosomosis in Rajasthan India. Vet Parasitolo 49: 319-323. Link: http://bit.ly/2u7WXPC
- Richard D (1979) The disease of the Dromedary (Camelus dromedaries) in Ethiopia. Ethiop Vet Bull 2.
- Megersa B, Regassa A, Kumsa B, Abunna F (2008) Performance of camels (Camelus dromedrius) kept by pastoralists with different degree of experience in camel keeping in Borana, Southern Ethiopia. Animal Science Journal 79: 534-548. Link: http://bit.ly/2OkXK6z
- Mehari Y, Mekuriaw Z, Gebru G (2007) Potentials of camel production in Babilie and Kebribeyah woredas of the Jijiga Zone, Somali Region, Ethiopia. Livestock Research for Rural Development 19: 1-11. Link: http://bit.ly/2GPEpGo
- Evans JO, Simpkin SP, Aitkins DJ (1995) Camel keeping in Kenya. Ministry of Agriculture. Livestock Development and Marketing.
- Food and Agricultural Organization of the United Nations (FAO) (2000) A field guide for the diagnosis, treatment and prevention of African animal trypanosomosis. 2nd edition. FAO, Rome, Italy.
- Van den Bossche P, Vale GA (2000) Tsetse and trypanosomosis in Southern Africa: Bovine Trypanosomosis in Southern Africa. RTTCP, Zimbabwe 2: 140. Link:
- Luckins AG (1998) Trypanosoma evansi in Asia. Parasitology Today 4: 137-142. Link: http://bit.ly/31sxVqC
- Catley A, Osman J, Mawien C, Jones BA, Leyland TJ (2002) Participatory analysis of seasonal incidences of diseases of cattle, disease vectors and rainfall in Southern Sudan. Prev Vet Med 53: 275-284. Link: http://bit.ly/2GOBJcd
- Macpherson CNL (1995) The effect of transhumance on the epidemiology of animal diseases. Prev Vet Med 25: 213-224. Link: http://bit.ly/2OrewAR
- Gruvels J, Bail J (1965) Seasonal incidence of disease in chad during rainy season. Revue Méd Vét de tropicaus 18: 435-439.
- Tekele T, Abebe W (2001) Trypanosomosis and helminthiasis: major health problems of camels (Camelus dromedaries) in the Southern rangelands of Borena. J Camel Pract Res 8: 180-181. Link: http://bit.ly/31pFKNO
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
