International Journal of Veterinary Science and Research
Splenic Hemangiosarcoma in a 10-year male German Shepherd dog: Case Report
Wyckliff Ngetich1*, Abuom Tequiero Okumu2, Jafred M Kitaa2 and Evalyne Chepkirui3
2University of Nairobi, Nairobi, Kenya
3University of Nairobi, Department of Veterinary medicine, Nairobi, Kenya
Cite this as
Ngetich W, Okumu AT, Kitaa JM, Chepkirui E (2017) Splenic Hemangiosarcoma in a 10-year male German Shepherd dog: Case Report. Int J Vet Sci Res 3(2): 078-081. DOI: 10.17352/ijvsr.000026Hemangiosarcoma is a malignant tumor of endothelial cells and mostly seen in dogs than in other species. A 10-year male German shepherd dog (GSD) was presented to the small animal clinic with history of swollen abdomen and weakness. The dog was conscious but lethargic, weak, tachycardic with muffled heart sounds, labored breathing and tensed swollen abdomen. Radiography, Ultrasonography and a hemogram and biochemistry indicated splenic mass, internal hemorrhage and hemostasis pathology. The dog was euthanized and on post mortem examination, diagnosis of splenic hemangiosarcoma was made. The current advances in screening tests for diagnosis and treatment options have increase the life span of affected animals.
Introduction
Hemangiosarcoma (HSA) is a malignant tumor of endothelia cells that can occur anywhere in the body [1]. The tumor is more common in dogs as compared to other species, accounting for 5-7% canine non-cutaneous primary malignant neoplasias [2,3]. The three most commonly predilection sites are the spleen (28– 50%), right atrium and auricle (3–50%), and 13% in the subcutaneous tissue [1]. The liver, kidney, lung, vertebral body, and central nervous system are the other primary sites [4-6].
The etiology is not known though hereditary or familial predisposition has been suggested [7]. The disruption of biochemical pathways governing angiogenesis play an important role in the development of HSA and this suggests autocrine stimulation of receptors leading to dysregulated proliferation of HSA cells and their survival [3].
Metastases as well as local infiltration of HSA cells have been documented to occur early in disease [1]. Males and neutered females are more commonly affected with atrial HSA with the mean age occurrence of 8-13 years [8]. Splenic tumors are more common in large breeds; German shepherd, Golden Retrievers, Great Danes, English Setters, Boxers and Labradors [4] while cutaneous forms are found in light-coated, sparsely haired dogs like Beagles, Bloodhound, Dalmatians and Whippets among others [1,5]. Cutaneous forms are commonly seen on the ventral abdomen and the region of the prepuce where the hairs are few [9]. Dermal haemangiosarcoma metastasizes more rarely unlike the visceral form [4,9]. The tumor in the heart results in pericardial effusion and cardiac tamponade [8] with liver, omentum and lung as the primary sites of metastasis either hematogenously or via local seeding [1]. A quarter of HSA have a corresponding cardiac tumor [6], and this makes it difficult to determine the primary site in dogs with advanced HSA [6].
Surgical removal followed by doxorubicin-based chemotherapy has been documented as the current standard care though prognosis is guarded because of the high metastatic nature of HSA [1,6,10].
The owners’ observations vary according to the site of the tumor, but generally manifest as episodic weakness with recovery, abdominal distension, malaise, depression, anorexia and weight loss [4]. Acute collapse or death, bleeding from the nose, lameness and paralysis [5], seizures or syncope can be seen as well [1].
The aim of this article is to describe the clinical case of splenic HSA in a 10-year-old German shepherd dog presented with swollen abdomen and weakness.
Case Description
An adult (10 yrs) 20.3 kg male German shepherd dog was brought to the University of Nairobi Small Animal Clinic with a history of decreased appetite for 6-7 days, swollen abdomen and weakness. The physical examination revealed a general lethargy, weakness, tachycardia, muffled heart sounds dyspnoea, arrhythmias with pulse deficits, a swollen abdomen (Figure 1) and tense abdominal palpation. The body temperature (38.9°C), the respiratory rate: 48 Breaths/ min and the heart rate of 108 Beats/min were within the reference range.
The hematological analysis showed Hb 13.0 g/L, Hct 39.7 %, Leu 27.3, H (103/µL), RBC 6.34 (106/µL), PLT 168L (103/µL), Eo 2.5%, MCV 62.6 Fl, MCH 20.5 pg and MCHC 32.7 g/Dl, whereas the blood biochemical analysis: ALT 5.7 IU, АSТ 24 IU, creatinine 1.4 mg/dl, urea 7.6 mg/dl and Total protein 7.1 mg/dl. Urine analysis showed protein 300mg/dl, relative density 1.050 and PН 6 but was negative for glucose, bilirubin, urobilinogen, ketone bodies, blood cells and Nitrate. However, urine sediment was consisted of single red blood cells and leukocytes. Ultrasound-guided paracentesis revealed serosangueneous effusion (Figure 2), with specific gravity of 1.020, PH of 6, 300 mg/dl Total protein with +++ leucocytes.
Radiographs of the abdomen revealed increased radiodensity, with a fluid line obscuring abdominal organs. The structures of the liver and the spleen were not clear on X-ray due to fluid filled peritoneal cavity. The following morning, the dogs showed episodic convulsions that were initially at intervals of 20 minutes but at mid-day, the frequency increased to intervals of 5 minutes and this necessated the use of Anesthetics (Thiopentone sodium at 10 mg/kg) to calm the dog.
Abdominal Ultrasonography (Figure 3) revealed a generally enlarged spleen and with a distinct mass of approximately 3 cm in diameter. Its structure was heterogeneous including hyperechoic, hypoechoic and anechoic areas. The peritoneal cavity showed inhomogeneous or mixed echogenic cavity masses revealing presence of fibrous material in the fluid possibly fibrinogen.
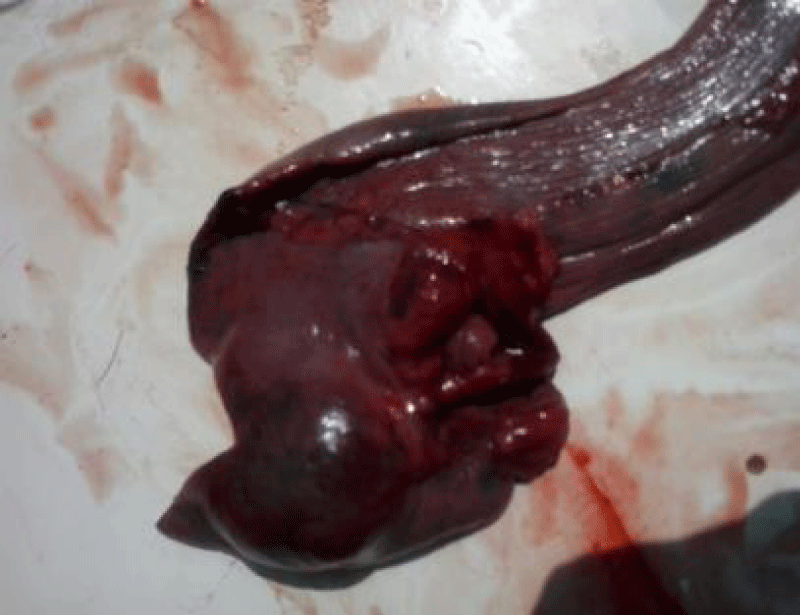
On third day in the afternoon, the dogs was in a bad state of episodic convulsions, dyspnoea, and frothing from the nostrils and the owner was informed on the guarded prognosis and the need of resting the dog on welfare grounds. The dog was euthanized with 200mg/ml Sodium Pentobarbitone (10mls) upon the owner’s consent. Post mortem revealed serosangiunous abdominal fluid approximately 1.5 liters, hyperemic intestinal serosa, distended gall bladder, normal appearance of liver and lungs. The spleen was enlarged with an irregular surface with a mass of approximately 3.5cm, which had ruptured (Figure 4). The tumorous masses in spleen were localized in the proximal part around the hilus with a smooth cut surface that bulges out.
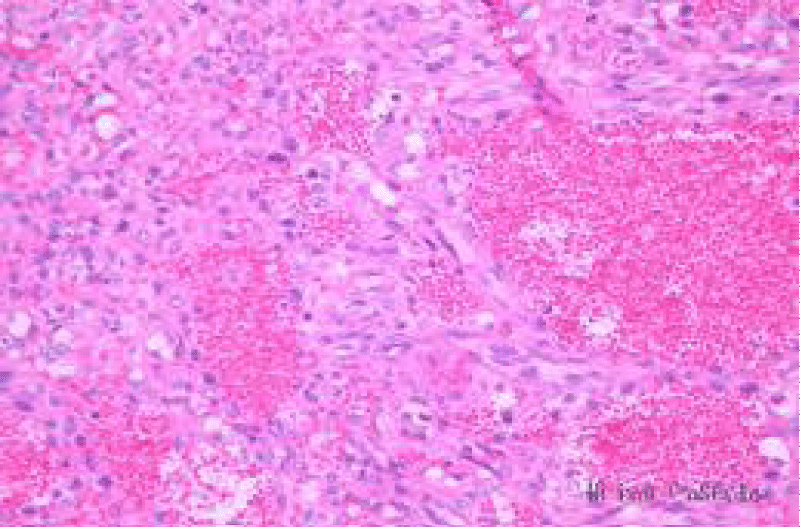
Histopathology of the mass showed malignant pleomorphic spindle cells that are diffuse and forming irregular blood-filled cavities. The cells have variable amounts of eosinophilic cytoplasm without distinct borders with centrally located nucleolus containing small amounts of fine chromatin (Figure 5).
The diagnosis of splenic HSA was made based on clinical, laboratory, ultrasonographic, gross and histological examinations.
Clinical management
On general, physical and radiographic examination on day one of admission, a tentative Liver syndrome diagnosis was made. Both EDTA and serum samples were taken for hematology and Biochemistry. Hematological profile (above), revealed Leucocytosis and thrombocytopenia. An oral diuretic was recommended to drain accumulated abdominal fluid as results of Liver and Kidney functions were to be given the following day. Furosemide tablets (by Cosmos Limited) were given at a dosage rate of 4mg/kg body weight. Each tablet contains Furosemide BP 40mg.
On the following, due to episodic convulsion, dyspnoea and frothing that suggest nervous involvement, Thiopentone sodium, 200mg was administered intravenously at a dosage rate of 10mg/kg body. This was done twice two hours apart when convulsions recurred upon recovery from anesthesia. Chloramphenicol 600mg was also given (at 30 mg/kg) and Dexamethasone 15 mg both given intravenously.
Discussion
Hemangiosarcoma is a common splenic tumor found in dogs and has been classified into three stages depending on the nature of spread. Stage one is when the tumor is confined to the spleen, stage two is when the mass ruptures and has involved the regional lymphnode whereas stage three is seen with involvement of distant lymph nodes or other tissues [5]. This particular case was at stage two as the mass had ruptured in situ.
When the HSA is localized in visceral organs, the initially symptoms are general and non-specific [1], but depend largely on size, growth rate, metastases and secondary complications [8].
It is only when state of the animal aggravates that the pet owners seek veterinary help [9]. The most common reported complaints are anorexia, general weakness, and wasting [1,4,11]. In advanced stage of the disease, a high-degree anemia, hemorrhages on mucosae, Disseminated intravascular Coagulopathy syndrome develops, syncope, cardiac arrhythmia and abdominal effusions could be observed [6,12]. It was not easy to make correct diagnosis with non-specific clinical manifestation and hematologic profile and therefore necessated radiography and Ultrasonography. Laboratory results including low hemoglobin and erythrocyte counts resulting in regenerative anemia, fragmented red blood cells and acanthosis, leukocytosis and thrombocytopenia have been reported by oncologist that they correspond to neoplastic changes in internal organs [1,5].
In this particular case, the swollen abdomen was misleading as suggested probable liver and/or heart involvement and therefore the request of Liver Enzyme profile and the initial tentative diagnosis of Liver syndrome. The inconclusive radiographs due to diminished Silhouettes of visceral organs brought on board the use of Ultrasonography. The pressure by the mass to blood supply and occlusion of vessels could have resulted in the seapage of plasma to the peritoneal cavity and pooling of blood in the spleen [1]. The accumulation of peritoneal effusions exerts pressure on the organs and the diaphragm that manifested as dyspnea [9]. Pulmonary congestion and probable hypovolemia resulted in pulmonary edema and thus the frothy discharge from the nostrils [6]. The predilection in the spleen explained the hematological changes and probable normal range of Liver enzymes and Blood Urea and Creatinine that has also been reported in some other studies [4,9].
Histopathological diagnosis is recommended to differentiate splenic HSA from other lesions that may present in a similar way [9,11]. Such lesions include; hematomas, hemangiomas, nodular hyperplasia, lymphomas and extra-medullary hematopoiesis [1,4,9].
In early diagnosed cases of HSA, the critical period for splenectomy is within 20-60 days after diagnosing the tumorous growth [1]. Three months have been documented as the average life span in dogs with splenic HSA with only 10% surviving up to 1 year or longer [12]. Chemotherapy usually prevents the spread of the tumor by inhibiting early metastases [1]. The most commonly used anticancer drug to control HSA in veterinary practice is cyclophosphamide that is applied either independently or in combination with other preparations [10]. Splenectomy with adjunct chemotherapy have been shown to increase the mean survival time with 140 to 202 days but with limited success in improving the quality of life [1,7,9].
However, recent advances in management and treatment of patients with HSA are increasing survival times and better quality of life [1]. Currently there are new screening methods that detect HSA in early stages that then allow treatment to start before the development of overt clinical signs or grossly clinical detectable disease [4,5].
Unfortunately in our case, the ultrasonographic confirmation of the splenic Hemangiosarcoma, guarded prognosis, old age and cost implication necessitated euthanasia, therefore neither surgery nor chemotherapy were attempted [13].
Conclusion
Splenic HSA is an important malignancy in dogs and regular screening especially for large breed is essential for early diagnosis and treatment. The non-specific clinical signs of HSA in this case necessitated imaging techniques to make diagnosis.
I would like to acknowledge University of Nairobi, Small animal clinic for provision of facilities for diagnosis on this case.
- Jacobs RM, Woods D (2010) Hemostasis and Hemostatic Evaluation. Principles of Disease course notes, module 5.1 and 5.2; Ontario Veterinary College, University of Guelph. Pathology 34: 28–34.
- Anette N Smith (2005) Standards of Care, Emergency and Critical Care; Hemangiosarcoma in North America. J Veterinary Association 4:25-27.
- Thamm DH (2007) Miscellaneous tumors: hemangiosarcoma. In: Withrow SJM, Macewen EG, Small Anim. Clin. Oncology 4th ed. Philadelphia: WB Saunders Co, USA 785-795. Link: https://goo.gl/gt6beV
- Patricia Ann Cole (2014) Association of canine splenic hemangiosarcomas and hematomas with nodular lymphoid hyperplasia or siderotic nodules. J Vet Diagnostic Investigation 24: 759-762. Link: https://goo.gl/tqLP6r
- Martins BDC, Torres BBJ, Rodriguez AAM, Gamba CO, Cassali GD, et al. (2013) Clinical and pathological aspects of multicentric hemangiosarcoma in a Pinscher dog. Arq Bras Med Vet Zootec 65:322-328. Link: https://goo.gl/KE9dtw
- Sabattini S, Bettini G (2009) An Immunohistochemical analysis of canine haemangioma and haemangiosarcoma. J Companion animal Pathology 140:158–168. Link: https://goo.gl/ZKhjFv
- Clifford CA, Mackin AJ, Henry CJ (2000) Treatment of canine hemangiosarcoma: 2000 and beyond. J Vet Int Med 14:479-485. Link: https://goo.gl/Y1apTw
- Withrow SJ, MacEwen EG (2001) Small Animal Clinical Oncology, 3rd ed. W.B. Saunders Co, Philadelphia, PA, USA 639-645. Link: https://goo.gl/6buxYx
- Bertazzolo W, Dell’Orco M, Bonfanti U (2005) Canine angiosarcoma: Cytologic, histologic and immunohistochemical correlatations. Vet Clin Dogs and Cats. Veterinary Clinics of North America Small Animal Practice 33: 533-552. Link: https://goo.gl/Xky25v
- Todorova I, Simeonova G, Simeonov R, Dinev D (2005) Efficacy and toxicity of doxorubicin and cyclophosphamide chemotherapy in dogs with spontaneous mammary tumours. Trakia Journal of Veterinary Internal Medicine 3: 35-39. Link: https://goo.gl/RrP1Xg
- Dennler M, Lange EM, Schmied O, Kaser-Hotz B (2007) Imaging diagnosis – Metastatic hemangiosarcoma causing cerebral hemorrhage in a dog. J Vet Radiol Ultrasound 48:138-140. Link: https://goo.gl/JbgJ8Q
- Smith Annette N (2003) Hemangiosarcoma in dogs and cats. In: The Veterinary Clinics of North America, Small Animal Practice, Advances in Medical Oncology. W.B. Saunders Co, Philadelphia, USA 533- 555. Link: https://goo.gl/gVcvJf
- Ward H, Fox LE, Calderwood-Mays MB, Hammer AS, Cuoto CG (2001) Cutaneous hemangiosarcoma in 25 dogs: A retrospective study. Journal of Veterinary Internal Medicine 8: 345-348. Link: https://goo.gl/UWVggi
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.




 Save to Mendeley
Save to Mendeley
