International Journal of Veterinary Science and Research
Repair of Large Abdominal Wall Defect Using Glycerolized Bovine Tunica Vaginalis and Prolene Mesh Implants in Dogs
KS Abouelnasr2, AE Zaghloul2 and GI Karrouf1,2*
2Surgery, Anesthesiology and Radiology Department, Faculty of Veterinary Medicine, Mansoura University, Mansoura, 35516 Dakahlia, Egypt
Cite this as
Abouelnasr KS, Zaghloul AE, Karrouf GI (2017) Repair of Large Abdominal Wall Defect Using Glycerolized Bovine Tunica Vaginalis and Prolene Mesh Implants in Dogs. Int J Vet Sci Res 3(1): 001-007. DOI: 10.17352/ijvsr.000014The repair of large abdominal defects remains a complex surgical problem of insufficient autogenous tissue for adequate reconstruction. The aim of the present study was to evaluate the uses of polypropylene mesh with glycerolized tunica vaginalis (GTV) for repairing abdominal wall defects in dogs using either inlay or underlay technique of implatations. Full thickness (all the layers of muscles except the skin) abdominal wall defects (6 x 10 cm) were created in 24 healthy mongrel dogs allocated into two main equal group (n= 12 each) according to the type of the prosthetic material used. Each group is subdivided into two subgroup (n= 6 each).
The present study indicated that, GTV Patches are considered to be a successful alternative for reinforcement and repair of large abdominal wall defects than prolene mesh based on postmortem findings, biomechanical analysis and histopathological examination.
Introduction
Reconstruction of major abdominal wall defect remains a significant problem and commonly encountered as a challenge for the surgeon with recurrence being a common outcome. Many abdominal wall defects can be repaired by primary closure; however, if the defect is larger and there is a tension on wound closure, the use of prosthetic materials is indicated [1]. Post-repair clinical complications such as wound infection, bowel fistula, erosion into abdominal viscera, increased recurrence rate (25%), repair failure and mesh extrusion had been reported as well as the high cost associated with synthetic material initiated the search for safe and cheap biodegradable material that can be replaced by the recipient’s tissue [2].
Several types of connective tissues and muscles have been used experimentally and clinically in reconstructing congenital or acquired soft tissue defects. These were duramater [3], Fascialata [4], pericardium [5,6] and tunica vaginalis [7]. The parietal tunica vaginalis is a serous sac that is formed by an out pouch of the parietal peritoneum of the abdominal cavity during testicular descent. The glycerolized and irradiated freeze dried tunica vaginalis has a higher histocompatability and safety for repair of abdominal wall defect with more advantage of glycerolized tunica vaginalis [8].
The aim of this study was to evaluate the effectiveness of using glycerolized bovine tunica vaginalis in comparison to polypropylene mesh for repair of induced abdominal wall defect in dogs by using of either inlay or underlay technique of implantations.
Materials and Methods
This study was approved by the ethical committee for animal care (Laboratory Animal Center, Mansoura University, Egypt).
Experimental animals, housing and feeding
Twenty four healthy mongrel dogs with an average age of 1.5-2 years and 17.4 ±1.6 kg body weights were used in this study. All dogs were kept in closed boxes at Mansoura Veterinary Teaching Hospital of the Faculty of Veterinary Medicine, and were fed a dog food according to maintenance and free access to water.
The animals were divided into two main groups according to the type of the prosthetic material used. Each one is subdivided into two subgroups according to the technique of implantation. The different prosthetic materials used are polypropylene mesh (Prolene Ethicon; Johnson& Johnson, Belgium) and GTV. The two techniques of implantation are the inlay and underlay techniques.
Preparation of the prosthetic material
Collection of Tunica Vaginalis Sacs: Fresh bovine parietal tunica vaginalis parietalis sacs were obtained from the abattoir from animals with average age 1.5 - 2 years. Immediately after slaughtering. The tunica vaginalis sacs were cut through a longitudinal incision parallel to the epididymal attachment to the testis and their external loose connective tissue and fascia were stripped mechanically.
Implant preparation
For preparation of the glycerolized tunica vaginalis (GTV) sacs were shaken serially in sterile normal saline for 60 min, transferred to a sterile glass bottle containing 50% sterile glycerol under aseptic condition and kept there for 3 hours at room temperature. The pieces of bovine tunica vaginalis were then transferred to a second bottle containing 70% sterile glycerol for 3 hours at room temperature and were finally plugged into 99.5% sterile glycerol and stored at 4◦C before implantation according to Hafeez et al. [8].
Preoperative preparation and anesthesia
All dogs received a preoperative dose of amoxicillin and flucloxacillin (flummox, EIPICO, ARE) then pre-medicated with intramuscular injection (IM) of atropine sulphate (1mg/ml, Misr Co- Egypt) at a dose of 0.1mg/kg B.W followed by IM injection of xylazinHcl (Xylaject-ADWIA, ARE) at a dose of 1mg/kg B.W. General anesthesia was induced by using thiopental sodium 2.5% (Thiopental- EIPICO, ARE) andmaintained by isoflurane inhalation anesthesia.
Surgical technique
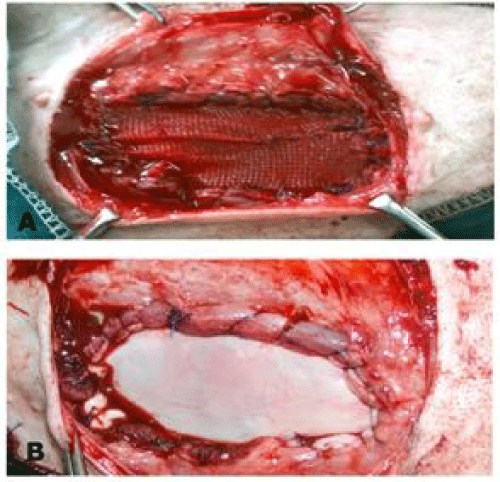
A full thickness abdominal wall defect (6 x 10cm), including muscles and peritoneum was created. After control of bleeding, a prosthetic materials with appropriate size to the defects were implanted using either inlay or underlay techniques.
For inlay technique: The prosthetic materials were sutured to the cut edges of the abdominal wall using 2/0polypropylene monofilament (Prolene, Ethicon; Johnson &Johnson, Belgium) by simple continuous suture, interrupted one at the corners. Mild tension was maintained on the patch to avoid bulging and rolling of the implant (Figure 1).
For underlay technique: The prosthetic material was fashioned to the shape of the defect allowing for 4-5 mm underlay then placed under the peripheral peritoneum after omentalization and secured to the recipient tissue with interrupted overlapped mattress suture using prolene (Figure 2). Abdominal bandage was applied to the operated animals for one week postoperatively.
Postoperative care and follow up
Cage rest was provided for all dogs in the immediate postoperative period. Dogs were injected with ketoprofin (AMR.Co. Egypt) in a dose of 1mg/kg B. W. after the operation and with 12 hours interval. The preoperative antibiotic was continued for 5 successive days at 12 hours interval. The wound was dressed 2 times daily using povidone iodine.
Dogs were fed on soft food with reduced amount to one third for the first week postoperatively and gradually returned to normal amount within two weeks. The operated animals were clinically observed daily during the study period for presence or absence of postoperative complications. The skin stitches were removed at 10 - 12 days post-operatively.
Euthanasia and macroscopic evaluation
Four dogs from each group were euthanized humanly at 60, 120 and 180 days. The abdominal wall defect areas were examined grossly before removal of the skin for assessment of skin wound healing, presence of prosthetic imprint and for detection of adhesion between the skin and the implant.
After removal of the skin, a rectangular shape of 6 cm width including the prosthetic implant was examined for the presence of covering connective tissue, and shrinkage of the implant. Then three borders full thickness abdominal wall defect was carried and reflected for detection of the development of neoperitonium, presence or absence of inner revascularization and the grade of adhesion using the adhesion scoring system described by Jenkins et al. [9], in which a qualitative assessment of adhesion was classified into four grades: (0) no adhesion, (1) minimal adhesion could be freed by gentle hand dissection, (2) moderate adhesion could be freed by aggressive hand dissection and (3) dense adhesion required sharp dissection to free the biomaterial from the abdominal viscera.
Biomechanical evaluation
Transverse strips of 4 cm width and 10 cm length including the implanted material and bilateral adjacent body wall were harvested at each time of euthanasia and packed in containers of refrigerated normal saline until tensile testing that occurred within 3 hours of tissue collection. The remaining polypropylene suture material was removed. Both ends of the sample were then placed into grips of tensile strength testing apparatus (LLOYD).
The samples were elongated until failure at a crosshead speed of 50 mm/min. Load at failure and extension percentages were recorded simultaneously on a digital screen connected to the load frame. Tensile strength was calculated by dividing the sample load at failure by the sample width. The anatomic location of the break was noted for each specimen.
Histopathological examination
Tissue specimens were collected from the patch grafts at 60, 120 and 180 days following hernioplasty. All collected specimens were fixed in 10% formalin solution for 48 hours, trimmed to suitable sizes, washed, dehydrated, cleared in xylol, embedded in paraffin wax, sectioned at 5-6 µ thickness, and mounted onto glass slides stained with Hematoxylin and Eosin (H&E), Masson’s Trichrome and Van Kossa for evaluation using conventional light microscope according to Bancroft et al. [10]. Amount of fibrosis were graded during histological examination according to fibrosis grading scale described by Hooker et al. [11], as the following 0: nil, 1: minimal, 2: moderate and 3: dense.
Statistical analysis
Data analyses were performed with statistical software program (Graph pad prism version 3.0, USA). The mean values and standard deviation for each assessed variable at each time point were calculated. Repeated mea sures analysis of variance (ANOVA, with repeated measures on treatment and time) were used to determine the main effect of implants and time. Where the repeated measures ANOVA indicated a statistically significant difference between groups, one way ANOVA with Duncan post hock multiple comparison test was used to identify which group was statistically different from the rest. Differences between means at P < 0.05 were considered significant.
Results
Experimental animals
All the experimental dogs tolerated the surgical procedure well and survived until the determined date of euthanasia. The prosthetic materials used were effective in reconstruction of experimentally induced abdominal wall defects in dogs. The results of this prosthetic herniorrhaphy were illustrated in (Table 1).
Clinical findings
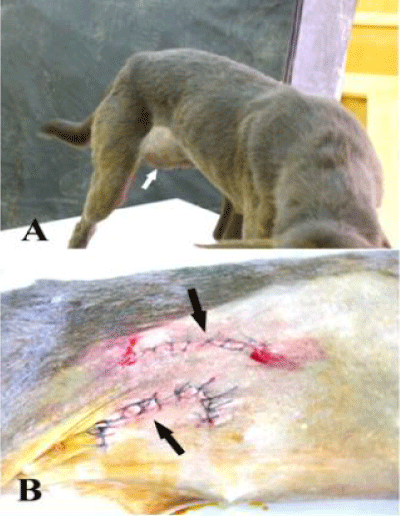
A mild to moderate inflammatory signs including hotness, pain, redness and abdominal discomfort were observed in animals implanted with GTV24 hours postoperatively and disappeared within 5-7 days while in animals implanted with prolene mesh, the inflammatory response was more intense then decreased gradually until its disappearance within 3 weeks. Seroma formation was recorded in 5 animals (3 repaired with prolene, and 2 repaired with GTV) 2 days postoperatively and it was disappeared within 2 weeks without interference. All dogs had no evidence of herniation except one dog of prolene mesh group using inlay technique. The animal showed herniation of an orange size swelling at the right side developed at the third day after implantation at the same site of the primary operation and re-operated (Figure 3).
There were no post-operative complications until 2 months and the abdominal wall was regained its normal integrity without abnormalities at 4 months. At 6 months, there was some difficulty to distinguish the site of implantation by naked eye especially in animals implanted by using underlay technique. By finger examination, the imprint of the prosthetic materials could be detected through the intact skin, the implantation site become more firm in animals implanted with prolene mesh while in animals implanted with GTV it was felt more pliable and it was difficult to outline the imprint of the prosthetic materials.
Post-mortem findings
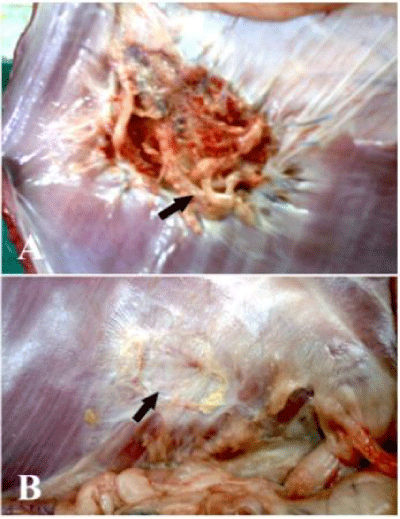
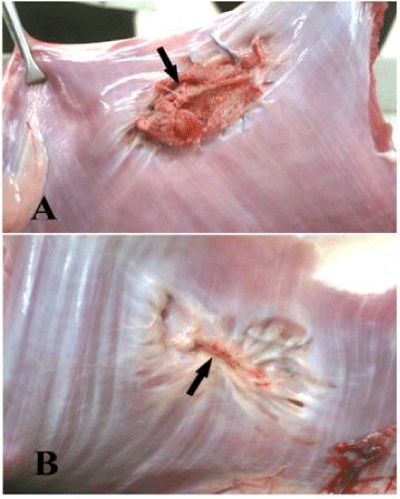
Gross inspection of the subcutaneous surface of the abdominal wall at the implantation site at 2 months revealed covering of the implanted materials with a thin layer of white fibrous connective tissue with outer neovascularization which become more prominent at 4 and 6 months. The visceral surface of the implanted prolene mesh were encapsulated with fibrous tissue, remained without marked structural changes and showed some irregularities and folding of the mesh while the implanted GTV was gradually coated with a connective tissue layer and did not showed irregularities or folding at 2 months. At 4 months, the peritoneal surface of prolene mesh became encapsulated with more fibrous tissue without structural changes and show more folding and irregularities while GTV implants were more coated with the connective tissue layer that cover 60% of the implant (Figure 4).
At 6 months the peritoneal surfaces of the prolene mesh remained without structural changes, encapsulated with fibrous tissue and show more folding and wrinkling while GTV implants were nearly complete covered with a new peritoneum and a thin connective tissue layer. Inner neovascularization also could be clearly seen by naked eye (Figure 5).
Formation of intra- abdominal adhesion
There was no evidence of adhesion between the biomaterial and the underlying visceral organs in all dogs at all-time intervals except in one dog implanted with prolene mesh, euthenized at 60 days, in which grade 3 of adhesion was recorded. There was frequent formation of adhesion between the peritoneal side of the biomaterial and the greater omentum of grade (0-3) according to the implanted material and the technique of implantation as shown in (Table 2).
Tensiometric evaluation
Twenty four strips tested were broken either at the implant-muscle interface or at the muscle on either end of the implant.
The average mean differences of load at failure and healing tensile strength of prolene mesh and GBP implants were not statistically significant at each time interval. However, the healing tensile strength of the abdominal wall implanted with different types of graft materials was generally significantly affected with time (P < 0.01). Moreover, One way ANOVA showed significant differences (P < 0.05) at different times (Tables 3,4). The different values of the extension percentage of the two types of implants showed statistically significant (P < 0.001) at all-time points with different types of the implants (Table 4).
Histopathological examination
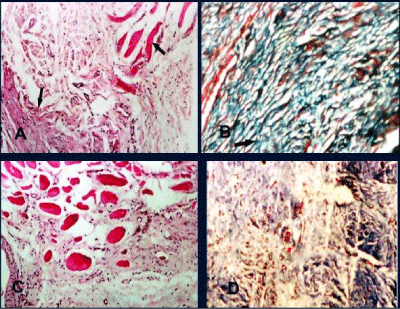
Prolene Mesh: The inflammatory response was represented by few numbers of mononuclear cells, plasma cells and lymphocytes that widely scattered especially around the mesh fibersat 2 months. This inflammatory response was decreased by time at 4 and 6 months. Grade 1 of fibrosis including immature fibrous tissue and a newly developed connective tissue layer consisting of delicate collagenous fiber was present at 2 months and gradually changed into grade 2, 3 at 4 and 6 months respectively that was confirmed with Masson’s trichrome. Moreover, dense collagen fibers with the presence of mild degree of calcification, which confirmed by van Kossastain were seen.
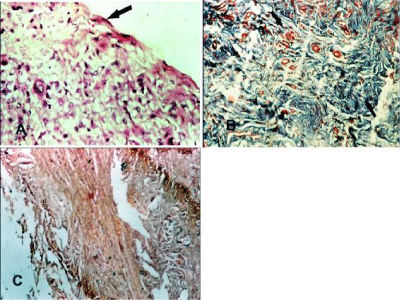
Glycerolized Tunica Vaginalis: At 2 months, the dense collagen bundles of the tunica vaginalis implant were present and surrounded by few numbers Plasma cells, lymphocytes and macrophage. Myoblasts were observed at junctional area and neovascularization including newly formed capillaries was observed in the implant (Figure 6A) the newly formed fibrous tissue and collagen were confirmed by Masson’s trichrome (Figure 6B). At 4 months, myoblasts were infiltrating throughout the implant Collagen fibers which began to be gradually resorbed and replaced by loose connective tissue which was delicate, immature and observed in unorganized form (Figure 6C).Well-vascularized fibrous tissue consisting of few fibrocyte that represent grade 1 of fibrosis were observed and confirmed with Masson’s trichrome (Figure 6D). At 6 months the loose connective tissue was gradually changed to form dense fibrous tissue that was infiltrated by myoblasts and neovascularization (Figure 7A). Neoperitoneum and mesothelial cells were observed at the under surface of the biomaterials. The collagen fibers were increased in amount, density and coarseness to represent grade 2 of fibrosis and became more clearly defined and well organized (Figure 7B). No calcification or foreign body giant cell were observed at all times (Figure 7C).
Discussions
Post-operative wound infection and inflammatory changes encountered with GTV implantation were less intense than those associated with polypropylene mesh. Moreover the higher elasticity of GTV made the site of implantation felt more pliable in comparison with abdominal wall rigidity recorded with prolene mesh. These findings were agreed with Hafeez et al. [8] and Kader et al. [12] who added that clinical complications associated with synthetic materials and the difficulty in obtaining and preservation of autografts and allografts make the alternative of xenografts better. GTV was derived from the peritoneum, so, it imitates the properties of the natural abdominal wall and it does not carry too much rejection as mentioned by Werkmeister et al., Grossly, our results showed that the glycerol preserved tunica vaginalis implants were gradually resorbed and replaced with recipient tissue at different rates The previous studies by Coito & Kupiec [13], revealed that glutaraldehyde treatment does not completely eliminate the immune response to allograft and xenografts, and induce calcification which is not the case in this study, therefore glycerol preservation technique is preferable than glutaraldehyde. Glycerol pretreatment seems to delay implant biodegradation and replacement by host tissue compared with other types of preservation as lyophilized, irradiated freeze drying method [8].
The technique of implantation of the prosthetic materials is thought to play an important role in hernia recurrence [14]. The underlay technique with omentalization gave more satisfactory results than the inlay technique. Although the inlay technique is the simplest but it lack fixation of the implant due to minimal surface area of contact which may lead to high relapse rates. While in underlay technique the position of the implant behind the rectus muscles minimize hernia recurrence rates. The same explanation was obtained by Reilingh et al. [15] and Rainier et al. [16].
The continuous suture pattern used in the inlay technique explained presence of a swelling post-surgery that was recorded in this study. These observations were completely coincided with Ladurner et al. [17], who found that the interrupted sutures used for fixation of the implant in the underlay technique provide a multiple point non tension fixation which help in dividing the stress evenly over the mesh and reducing crimping and bulging of the mesh.
Hernia recurrence was not recorded in dogs implanted with underlay technique while it was recorded in a dog implanted with inlay technique. This may be attributed to the less contact of the prosthetic material to the host tissue and to the continuous suture pattern that used in the inlay technique as reported by Ladurner et al. [17], who found that the interrupted sutures in the underlay technique distributed the stress evenly over the mesh and reducing crimping and bulging of the mesh.
Higher degree of adhesion formation were encountered in animals implanted with inlay technique without omentalization than in the underlay technique with omentalization, it could be attributed to the direct contact between the mesh and the abdominal viscera which lead to inflammatory response to the prosthesis giving rise to intra-abdominal adhesion. Interposition of the omentum to act as a physical barrier between the implant and the viscera [17].
Formation of neoperitoneum recorded in animals implanted with GTV acts also as a physical barrier that decreases adhesion formation and proved that the processed tunica vaginalise can be placed in direct contact with underlying viscera without stimulating intra- abdominal adhesion. However adhesion was formed between the peritoneal side of the implant and the greater omentum may attribute to lesion caused by abrasion, ischemia and foreign body. Similar result was obtained in dogs using preserved tunica vaginalise [5].
The adhesion is more intense in animals implanted with prolene mesh, and was of grade 1, 2 and 3 that required aggressive blunt dissection. This result agreed with Baptista et al. [18]. Postmortem examination prolene mesh revealed that, despite of rolling the edges and suturing under moderate tension; wrinkling and folding was observed due to encapsulation with fibrous tissue which encourages mesh wrinkling. These findings were similar to that recorded by Amid [19]. While in tunica vaginalise showed no signs of fragmentation, retained their original shape and position and decreased in their diameter by time. This could be attributed to the contraction of the fibrous connective tissue after maturation of the healing sites around the patches [20].
Mild calcification was recorded at 6 months in animals implanted with prolene mesh while in animals repaired with GTV there was no calcification at all times that indicated the histocompatability of these materials with the recipient tissue. It also gave an advantage to glycerol preservation over the other types of preservative such as glutaraldehyde which was recorded to be associated with a cytotoxic effect and calcification formation [6,20].
All tissue failure in tensiometric evaluation in this study occurred either at the implant-muscle interface or at the muscle on either end of the implant. This type of failure indicates a lower tensile strength of the muscle and fascia than at the repair site [16,21]. The differences among overall mean values of load at failure and healing tensile strength of prolene mesh and GTV implants were not statistically significant at each time interval; these results suggested that the implanted materials were sufficiently strong to maintain abdominal wall integrity. Similar results were obtained by Hafeez et al. [5,8].
In conclusion, GTV provides a good alternative to prolene mesh where the saving in mesh cost and the need to large sized mesh to repair large defect are considerable in veterinary practice.
- Kingsnorth A, Leblanc K (2003) Management of abdominal hernias 3rd edn. Arnold. London. 172-188. Link: https://goo.gl/qy3W0b
- Luijendijk RW, Hop WC, van den Tol MP, de Lange DC, Braaksma MM, et al. (2000) A comparison of suture repair with mesh repair for incisional hernia. N Engl J Med 343: 392-398. Link: https://goo.gl/fwxuWW
- Parizek J, Mericka P, Husek Z, Suba P, SpacekJ, et al. (1997) Detailed evaluation of 2959 allogenic and xenogenic dense connective tissue grafts (fascia lata. Pericardium, and dura mater) used in the course of 20 years for dura plasty in neuro surgery. Acta Neurochir (Wien) 139: 827-838. Link: https://goo.gl/vSBjwk
- Peláez Mata D, Alvarez Zapico JA, Gutiérrez Segura C, Fernández Jiménez I, García Saavedra S, et al. (2001) Fascia lata transplant from cadaveric donor in the reconstruction of abdominal wall defects in children. Cir Pediatr 14: 28-30. Link: https://goo.gl/MOLuvP
- Hafeez YM, Zuki AZ, Logman MY, Yusof N, Asnah H, et al. (2004) Glycerol preserved bovine pericardium for abdominal wall reconstruction: Experimental study in rat model. Med J Malaysia 59: 117-118. Link: https://goo.gl/QmnRQC
- Abouelnasr KS, Zaghloul AE, Karrouf GI (2014) Comparative evaluation of glycerolized bovine pericardium implant with prolene mesh for repairing of abdominal wall defect in dogs. Iranian Journal of Veterinary Research 15: 211-217. Link: https://goo.gl/2SqQAL
- Tung W, Jamaludin Z, Pillay AG, Yusof N, Loqman MY (2002) Processed bovine tunica vaginalis as a biomaterial for the repair of large abdominal wall defects in surgical treatment. Journal of Medical Sciences 2: 7-11. Link: https://goo.gl/kfFlne
- Hafeez YM, Zuki AB, Logman MY, Noordin MM, Yusof N (2005) Comparative evaluation of the processed bovine tunica vaginalis implant in a rat model. Anat Sci Int 80: 181-188. Link: https://goo.gl/g0xXjE
- Jenkins SD, Klamer TW, Parteka JJ, Condon RE (1983) A comparison of prosthetic materials used to repair abdominal wall defects. Surgery 94: 392-398. Link: https://goo.gl/8vitE6
- Bancroft JD, Gamble M (2008) Theory and practice of histological techniques, 6th edn. Churchill Livingstone, Philadelphia. J Neuropathol Exp Neurol 67: 633. Link: https://goo.gl/HKwUs2
- Hooker GD, Taylor BM, Driman DK (1999) Prevention of adhesion formation with use of sodium hyaluronate – based bioresorbable membrane in a rat model of ventral hernia repair with polypropylene mesh- A randomized, controlled study. Surgery 125: 211-216. Link: https://goo.gl/8kU2Ij
- Kader NH, Abass BT, Al-Sadi HI (2005) A comparative experimental study of the use of tunica vaginalis and pericardium as allograft for hernioplasty in sheep. Iraqi J Vet Sci 19: 57-70. Link: https://goo.gl/DMFueC
- Coito AJ, Kupiec JW (1996) Extracellular matrix proteins: bystanders or active participants in the allograft rejection Cascade. Annals of Transplantation 1: 14-18. Link: https://goo.gl/5dYts6
- Schumpelick V, Kling U, Junge K, Stumpf M (2004) Incisional abdominal hernia: The open mesh repair. Langenbecks Arch Surg 389: 1-5. Link: https://goo.gl/kSRM4Q
- Reilingh VTS, Geldere VD, Langenhorst B, Jong D, Wilt G, et al. (2004) Repair of large midline incisional hernias with polypropylene mesh: comparison of three operative techniques. Hernia 8: 56-59. Link: https://goo.gl/SX9TjZ
- Rainier Ko, Kazacos EA, Snyder S, Ernst DM, Lantz GC (2006) Tensile Strength Comparison of Small Intestinal Submucosa Body Wall Repair. J Surg Res 135: 9-17. Link: https://goo.gl/7zFZ6s
- Halm, J, Jeekel J (2005) Incisional hernia, long- term complications of abdominal surgery. Global Surgery Future Direction 11: 1-4.18.
- Baptista ML, Bonsack MS, Delaney JP (2000) Seprafilm reduces adhesions to polypropylene mesh. Surgery 128: 86-92. Link: https://goo.gl/hk4D2l
- Amid PK (1997) Classification of biomaterials and their related complication in abdominal wall hernia surgery. Hernia 1: 15-21. Link: https://goo.gl/rQA6uA
- Zaghloul A, Mosbah E (2006) Reconstruction of abdominal wall defect by using bovine pericardium bioprosthesis. Mansoura Veterinary Medical Journal 1: 111-135.
- Badylak S, Klokini K, Tullius B, Whitson B (2001). Strength over time of a resorbable bioscaffold for body wall repair in a dog model. J Surg Res 99: 282-287. Link: https://goo.gl/ZPAX5U
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
