International Journal of Veterinary Science and Research
Inhibitory Effect of Mentha Longifolia L. Essential Oil against Listeria Monocytogenes Using Transmission Electron Microscopy
Razzagh Mahmoudi1, Farzad Katiraee2*, Hossein Tajik3, Amir Abbas1, Farshid4 and Omid Fakhri1
2Department of Pathobiology, Faculty of Veterinary Medicine, University of Tabriz, Tabriz, Iran
3Department of Food Hygiene and Quality Control, Faculty of Veterinary Medicine, Urmia University, Urmia, Iran
4Department of Pathobiology, Faculty of Veterinary Medicine, Urmia University, Urmia, Iran
Cite this as
Mahmoudi R, Katiraee F, Tajik H, Abbas A, Farshid, et al. (2016) Inhibitory Effect of Mentha Longifolia L. Essential Oil against Listeria Monocytogenes Using Transmission Electron Microscopy. Int J Vet Sci Res 2(1): 014-017. DOI: 10.17352/ijvsr.000008Inevitable side effects of chemical food preservatives and drug resistance have increased interests on use of natural preservatives derived from plants. Therefore, in the present paper, the biological properties of Mentha longifolia L. essential oil were studied. Chemical analysis (GC/MS) and antibacterial properties of the Mentha longifolia L. essential oil (EO) was under different temperature and pH values were evaluated with special reference to the mechanism of inhibition Listeria monocytogenes growth at ultra-structural level by TEM.
Minimum inhibition concentration and minimum bactericidal concentration values of the M. longifoli L. EO showed to be in the range of 150-9600 µg/ml. These MIC, MBC results and cell membrane damage observed in TEM evaluation indicate that this EO has a high potential of anti-Listeria effect. It is concluded that M. longifoli L. EO could be effectively used as a natural biopreservative against foodborne bacteria.
Abbreviations
EOs: Essential Oils; GC/MS: Gas Chromatography–Mass Spectrometry; TEM: Transmission Electron Microscopy, LPS: Lipopolysaccharides; MIC: Minimum Inhibition Concentration; MBC: Minimum Bactericidal Concentration; DMSO: Dimethylsulfoxide
Introduction
EOs has already found a considerable range of applications. The majority of them are used as fragrances in perfumery as well as in food and beverages industry. The recent years have also witnessed a revival of traditional natural products in medicine and in food and cosmetics preservation. Despite the development of antibiotics, bacterial and fungal infections are still a major issue in medicine, and the presence of numerous drug-resistant strains poses a new challenge. Drugs derived from plant sources have been extensively used in this field for many centuries. Recently, there has been a growing interest in natural products due to their availability, fewer side effects or toxicity as well as better biodegradability as compared to the available antibiotics and preservatives. In this regard, plant EOs may offer a great potential and hope. Therefore, their composition and antimicrobial activities have been thoroughly and systematically studied. The antimicrobial properties of EOs and their constituents have already been reviewed [1-3]. The action mechanism of the EOs is related to their chemical the composition and their antimicrobial activity is not attributable to a unique mechanism but to a cascade of reaction in the entire bacterial cell [2]. The hydrophobicity of the EOs is the main properties responsible for the distribution of bacterial structures increasing their permeability due to their inability to partition the lipids from the bacterial cell membrane. This permeability barrier role of cell membranes is integral to many cellular functions, including the maintenance of the energy status of the cell, other membrane-coupled energy-transducing processes, solute transport and regulation of metabolism and control of turgor pressure. Toxic effects on membrane structure and function have generally been used to explain the antimicrobial action of Eos and their monoterpenoid components [4-6]. EOs inhibit the synthesis of DNA, RNA proteins and polysaccharides in fungal and bacterial cells, EOs which having the hydroxyl group at a different location on phenol, both substances appear to make the cell membrane permeable, Carvacrol and Thymol are able to disintegrate the outer membrane of Gram-negative bacteria, releasing lipopolysaccharides (LPS) and increasing the cytoplasmic membrane permeability also carvacrol caused increasing membrane fluidity [5-7]. The effectiveness of M.longifoli L. EO against gram-positive and gram-negative bacteria has been well described [8-10]. The main goals of present research was to study the chemical composition of M. longifoli L. EO (this plant was collected from northern regions of the Kurdistan Province, located in the northwest of Iran) to determine the EO chemotype and investigate antibacterial and morphological effects of M. longifoli L. EO on Listeria monocytogenes observed by TEM.
Material and Methods
Plant material
M.longifoli L. was collected from Kurdistan province (is located in the northwest of Iran) and identified by the Institute of Medicinal plant, Medicinal University of Tehran, Iran. The prepared powder was kept in tight containers protected completely from light.
Method of the EO preparation
The dry powdered plant subjected to steam distillation for 3 h using a Clevenger-type apparatus. The obtained EO was dried over anhydrous sodium sulfate until the last traces of water were removed and then stored in dark sealed vials at 4°C [11].
Chemical analysis of EO
EO was analyzed by gas chromatography–mass spectrometry (GC/MS). The chromatograph (Agilent 6890 UK) was equipped with an HP-5MS capillary column (30 × 0.2 mm ID × 0.2 mm film thickness) and the data were taken under the following conditions: initial temperature 50°C, temperature ramp 5°C/min, 240°C/min to 300°C (holding for 3 min), and injector temperature at 290°C. The carrier gas was helium, and the split ratio was 0.8 mL-1/min. The mass spectrometry (Agilent 6890 gas chromatograph equipped with an Agilent 5973 mass-selective detector; Agilent UK) also was used. The chemical components of EO are identified by comparing its retention index with what is published in the reports or in the data bank of the data base, Wiley library, stored in the computer memory of GC/MS.
Antibacterial screening (MIC, MBC)
Microbial strains: EO was individually tested against L. monocytogenes ATCC 19118.
Micro-well dilution assay: The MIC and MBC values were studied for the bacterial strains based on Gulluce et al. (2007) methods [12]. EO dissolved in 10% dimethylsulfoxide (DMSO) was first diluted to the highest concentration (38400μg/ml) to be tested, and then two-fold serial dilutions were made in a concentration range from 17.5 to 38400μg/ml in 10 ml sterile test tubes containing nutrient broth. The MIC and MBC were defined as the lowest concentration of the compounds to inhibit the growth of microorganisms and bactericidal effects on microorganisms respectively.
Assessment of morphological damage: Bacterial cell damage (L. monocytogenes.) evaluated with TEM according to Mahmoudi et al. [12], The EO concentrations used in this part were 300μg/ml and 2400 μg/ml on S .aureus and L. monocytogenes, respectively (selection was based on MIC results).
Statistical analysis
All statistical analyses were performed using SPSS 17.0. All results were computed as mean standard deviation and were subjected to one-way analysis of variance to establish whether the differences in experimental results were significant or not. The Statistical significance was determined at P<0.05.
Results
The chemical composition of M.longifoli L. EO was analyzed by employing GC– MS. This study allowed identification of 22 composed representing 95.3% of the totality of the components of the EO. The EO is particularly rich in cis- Isopulegone (9.7%), Menthofuran (11.8%), pulegone (31.5%), 1, 8-cineole (15.9%), and p-Menth-3-en-8-ol (7.0%).
The antibacterial activity of the EO included in this work was conducted by broth microdilution susceptibility method. The results summarized in Table 1, showed that the EO tested, presented an antibacterial activity with different degree. In fact, the MIC and MBC values under different temperature and pH indicate that the M.longifoli L.EO have a broad activity especially for L.monocytogenes (P<0.05). The noted values under different temperature and pH values showed to be in the range of 150- 9600 μg/ml respectively. The results obtained from the MIC and MBC values indicated that M. longifoli L. EO was much more potent with decreasing temperature and increasing acidic conditions L. monocytogenes (P<0.05). The Lowest MIC value was found at 8°C and pH 6 (150μg/ml).
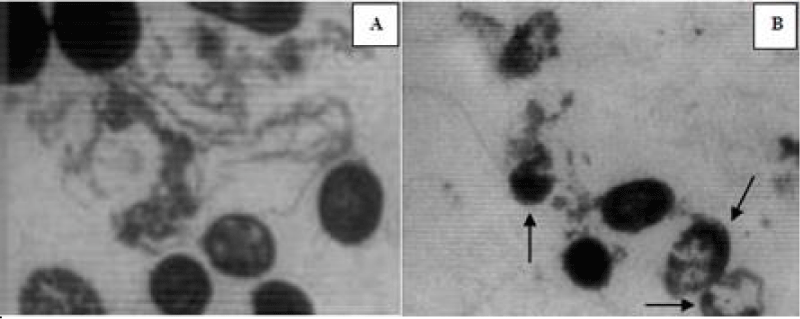
To confirm the mechanism of cell damage resulted from effects of EO, L. monocytogenes cells (untreated, EO treated) were observed through TEM (Figures A,B). In the TEM photomicrographs of control groups, L. monocytogenes cells had distinct and regularly outlined cell wall structure of gram-positive bacteria with the shape of bacilli with intact and smooth cellular walls (Figure A). After treatment with EO at MIC level, morphology, and shape of the cells was distorted and cell wall damage and removal of cellular contents and cellular disturbance was observed (Figure B), while the cell morphology appeared normal in control groups. Cell wall thickness varied and it appeared disrupted at some places (Figure B). Transmission electron microscopy of cells exposed to MIC levels of the oils showed irreversible damage to the cell wall.
Discussion
The antimicrobial activity can be attributed to the presence of high concentrations of Menthofuran (11.8%), cineole (15.9%) and pulegone (31.5%). Antimicrobial properties of mentioned components have been reported in many researches [12,13]. Also, it is reported that Pulegone is a bioactive compound (antioxidant and antimicrobial) [13].
According to the Results of this study, the highest antibacterial activity of EO was the pH 6 and 8°C (P<0.05), the highest MIC and MBC values of EO was in 37°C and pH 7.2 (Table 1). With the simultaneous increase in temperature and pH values, a significant increase in MIC and MBC values of the EO was quite evident (P<0.05).
Temperature, pH, salt concentration, type and amount of inoculated organism affected antimicrobial activities (MIC and MBC) of the Eos [15,16]. In our study, increased inhibition properties of EO on bacterial growth were observed by reducing incubation temperature and the pH values. This result is supported by findings of Rajkovic et al. [17]. They have shown that inhibitory effect of nisin and Carvacrol on Bacillus cereus has increased by reducing the temperature and pH. It can be a direct result of increase in solubility of EO and nisin in the lipid phase of cell membrane at low pH [18,19]. Synergistic effects of nicin and Garlic extract on six strains of L. monocytogenes in Tryptose Phosphate Broth liquid medium by Bhurinder et al. (2001), were studied, and a synergistic antibacterial effect on strains of Listeria was observed when using these two materials together [20]. Furthermore, the effect of other growth factors such as pH and temperatures (4 and 20°C) were studied and showed that increasing pH decreases the effect of both substances (EO and nisin). The effect of each substance at 4°C was higher than 20°C. Misaghi and Basti (2007), observed that Thyme EO and nisin each alone are effective on Bacillus cereus, but using the combination results in synergistic effects on inhibition of bacterial growth [21]. Decreasing temperature from 30°C to 10°C either has significantly increased antimicrobial effects. The increase in the pH value has reduced the effects of nisin.
There was a close relationship between the constituents of the EO and biological functions. It was reported that essential oils can affect the cell wall integrity due to their hydrophobic nature which influences the lipids and disrupts normal cell wall function leading to nutrients leakage and cell death [2,4,22]. As the result showed (Table 1), the inhibitory properties of EO affected by temperature and the pH values and increased inhibition effects of EO on bacterial growth were observed by reducing incubation temperature and the pH values (P<0.05).
The effect of the M. longifoli L. EO on L. monocytogenes cells has never been scientifically evaluated before. On the basis of present microscopic analyzes of the bacterial cells, it can be concluded that after treatment with M. longifoli EO at MIC level, morphology, and shape of the cells was distorted, and cell wall damage and removal of cellular contents and cellular disturbance was observed (Figure 1), while the cell morphology appeared normal in the control group.
Due to the low infectious dose of many food-borne pathogens, the application of spice oil components to improve food safety requires the development of bactericidal treatments. An understanding of the mechanism of action of these agents would allow prediction of organisms that can be expected to demonstrate sensitivity and food products to which these agents may be effectively applied. These results can be used to setup a combination of protective factors (known as Hurdle Technology) in the control of pathogenic bacterial growth, food poisoning and spoilage. Furthermore, knowledge of the mechanism of action would allow the rational development of treatments using combinations of antimicrobial agents that target different cell functions in a complimentary manner.
The authors express their sincere appreciation and thanks for the financial support received from Faculty of Veterinary Medicine, University of Tabriz for this research.
- Hyldgaard M, Mygind T, Meyer RL (2012) Essential oils in food reservation: modeofaction, synergies and interactions with food matrix components. Frontiers Microbiol 3: 12.
- Burt S (2004) Essential oils: their antibacterial properties and potential applications in foods-a review. Int J Food Microbiol 94: 223– 253.
- Kalemba D, Kunicka A (2003) Antimicrobial and antifungal properties of essential oils. Current Med Chem 18: 813-829.
- Gill AO, Holley RA (2006) Disruption of Escherichia coli, Listeria monocytogenes and Lactobacillus sakei cellular membranes by plant oil aromatics. Int J of Food Microbiol 108: 1-9.
- Lambert RJW, Skandamis PN, Coote P (2001) A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J Appl Microbiol 91: 453-462.
- Ultee A, Bennink MHJ, Moezelaarm R (2002) The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl Environ Microbiol 68: 1561-1568.
- Zani F, Massimo G, Benvenuti S, Bianchi A (1999). Studies on the genotoxic properties of essential oils with Bacillus subtilis rec-assay and Salmonella/microsome reversion assay. Planta Med 57: 237- 241.
- Ghoulami S, Idrissi A, Fkih-Tetouani S (2000) Phytochemical study of Mentha longifolia of Morocco Fitoterapia 72: 596-598.
- Rasooli I, Rezaei MB (2002) Bioactivity and chemical properties of essential oils from Zataria multiflora Boiss and Mentha longifolia (L.) Huds J Essent Oil Res 14: 141-146.
- Mimica-Dukic N, Bozin B, Sokovic M (2003) Antimicrobial and antioxidant activities of three Mentha species essential oils. Planta Med 69: 413-419.
- Mahmoudi R, Tajik H, Ehsani A (2012) Effects of Mentha longifolia L. essential oil on viability and cellular ultrastructure of Lactobacillus casei during ripening of probiotic Feta cheese. Int J Dairy Technol 66: 70-77.
- Gulluce M, Sahin F, Sokmen M (2007) Antimicrobial and antioxidant properties of the essential oils and methanol extract from Mentha longifolia L. ssp. longifolia. Food Chem 103: 1449-1456.
- Karousou R, Balta M, Hanlidou E (2007) “Mints”, smells and traditional uses in Thessaloniki(Greece) and other Mediterranean countries. J Ethnopharmacol 109: 248-257.
- Karaman IF, Sahin M, Gulluce H (2003) Antimicrobial activity of aqueous and methanol extracts of Juniperus oxycedrus L. J Ethnopharmacol 85: 231-235.
- Friedman JS, Gonzalez CA, Tepley Q (2000) Simultaneous atomic and ion layer enhancements observed in the mesopause region over Arecibo during the Coqui II sounding rocket campaign. Geophys Res Lett 27: 449-452.
- Lambert RJW (2000) Susceptibility testing: inoculum size dependency of inhibition using the Colworth MIC technique. J Appl Microbiol 89: 275-279.
- Rajkovic A, Uyttendaele M, Courtens T (2005) Antimicrobial effect of nisin and carvacrol and competition between Bacillus cereus and Bacillus circulans in vacuum-packed potato puree. Food Microbiol 22: 189–197.
- Periago PM, Moezelaar R (2001) Combined effect of nisin and carvacrol at different pH and temperature levels on the viability of different strains of Bacillus cereus. Int J Food Microbiol 68: 141–148.
- Koutsoumanis K, Lambropoulou K, Nychas GJE (1999) A predictive model for the non-thermal inactivation of Salmonella entritidis in a food model system supplemented with a natural antimicrobial. Int J Food Microbiol 49: 63–74.
- Bhurinder S, Falahee MB, Adams MR (2001) Synergistic inhibition of Listeria monocytogenes by nisin and garlic extract. Int J Food Microbiol 21: 133–139.
- Misaghi A, Basti AA (2007) Effects of Zataria multiflora Boiss. essential oil and nisin on Bacillus cereus ATCC 11778. Food Cont 18: 1043–1049.
- Palmer AS, Steward J, Fyfe L (2001) The potential application of plant essential oils as natural preservatives in soft cheese. Food Microbiol 18: 463–470.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
