International Journal of Veterinary Science and Research
Functional Properties (Acid and Bile Tolerance) and Antibiotic Susceptibility of Lactic Acid Bacteria Isolated from Newborn Calves for the Design of a Probiotic Product
Natalia C. Maldonado and María Elena Fátima Nader-Macías*
Cite this as
Maldonado NC, Nader-Macías MEF (2015) Functional Properties (Acid and Bile Tolerance) and Antibiotic Susceptibility of Lactic Acid Bacteria Isolated from Newborn Calves for the Design of a Probiotic Product. Int J Vet Sci Res 1(1): 011-022. DOI: 10.17352/ijvsr.000004Diarrhea in young calves in dairy farms is one of the main causes of economic losses, morbidity and mortality. The use of probiotic products as feed additives or complements is a novel alternative for the prevention of intestinal syndromes. In order to include beneficial bacteria in the design of a probiotic product, their functional and safety characteristics must be studied. The aim of this work is to evaluate the behavior of the strains in some “in vitro” gastrointestinal conditions such as acid stress and bile salts in the specific physiological concentration of young calves. The antibiotic susceptibility of a group of lactic acid bacteria from calves which were identified due to their beneficial properties was also studied. The strains, genetically identified and used for the resistance assays were: Lactobacillus johnsonii CRL1692, CRL1693, CRL1699, CRL1700, CRL1701 and CRL1706; L. amylovorus CRL1697; L. murinus CRL 1695 and CRL1705; L. mucosae CRL1696 and CRL1698; L. salivarius CRL1694 and CRL1702; and Enterococcus faecium CRL1703. The results of gut resistance assays showed that all the strains were resistant to pH 4 and to a bile salts concentration of less than 0.5%. However, some of them were sensitive to pH 2. The most pH-sensitive strains were found to be L. johnsonii and L. amylovorus, and enterococci. However, pre-treatment at low pH increased the growth rate of the L. salivarius strains. The minimal inhibitory concentration showed that the strains were sensitive to Tetracycline, Erythromycin, Chloramphenicol and Ampicillin, while most of them were resistant to Kanamycin. The results allowed the selection of the most adequate strains to be included in a probiotic product that can be utilized most successfully in young calves.
Introduction
One of the main causes of mortality in newborn calves of dairy farms is neonatal diarrhea, which causes severe economic losses [1-4]. As a novel alternative in this field, the use of probiotics as feed additives or complements is being proposed for the prevention of intestinal infections by restoring the balance of the microbiome. Probiotics are defined as “live microorganisms which when administered in adequate amounts confer a beneficial health on the host” [5]. In a previous work, fourteen Lactic Acid Bacteria (LAB) strains were selected for their beneficial properties to include some of them in the design a new probiotic product for newborn calves [6]. Later, the evaluation of the technological properties of most strains was also performed [7]. At the same time, functional properties and others related to the safe status of the strains must be determined for the final design of such product [8]. As the probiotic supplement is going to be administered orally, the bacterial constituents should be resistant to the conditions of the gastrointestinal tract (GIT). Moreover, the number of live organisms that reach the gut should be large enough to produce their beneficial effect on the host [9]. On the other hand, LAB can be reservoirs of resistance genes, which could be transferred to other bacteria and even to human pathogens [10], which is why some of their safety-related characteristics should be studied. The probiotic product on which we are working is directed to newborn calves, recognized as non-ruminant up to the time when the rumen becomes functional, which means that the digestive process is developed in a different way than in young animals. In calves, gastric juices are secreted daily by the stomach, resulting in the destruction of most of the microorganisms ingested with the food. Then, resistance to the acid conditions of the stomach is one of the selection criteria applied in the selection of probiotic bacteria [11].
Bile salts, which act as detergents that emulsify and solubilize fats, have been shown to exert a bactericidal effect. Consequently, the capability of LAB to resist bile is essential to maintain their viability in the intestine [12,13].
In the last decade the evaluation of the antibiotic susceptibility of LAB has grown because of their potential to spread resistance genes to other microorganisms by horizontal transfer resistance [14-17]. The evaluation of the antimicrobial susceptibility of microorganisms can be performed using different methods, including agar diffusion, E-test, macro and micro dilution [18-20]. Also, techniques included in the International Organization of Standardization/International Dairy Federation (ISO/IDF) Standard and Clinical and Laboratory Standards Institute (CLSI) guidelines [21], for Antimicrobial Susceptibility Testing of Lactobacilli have often been applied. For the determination of minimal inhibitory concentration (MIC), the EFSA (European Food Safety Authority) Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) has proposed epidemiological breakpoints to evaluate and identify phenotypic resistance. Moreover, the European Union has strongly recommended the study of the antibiotic susceptibility of bacteria included in feed additives for veterinary use and later used in the Food Chain.
The aim of this work was to evaluate some of the functional (acid and bile tolerance) and safety (antibiotic susceptibility) related properties of a LAB strains group previously selected for their beneficial characteristics to be used in the design of a probiotic product to prevent diarrhea in newborn calves.
Material and Methods
The study area
LABs were isolated from young calves’ feces and selected by their beneficial properties [6]. They were genetically identified as: Lactobacillus johnsonii CRL1692, CRL1693, CRL1699, CRL1700 and CRL1701 CRL1706, Lactobacillus amylovorus CRL1697 Lactobacillus murinus CRL 1695 and CRL1705, Lactobacillus mucosae CRL1696 and CRL1698, Lactobacillus salivarius CRL1694 and CRL1702, and Enterococcus faecium CRL1703. The strains were stored in milk yeast extract (13% nonfat milk, 1% yeast extract) containing 20% glycerol (vol/vol) at –20ºC. For the daily experiments, the strains were cultured in MRS broth [22] (Merck, Darmstadt, Germany) at 37°C for 24 h, and sub-cultured twice in the same media at 37°C for 12 h and 16 h respectively.
Resistance to gastrointestinal conditions
The strains were washed twice and the pellets were re-suspended in saline solution at neutral pH 7.0 (control) or at pH 2 or pH 4 adjusted with 1N HCl (Anedra, San Fernando, Argentina) and incubated for 90 min at 37ºC (the estimated time that the liquid food remains in the abomasum during the calves’ gastric digestion) [23]. After incubation, the bacterial cells were washed three times in standard saline solution (0.9% NaCl, Anedra, San Fernando, Argentina). These cultures were adjusted to 0.9-1 Optical Density at 560nm and later 2 µl corresponding to 5 x 108 CFU/mL) were inoculated into polystyrene microplates (Deltalab SL, Barcelona, Spain) containing MRS (Merck, Darmstadt, Germany) broth supplemented with different bovine ox-bile concentrations (0.1%, 0.3%, 0.5% and 1%) (Fluka-70168, Sigma-Aldrich, St. Louise, USA) and also into MRS pH 4 (adjusted with 1N HCl). The bacterial growth was determined by changes in the Optical Density (OD) at 560nm in a microplate reader (VersaMax Tunable Microplate reader, Sunny Valley, USA) at different time periods (3, 5, 7, 12, 20 and 24 h). Final ΔOD was calculated as the increase in OD between OD0 (OD0 is OD at t=0) and OD24 (OD24 is OD at t=24). The growth curves were performed from the OD of each time period. The growth rate was calculated as: µ/h (growth rate); µ = ln2/g; generation time g = (t2 - t1) log2/ (log OD2 - log OD1).
Statistical analyses
Growth determinations were performed in triplicate. Data express the mean ±SD. The experimental results were used to create the Main Effects Plot using the Minitab 16 Statistical Software.
Antibiotic susceptibility
The strains selected to determine the antibiotic susceptibility were those expressing beneficial properties: surface characteristics (hydrophobicity degree and autoaggregation patterns), antagonistic activity (production of hydrogen peroxide, organic acids, or bacteriocins) and functional properties (resistance to acid and bile salts). In all cases, only one of each of the species of Lactobacillus under study was included in these assays.
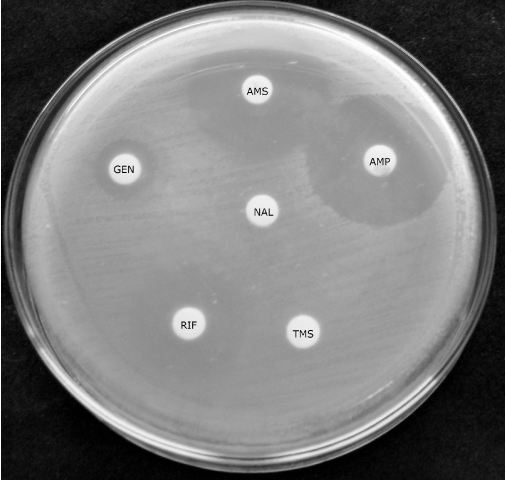
Disk diffusion: The Kirby-Bauer disk diffusion susceptibility test was carried out according to the Performance Standards for Antimicrobial Disk Susceptibility Tests of CLSI with minor modifications [24]. The technique was performed in three different culture media: MRS agar pH 6.2; LAPTg agar (15 g/L peptone, 10 g/L tryptone, 10 g/L glucose, 10 g/L yeast, 1 ml/L Tween 80, 15 g agar, distilled water 1L), LAPTg agar pH 6.5 and LSM agar [25], (LAB susceptibility test medium). This last medium is formulated with 90% Müller Hinton agar (Britania, Buenos Aires, Argentina) and 10% MRS agar. The antibiotics assayed were: Sulfamethoxazole+Trimethoprim (TMS) 25 µg, Clindamycin (CLIN) 2 µg, Erythromycin (ERY) 15 µg, Gentamicin (GEN) 10 µg, Ampicillin (AM) 10 µg, Vancomycin (VAN) 30 µg, Nalidixic Acid (NAL) 30 µg, Cephalexin (CEF) 30 µg, Ciprofloxacin (CIP) 5 µg, Ampicillin Sulbactam (AMS) 30µg, Teicoplamin (T) 30 µg, Rifampicin (RFA) 5 µg, and Minomicyn (MIN) 30 µg. All disks were obtained from Britania (Buenos Aires, Argentina). For the inoculum preparation, LABs were spread on MRS agar and incubated at 37°C for 48 h, and isolated colonies were suspended to McFarland standard 1. Later, the strains were spread onto the different media where the antibiotic disks were added. The plates were incubated for 48 h and the diameter of the inhibition zone was determined after incubation [26].
Minimum inhibitory concentration (MIC): The Minimum Inhibitory Concentration (MIC) was determined by the microdilution method in solid media in LSM agar (LAB Susceptibility test medium) as the culture medium [25]. The bacteria assayed at this stage were: L. salivarius CRL1694, L. amylovorus CRL1697, L. johnsonii CRL1693, L.mucosae CRL1696 and L. murinus CRL1695. The following antibiotics and concentration ranges were assayed: Vancomycin (0.25-128 μg/mL), Rifampin (0.25-128 μg/mL), Ciprofloxacin (0.25-128 μg/mL), Ampicillin (0.25-128 μg/mL), Chloramphenicol (0.12-64 μg/mL), Tetracycline (0.12-64 μg/mL), Oxytetracycline (0.12-64 μg/mL), Lincosamide (0.12-64 μg/mL), Kanamycin (2-1024 μg/mL), (Sigma-Aldrich, St Louis, USA) and Erythromycin (0.25-128 μg/mL) (ICN Biomedicals, Santa Ana, USA).
Antibiotics were stored at -20°C until the preparation of stock solutions, re-suspending antibiotics in distilled water or methanol (Cicarrelli, San Lorenzo, Argentina) for those not soluble in water (Chloramphenicol, Erythromycin, Rifampicin), and dilutions were performed in twofold series according to CLSI specifications [27]. Agar plates were prepared with 1 mL of each of the antibiotic solutions and 9 mL of LSM agar melted and cooled to 45±5°C. The bacterial inoculum was adjusted to 0.16 to 0.2 OD at 625nm, corresponding to approximately 3x108 CFU/mL and to McFarland standard 1, following the procedures proposed by ISO 10932/IDF 233 described in Shao et al. [28]. In order to determine the effect of different bacterial concentrations (105 CFU/mL, 106 CFU/mL and 107 CFU/mL) in the susceptibility assays, serial dilutions in saline solution were prepared and added to the plates. All the plates were inoculated with 2 µL of bacterial suspensions and incubated for 48 h in microaerophilic conditions. MIC was calculated from the lowest antibiotic concentration that caused inhibition of the microorganism. The interpretation of the results was performed was performed on the basis of the EFSA document [15], according to the results obtained with the highest microorganism concentration. The L plantarum ATCC14917 strain was also used as quality control. All the antibiotic assays were performed in triplicate.
Results
Resistance of beneficial LAB strains to gastrointestinal conditions (acid and bile salts)
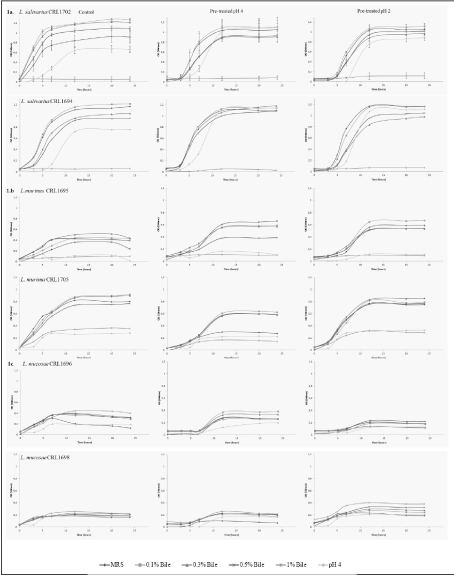
The resistance of beneficial LAB strains to bile salts after pre-treatment at pH 4 and pH 2 was determined in all the strains used. Growth curves were plotted to compare the behavior of each strain, and also those from the same species in a combined figure. The standard deviation was not included in the graphs, except for L. salivarius CRL1702 in order to allow a better interpretation of the results. The resistance of the two L. salivarius (CRL1694 and CRL1702) and L. mucosae (CRL1696 and CRL1698) strains to the acid conditions and bile salts was similar for each species (Figures 1a,1b). However, in the case of L. murinus (CRL 1695 and CRL1705), the two strains showed a different behavior: CRL 1705 grew at pH 4 and 1% bile salts, but both strains proved to be more resistant after pretreatment at pH 2. In the L. salivarius strains, acid pretreatment improved their growth at all bile concentrations used (with the exception of 1% where the strains failed to grow) and also at pH 4. The two L. mucosae strains grew in all conditions assayed when compared with controls; however, their growth was lower than all the other species (Figure 1c). The growth curves of Enterococcus and L. amylovorus strains are shown in Figures 2a,1b. The six L. johnsonii strains exhibited a different behavior, with two different patterns or profiles. The strains CRL 1692, CRL1693 and CRL1700 pretreated at pH 4 showed a higher growth, in contrast with CRL 1966, CRL1701 and CRL 1706, which were stimulated when the acid pretreatment was at pH 2, as indicated in Figure 3.
All the strains assayed increased the lag phase length in bile salts after the pretreatments at low pH, either 2 or 4.
When all the strains were pretreated at pH 2, 4 or 6.5 and later transferred to evaluate their growth at pH 4, Lactobacillus johnsonii and L. murinus CRL1695 strains or enterococcus did not grow, in contrast to both L. salivarius and L. mucosae strains, and L. murinus CRL1705, which grew in acid conditions, as indicated in Table 1. The strains that were able to grow at higher bile salts concentrations were L. mucosae CRL1696 and CRL1698, L. amylovorus CRL 1697, E. faecium CRL 1703 and L. murinus CRL 1705; the other strains grew at a bile concentration of 0.5% or less. Most of the strains treated in acid conditions (pH 4) and later transferred to MRS-0.1%, 0.3% and 0.5% bile did not modify their growth, but in some cases their growth rates were lower (L. murinus CRL 1695 L. mucosae CRL1696 and CRL1698), as shown in Table 2. In addition, the growing and growth rate of L. murinus CRL 1695, L. salivarius, L. amylovorus CRL1697 and enterococcus strains pretreated at pH 2 were similar to those observed after pH 4 pretreatment. The growth rates of L. salivarius and L. murinus increased in MRS-bile salts pretreated at pH 2.
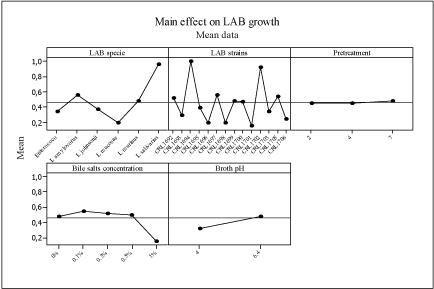
The application of the main effect analysis to the growth and growth rate data of all LAB strains used, analyzing strain, bile salt concentrations, acid pretreatment and growth at different pH values, resulted in the plot in Figure 4. L. salivarius CRL1694 and CRL1702 proved to be the strains with higher growth in all conditions studied. In contrast, L. mucosae CRL1696 and CRL1698, L. johnsonii CRL1699 and L. johnsonii CRL1701 showed a lower growth. Pretreatment in acid conditions (pH 2-pH 4) did not affect growth at pH 4 or in bile salts of all the strains. The highest bile concentration exerted a higher negative effect on the growth of all the strains.
With respect to the growth rate, the same pattern was obtained for all the strains (data not shown).
Antimicrobial susceptibility testing
In this paper, the evaluation of the most suitable media to determine the antibiotic susceptibility or resistance of beneficial LAB isolated from calves’ feces was carried out. The disk diffusion assay showed that Gentamicin, Ciprofloxacin and Cephalexin were the antibiotics showing different patterns according to the culture media, because different inhibitions zone diameters were obtained in MRS, LAPTg and LSM. Also, Rifampicin, Erythromycin and Ampicillin Sulbactam showed different diameters of the inhibition zone. Minomycin was the antibiotic that caused the highest inhibition in all strains assayed. There were no inhibition halos for Sulfamethoxazole+Trimethoprim and Nalidixic Acid, as shown in Figure 5. Considering the results for each strain, L. mucosae CRL1696 grew poorly in LMS medium, L. amylovorus CRL1697 was the most sensitive, and L. johnsonii CRL1693 and L. amylovorus were inhibited by Vancomycin. These results are summarized in Table 3.
The MIC values obtained in solid media were compared with those suggested by EFSA [15], as shown in Table 4. All the strains were sensitive to Erythromycin, Ampicillin and Chloramphenicol but were resistant to Kanamycin (except for L. mucosae CRL1696). The MIC values of all the strains were slightly higher for Oxytetracycline than for Tetracycline, except for L. johnsonii CRL1693l. The MIC assays using different concentrations of the inoculum performed with the macro dilution agar technique indicated differences of one dilution, mainly in Kanamycin and Oxytetracycline.
When comparing the two methods, the resistance to Vancomycin of those strains carrying intrinsic resistance to this antibiotic showed similar patterns. No inhibition zones were observed in the disk assay and MIC values indicated that the cut-off points were higher than those suggested by EFSA [15].
Discussion
Over the last few decades probiotics have been used in the gut, skin, respiratory or urogenital mucosa of both humans and animals [29-31]. One of the main objectives of these feed additives or complements is the restoration of the indigenous microbiome of different tracts, supported by the host-specificity evidenced some years ago [32-34]. Some authors suggest that the autochthonous microbiota of the intestine could be better adapted to gastrointestinal conditions, and also that the bacterial resistance profiles of some of the microorganisms could be influenced by the region of the tract [13].
A basic characteristic to be evaluated when working in the design of a probiotic adjunct or complement to a specific host is its resistance to the conditions where it will be applied, the so-called functional properties [11]. On the other hand, viable bacteria in high numbers should be able to exert their beneficial effect on the target organ or mucosae. The microorganisms should cross over several biological barriers through the GIT, including gastric acid, enzymes, secretions and bile salts [12,13]. The digestive system of newborn calves is different from that of adult; in fact, calves behave as monogastrics up to the development of rumen in older animals.
The capability of the bacteria to survive the passage through the GIT is variable and strain dependent [35]. Lactic Acid Bacteria have several mechanisms that confer resistance in acidic conditions [36]. According to our results, each strain showed a different resistance profile and different sensitivity to acid in the presence of bile. However, those strains that produce higher concentrations of lactic acid [6], were the most sensible to these conditions. Similar results were obtained by other authors, where resistance to gastrointestinal conditions varied with the microorganism [37,38].
Lactic Acid Bacteria can also induce stress tolerance responses [39]. Our results suggest that the growth of microorganisms previously treated in acid conditions was higher in bile. All the strains should increase the lag phase length as observed in the growth curves. This behavior could suggest an adaptation of the strain; Burns et al. [35], evaluated the pre-adaptation and cross-resistance mechanisms to gastric conditions submitting the strains to sub-lethal acid stress. The resistance patterns observed in this group of strains could support a better adaptability of certain bacteria that should be further studied. The beneficial properties evaluated in a previous work [6], indicated that there is no relation between hydrophobicity or auto aggregation and resistance profiles.
The antibiotic susceptibility of LABs should be determined to prevent the incorporation of multi-resistance strains into the Food Chain or feed additives for animals, as stated before [40]. The standardization of the method for the assessment of antibiotic susceptibility and its interpretation was determined by different research groups [16,19,21,25,28,41,42], in order to generate a data base to establish and compare susceptibility profiles in different hosts and areas. Moreover, the selection of the technique is essential to determine the resistance criteria, as suggested by Mayrhofer et al. [21], who compared the procedures of CLSI and ISO/ IDF Standard guides. The disk diffusion method was applied for LAB against antibiotics of different groups. The LSM media was compared with two frequent media use for lactobacilli, which are nutritionally demanding bacteria. The diffusion of the antibiotic can be modified by the composition or pH of the media and is of major importance to obtain reproducible results [24]. In the case of LABs, different media were used [42-44]. In this work, the LSM media proposed by Klare et al. [25], and studied by others authors was included [16,42], supporting the growth of most of the strains, with the exception of L. mucosae strains. Higher inhibitions zones were observed in LSM, which could indicate a better diffusion compared with LAPTg media. Similar results were obtained by Ocaña et al. [43], using MRS and LATPg.
The selection of antibiotics for MIC assays was performed according to EFSA 2012 and Klare et al. [16,25]. Microorganism concentration, incubation time and atmospheric conditions must be defined. Egervärn et al. [42], observed that high inoculum concentrations and longer incubation periods in the microdilution test increase MIC values. Our results showed that some resistances profiles were affected by the inoculum of the bacteria and supported the importance of standardization of the inoculum to compare results. MIC values were compared with others authors, Kanamicyn resistance were also observed in lactobacillus isolated from pigs, human GUT and food [45-47]. The intrinsic resistance of heterofermentative lactobacilli to Vanomicyn, which was phenotypically determined in the strains, does not present the risk of gene transfer [14,18,40].
Conclusions
According to our results, the behavior of LAB in acid conditions and bile salts cannot be predicted based on bacterial species and should be evaluated in each of the strains. Although some of the strains showed similar resistant patterns, since all the strains were be affected by the acid pretreatment, each strain was affected in a different way, and in some cases the strain became more resistant. On the other hand, all the strains proved to be resistant to 0.5% bile salts after acid pretreatment.
The antibiotic resistance profile of the strains indicated that the culture media affected the inhibition zone in the disk diffusion technique for most of the antibiotics assayed. Also, almost all the strains grew in LSM media, proposed by the ISO/IDF Standard guidelines. The phenotypic studies of microbial resistance of the strains according to the EFSA breakpoints indicated they are sensitive to the antibiotics evaluated, except for Kanamicyn. Some of these strains are being included in the design of a probiotic product for calves to prevent diarrhea (Table 5).
This work was supported by CONICET (Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina) (PIP 632 and 744) and ANPCyT (Agencia Nacional de Promoción Científica y Tecnológica) (PICT 2007-543 and PICT 2012-1187). Some of the strains were included in a patent form presented by CONICET at INPI.
- Cho YI, Yoon K J (2014) An overview of calf diarrhea - infectious etiology, diagnosis, and intervention. J Vet Sci 15: 1-17.
- Klein-Jöbstl D, Arnholdt T, Sturmlechner F, Iwersen M, Drillich M (2015) Results of an online questionnaire to survey calf management practices on dairy cattle breeding farms in Austria and to estimate differences in disease incidences depending on farm structure and management practices. Acta Vet Scand 57: 44.
- Torsein M, Lindberg A, Sandgren CH, Waller KP, Törnquist M, et al. (2011) Risk factors for calf mortality in large Swedish dairy herds. Prev Vet Med 99:136-147.
- Uyeno Y, Shigemori S, Shimosato T (2015) Effect of Probiotics/Prebiotics on Cattle Health and Productivity. Microbes Environ 30: 126-132.
- Reid G, Sanders ME, Gaskins HR, Gibson GR, Mercenier A, et al. (2003) New scientific paradigms for probiotics and prebiotics. J Clin Gastroenterol 37:105-118.
- Maldonado NC, de Ruiz CS, Otero MC, Sesma F, Nader-Macías ME (2003) Lactic acid bacteria isolated from young calves--characterization and potential as probiotics. Res Vet Sci 92: 342-349.
- Maldonado NC, Silva de Ruiz C, Nader-Macías MEF (2015) Design of a beneficial product for newborn calves by combining lactobacilli, minerals and vitamins. Prep Biochem and Biotech 2015. In press.
- Bujnakova D, Strakova E, Kmet V (2014) In vitro evaluation of the safety and probiotic properties of Lactobacilli isolated from chicken and calves. Anaerobe 29: 118-127.
- Fuochi V, Petronio GP, Lissandrello E, Furneri PM (2015) Evaluation of resistance to low pH and bile salts of human Lactobacillus spp. isolates. Int J Immunopathol Pharmacol 28: 426-433.
- Egervarn M, Lindmark H, Olsson J, Roos S (2010) Transferability of a tetracycline resistance gene from probiotic Lactobacillus reuteri to bacteria in the gastrointestinal tract of humans. Antonie Van Leeuwenhoek 97:189-200.
- Binns N (2013) Probiotics, prebiotics and the gut microbiota. International Scientific Association for Pro & Prebiotics University of Reading.
- Begley M, Gahan CGM, Hill C (2005) The interaction between bacteria and bile. FEMS Microbiol Rev 29: 625-651.
- Watson D, Sleator RD, Hill C, Gahan CGM (2008) Enhancing bile tolerance improves survival and persistence of Bifidobacterium and Lactococcus in the murine gastrointestinal tract. BMC Microbiol 8: 176.
- Ammor MS, Belén Flórez A, Mayo B (2007) Antibiotic resistance in non-enterococcal lactic acid bacteria and bifidobacteria. Food Microbiol 24: 559-570.
- (2012) EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J 10: 2740.
- Teuber M (1999) Spread of antibiotic resistance with food-borne pathogens. Cell Mol Life Sci 56: 755-763.
- Van Reenen C, Dicks LMT (2011) Horizontal gene transfer amongst probiotic lactic acid bacteria and other intestinal microbiota: What are the possibilities? A review. Arch Microbiol 193: 157-168.
- Danielsen M, Wind A (2003) Susceptibility of Lactobacillus spp. to antimicrobial agents. Int J Food Microbiol 82: 1-11.
- Huys G, D'Haene K, Cnockaert M, Tosi L, Danielsen M, et al. (2010) Intra- and interlaboratory performances of two commercial antimicrobial susceptibility testing methods for bifidobacteria and nonenterococcal lactic acid bacteria. Antimicrob Agents Chemother 54: 2567-2574.
- Mayrhofer S, van Hoek AH, Mair C, Huys G, Aarts HJ, et al. (2003) Antibiotic susceptibility of members of the Lactobacillus acidophilus group using broth microdilution and molecular identification of their resistance determinants. Int J Food Microbiol 144: 81-87.
- Mayrhofer S, Zitz U, Birru FH, Gollan D, Gołoś AK, et al. (2014) Comparison of the CLSI guideline and ISO/IDF standard for antimicrobial susceptibility testing of Lactobacilli Microb Drug Resist 20: 591-603.
- De Man JC, Rogosa M, Sharpe ME (1960) A medium for the cultivation of lactobacilli. J Appl Bacteriol 23: 130-135.
- Church DC (1980) Digestive Physiology and Nutrition of Ruminants Corvallis: O & B Books Inc.
- CLSI (2012) Performance Standards for Antimicrobial Disk Susceptibility Tests. Approved Standard-Eleventh Edition. CLSI document M02-A11 950. Wayne PA: Clinical and Laboratory Standards Institute.
- Klare I, Konstabel C, Müller-Bertling S, Reissbrodt R, Huys G, et al. (2005) Evaluation of New Broth Media for Microdilution Antibiotic Susceptibility Testing of Lactobacilli, Pediococci, Evaluation of New Broth Media for Microdilution Antibiotic Susceptibility Testing of Lactobacilli, Pediococci, Lactococci, and Bifidobacteria. Appl Environ Microbiol 71: 8982-8986.
- Sigrid Mayrhofer, Konrad J. Domig, Christiane Mair, Ulrike Zitz, Geert Huys, et al. (2008) Comparison of broth microdilution, E test, and agar disk diffusion methods for antimicrobial susceptibility testing of Lactobacillus acidophilus group members. Appl Environ Microbiol 74: 3745-3748.
- CLSI (2006) Performance Standard Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A7. Wayne PA: Clinical and Laboratory Standards Institutes.
- Yuyu Shao, Wenyi Zhang, Huiling Guo, Ling Pan, Heping Zhang, et al. (2015) Comparative studies on antibiotic resistance in Lactobacillus casei and Lactobacillus plantarum. Food Control 50: 250-258.
- Gaggìa F, Mattarelli P, Biavati B (2010) Probiotics and prebiotics in animal feeding for safe food production. Int J Food Microbiol 141: S15-S28.
- Hersom M, Imler A, Thrift T, Yelich J, Arthington J (2015) Comparison of feed additive technologies for preconditioning of weaned beef calves. J Anim Sci 93: 3169-3178.
- Hou C, Zeng X, Yang F, Liu H, Qiao S (2015) Study and use of the probiotic Lactobacillus reuteri in pigs: a review. J Anim Sci Biotechnol 6: 14.
- Dogi C, Perdigón G (2006) Importance of the host specificity in the selection of probiotic bacteria. J Dairy Res 73: 357-366.
- Giraffa G (2012) Selection and design of lactic acid bacteria probiotic cultures. Eng Life Sci 12: 391-398.
- Lebeer S, Vanderleyden J, De Keersmaecker SCJ (2010) Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat Rev Microbiol 8: 171-184.
- Vinderola G, Binetti A, Burns P, Reinheimer J (2011) Cell viability and functionality of probiotic bacteria in dairy products. Front Microbiol 2: 1-6.
- Van de Guchte M, Serror P, Chervaux C, Smokvina T, Ehrlich SD, et al. (2002) Stress responses in lactic acid bacteria. Antonie Van Leeuwenhoek 82: 187-216.
- Frizzo LS, Soto LP, Bertozzi E, Sequeira, G, Marti LE, et al. (2006) Evaluación in Vitro de las Capacidades Probióticas Microbianas Orientadas al Diseño de Inóculos Probióticos Multiespecie para Ser Utilizados en la Crianza de Terneros. FAVE Sección Ciencias Vet 5: 69-80.
- Park SC, Hwang MH, Kim YH, Kim JC, Song JC, et al. (2006) Comparison of pH and Bile Resistance of Lactobacillus acidophilus Strains Isolated from Rat, Pig, Chicken, and Human Sources. World J Microbiol Biotechnol 22: 35-37.
- Cotter PD, Hill C (2003) Surviving the acid test: responses of gram-positive bacteria to low pH. Microbiol Mol Biol Rev 67: 429-453.
- Mathur S, Singh R (2005) Antibiotic resistance in food lactic acid bacteria—a review. Int J Food Microbiol 105: 281-295.
- Klose V, Bayer K, Kern C, Goelß F, Fibi S, et al. (2014) Antibiotic resistances of intestinal lactobacilli isolated from wild boars. Vet Microbiol 168: 240-244.
- Egervärn M, Lindmark H, Roos S, Huys G, Lindgren S (2007) Effects of inoculum size and incubation time on broth microdilution susceptibility testing of lactic acid bacteria. Antimicrob Agents Chemother 51: 394-396.
- Ocaña V, Silva C, Nader-Macías ME (2006) Antibiotic Susceptibility of Potentially Probiotic Vaginal Lactobacilli 2006: 1-6.
- Lima KGDC, Kruger MF, Behrens J, Destro MT, Landgraf M, et al. (2009) Gombossy de Melo Franco BD. Evaluation of culture media for enumeration of Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium animalis in the presence of Lactobacillus delbrueckii subsp bulgaricus and Streptococcus thermophilus. LWT - Food Sci Technol 42: 491-495.
- Flores G (2007) Perfiles de susceptibilidad/resistencia a antibióticos en bacterias del ácido láctico y bifidobacterias. Caracterización molecular de genes de resistencia. España: Tesis Dr Univ Oviedo.
- Delgado S, Flórez AB, Mayo B (2005) Antibiotic Susceptibility of Lactobacillus and Bifidobacterium Species from the Human Gastrointestinal Tract. Curr Microbiol 50: 202-207.
- Korhonen JM, Danielsen M, Mayo B, Egervärn M, Axelsson L, et al. (2008) Antimicrobial susceptibility and proposed microbiological cut-off values of Lactobacilli by phenotypic determination. Int J Probiotics Prebiotics 3: 257-268.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
