International Journal of Vascular Surgery and Medicine
Carotid anastomosis using diode lasers of different wavelengths and the evaluation of the arterial wall resistance in swine
Luciane Basilio Aledi*, Djalma José Fagundes and Helio Plapler
Cite this as
Aledi LB, Fagundes DJ, Plapler H (2018) Carotid anastomosis using diode lasers of different wavelengths and the evaluation of the arterial wall resistance in swine. Int J Vasc Surg Med 4(1): 004-009. DOI: 10.17352/2455-5452.000031Objective: due to the advantages of Laser Assisted Vascular Anastomosis (less occurrence of myointimal hyperplasia with better hemodynamic evolution, shorter surgical time, absence of diameter incompatibility, absence of anaphylaxis, easy execution) the evaluation of the ideal diode laser wavelength to perform anastomosis in elastic arteries, without solder, is a great advance in the surgical practice.
Method: End-to-end anastomosis was performed on the common carotid arteries of swines, bilaterally, using diode lasers by the wavelengths: 808 nm (n=16), 980 nm (n=16), 1470 nm (n=16) and 1908 nm (n=16) with the same parameters (CW, spot size= 2mm, P≅ 5,1W, t=26s, E= 132,6J, I= 164,51 W/cm2, F= 4277,4 J/cm2). Following, the occurrence of bleeding was verified. When the anastomosis did not bleed a mechanical resistance test was performed. Results: In group 808 nm, there was no welding of the vessels. In group 1908 nm, carbonization of all arterial edges was observed. In groups 980 nm and 1470 nm, the anastomosis results were satisfactory. In group 980 nm, 50% of the anastomosis cases exhibited bleeding and the medium leaking point pressure was 155 ± 56,3 mmHg. In group 1470 nm, 31,3% of the anastomosis cases exhibited bleeding and the medium leaking point pressure was 179,1 ± 37,0 mmHg. There was no statistically significant difference in leaking point pressures between the groups 1470 nm and 980 nm (p=0,094). Based on the physiological blood pressure levels for group 980 nm, the bleeding risk was higher and the survival probability was lower than that of group 1470 nm (p=0,022).
Conclusions: The wavelength that presented the best results for anastomosis in elastic arteries without solder, in experimental swine model, was 1470 nm. The higher survival probability and lower bleeding risk was achieved on anastomosis using 1470nm. Further studies are required to investigate the other laser dosimetry parameters.
Introduction
Arterial anastomosis is routinely used to reconstruct the blood flow and can be performed by various techniques [1], clips, staples, cyanoacrylate glue, fibrin glue, extra luminal rings, laser, conventional suture and magnetic compression anastomosis (magnamosis) [2-4], all of which have limitations. The gold-standard technique is the conventional suture; however, this technique can lead to a reaction of the arterial wall with the foreign suture material. This foreign body reaction of intima and media might contribute to myointimal hyperplasia5. In laser-assisted vascular anastomosis (LAVA), fewer or no sutures are required. If the thermal damage to the intima and media caused by the laser energy is controlled, then, less myointimal hyperplasia might be expected at the site of the anastomosis, with better hemodynamic evolution along with shorter surgical time, easy execution, absence of anaphylaxis to the material used and no diameter incompatibility [5,6].
CO2 LAVA leads to a high rate of postoperative aneurysm formation, resulting in its lack of use, despite providing excellent wall tensile strength [7]. Other lasers, such as argon ion (488 nm and 514,5 nm) and diode (670 nm) presented low rate of postoperative aneurysm formation, although a low tensile strength was observed if no solder was used [8]. Diode lasers, especially at wavelengths of 1900 nm [9-11], 1470 nm [12], 980 nm [9-13] and 810 nm [9] showed satisfactory results regarding tensile strength without high rates of aneurysm formation.
Jonge et al. [9], reviewed LAVA from 1966 to 2002. In most studies, arteriotomies were performed in low-caliber arteries using solder, which may suffer liquefaction with the application of the laser, thus reducing the reproducibility. No percentage of anastomosis with bleeding was reported; only the patency rate, aneurysm rate, and leakage pressures were reported.
Leclere et al. [14], used LAVA for the vascular reconstruction of microvessels in emergency hand surgeries using a 1950-nm laser diode (used in previous works of the same group that consider it to be the ideal wavelength for microvascular anastomosis). The strength of the anastomosis was comparable to that of the sutures, and the occurrence of aneurysms was minimal.
Because of the advantages of LAVA previously mentioned, the widespread use of this technique could bring great benefits. Despite decades of LAVA studies, there are doubts regarding the ideal diode laser wavelength to perform arterial anastomosis in elastic arteries without solder.
Methods
This research was approved by Ethics Committee of the Universidade Federal de São Paulo (CEUA number 6730140217).
The same surgeon performed all the procedures.
The animals received Acepromazine 1% Vetnil® (0,1 mg/kg I.M) associated with Midazolan 1mg/mL Vet Smart® (0,5 mg/kg I.M) as preanesthetic medication. After 15 minutes, the marginal ear vein was cannulated, and then, saline 0,9% was infused (10 ml/kg/h). Anesthetic induction was performed with Tiletamine-Zolazepam Vet Smart® (0,2 mg/kg I.V) and Pancuronium Vet Smart® (0,066 mg/kg I.V), followed by endotracheal intubation [15]. Isoflurane Vet Smart® was used for anesthesia maintenance and Fentanyl Vet Smart® (0,01 mg/kg I.V) for analgesia. The animals were maintained under positive pressure ventilation, with inspiration/expiration ratio of 2:1 [15,16].
In total, 40 common carotid arteries from 20 Landrace swines, weighing 8 to 10 kg and 3 to 5 months old, were identified and randomly distributed.
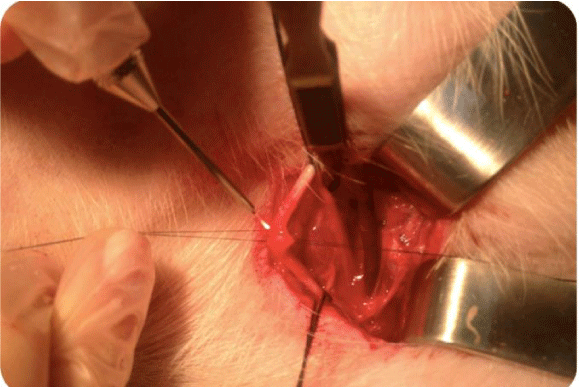
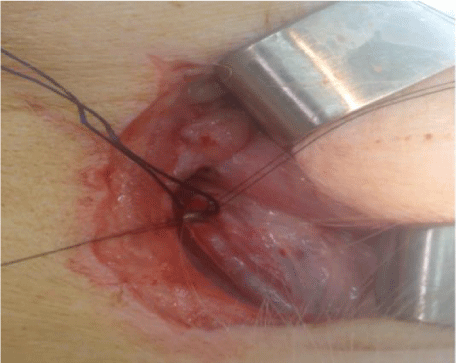
An incision on each side of the cervical region was made, which was parallel to the trachea over the site of carotid artery (diameter of 2 mm), measuring 10 cm and exposing the artery site. The carotid sheath was approached, and carotid dissection was performed and systemic heparinization with 250 UI/Kg; after 3 minutes, repair of the common carotid artery with vessel loops was performed, along with proximal and distal clamping of this artery and the total section. Two diametrically opposite repair points were established in the arteries using polypropylene 9.0. After the coaptation of the arterial edges by traction of the polypropylene, the laser was applied once between the repair points to perform the end-to-end anastomosis on the groups (Figures 1,2).
Group 1: 4 samples. Anastomosis was performed with an 808-nm diode laser (InGaAs, manufacturer DMC, São Carlos SP-Brazil, model MedLaser 808®) bilaterally in the common carotid arteries, with the following settings: CW mode, power (P)= 5,1 W, time (t)=26 s, energy (E)= 132,6 J, fiber diameter= 400 µm unfocused and power density (PD)= 164,51 W/ cm2 9.
Group 2: 16 samples. Anastomosis was performed with a 980-nm diode laser (InGaAs, manufacturer DMC, São Carlos SP- Brazil, model MedLaser 980®) bilaterally in the common carotid arteries, with the following settings: CW mode, P= 5,0 W (this was the intrinsic power of the device closest to the 5,1 W that was standardized), t=26 s, energy (E)= 130 J, fiber diameter= 400 µm unfocused and PD= 161,36 W/ cm2 9
Group 3: 16 samples. Anastomosis was performed with a 1470-nm diode laser (InGaAsP, manufacturer DMC, São Carlos SP-Brazil, model MedLaser 1470®) bilaterally in the common carotid arteries, with the following settings: CW mode, P=5,1 W, t=26 s, energy (E)= 132,6 J, fiber diameter= 400 µm unfocused and PD= 164,51 W/ cm2 9.
Group 4: 4 samples. Anastomosis was performed with a 1908-nm diode laser (AlGaIn-AsSb, manufacturer DMC SP-Brazil, model MedLaser 1908®) bilaterally in the common carotid arteries, with the following settings: CW mode, P= 5,1 W, t=26 s, energy (E)= 132,6 J, fiber diameter= 400 µm unfocused and PD= 164,51 W/ cm2 9.
The laser dosimetry measurements are presented in table 1.
The lasers were used in non-contact mode, with the handpiece helded approximately 2 mm from the target. The spot size was 2,0 mm.
The distal blood flow of the common carotid artery was released, and then the proximal flow.
The mechanical resistance test was performed in the anastomosis that did not bleed after flow release. We performed clampings in the distal and proximal common carotid artery and made the total arterial section in the proximal segment, where a 24G catheter (attached to a Y device) was introduced. The Y device contained a syringe with colored saline solution (which was infused under pressure) and a pressure transducer (Omega, model 03-PSIG) that measured the pressure at which the solution was leaked while the pressure (in mmHg) was continuously recorded. For the pressure at which the leaking occurred, a sudden drop in infusion pressure was observed, after which no further increase in pressure was noticed [17].
The euthanasia of the animals was performed after mechanical evaluation or after observation of anastomosis bleeding [16].
The results were analyzed by the tests of Fischer (to determine the independence between the groups), Mann-Whitney (to evaluate the differences of the medians of leaking pressures between the groups) and log-rank (to evaluate the survival estimate).
The level of rejection of the null hypothesis was set at 0,05 or 5% (α ≤ 0,05).
Results
Group 1 (808 nm): 4 samples. There was no coaptation of the arterial edges in any of the samples, consistently.
Group 2 (980 nm): 16 samples were obtained, of which 8 (50%) bled and 8 (50%) did not bleed after release of the flow. Considering the anastomosis that bled (leakage pressure = 0mmHg) plus those that did not bleed, the leakage pressures (measured by mechanical evaluation) had the following characteristics: medium= 77,5mmHg, standard deviation = 88,81, median =30mmHg, 1st quartile (q1)= 0,0mmHg, 3th quartile (q3)= 175,0mmHg, minimum leakage pressure = 0mmHg e maximum leakage pressure = 220mmHg. Considering only the anastomosis that did not bleed, the leakage pressures obtained were: medium= 155,0 mmHg, standard deviation = 56,32, median= 170 mmHg, q1= 100,0 mmHg, q3= 195,0 mmHg, minimum leakage pressure= 60 mmHg and maximum leakage pressure= 220 mmHg.
Group 3 (1470 nm): 16 samples were obtained, of which 5 (31,3%) bled and 11 (68,8%) did not bleed after release of the flow. Considering the anastomosis that bled (leakage pressure = 0mmHg) plus those that did not bleed, the leakage pressures (measured by mechanical evaluation) had the following characteristics: medium= 123,1mmHg, standard deviation= 90,9, median= 140mmHg, q1= 0,0mmHg, q3= 200, 0mmHg, minimum leakage pressure = 0mmHg e maximum leakage pressure = 240mmHg. Considering only the anastomosis that did not bleed, the leakage pressures obtained were: medium= 179,1 mmHg, standard deviation= 37,00, median= 180 mmHg, q1= 140,0 mmHg, q3= 200,0 mmHg, minimum leakage pressure= 130 mmHg and maximum leakage pressure= 240 mmHg.
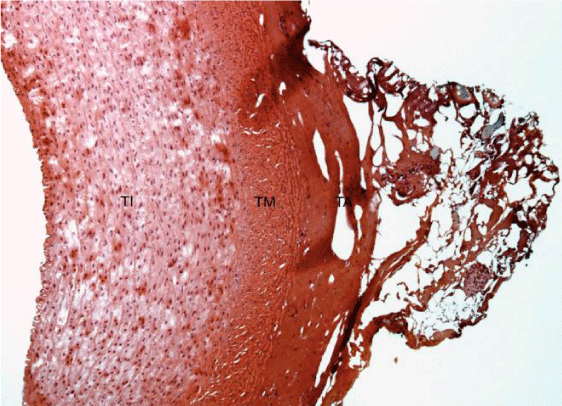
Histological slides show superficial damage only to the outermost layer (adventitia) either for group 2 and 3 at the anastomosis area (Figures 3,4).
Group 4 (1980 nm): 4 samples. There was carbonization of the arterial edges of all samples, consistently.
Because of the consistently unsatisfactory results found on groups 1 and 4, the procedures were not performed in a total of 16 samples in these groups.
There was no difference statistically significant in groups 2 and 3 regarding leakage pressures tested by Fisher’s test (p=0,473) and Mann-Whitney’s test (p=0,094).
For group 2 (980 nm), the leakage pressure levels in which there was a probability of survival (end point= bleeding) had the following characteristics: medium= 102,0 mmHg, confidence interval (CI) 95%= 43,1- 160,9, median= 0 mmHg, q1=0,0, q3= 160 mmHg, minimum leakage pressure= 0 mmHg and maximum leakage pressure= 220 mmHg. The possibility of survival with blood pressure of 0 mmHg, 50 mmHg, 100 mmHg , 150 mmHg , 200 mmHg, and 250 mmHg was 46,7%, 46,7%, 33%, 20%, 0%, and 0%, respectively.
For group 3 (1470 nm), the leakage pressure levels in which there was probability of survival (end point= bleeding) had the following characteristics: medium= 136,0 mmHg, CI 95%= 75,6 – 196,4, median= 140 mmHg, q1= 0 mmHg, q3=200 mmHg, minimum leakage pressure= 0 mmHg, maximum leakage pressure= 240 mmHg. The possibility of survival with blood pressure of 0 mmHg, 50 mmHg, 100 mmHg, 150 mmHg, 200 mmHg, and 250 mmHg was 68,8%, 68,8%, 68,8%, 43,8%, 12,5%, and 0%, respectively.
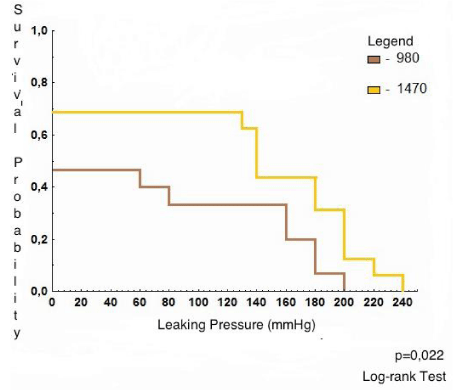
The evaluation of the probability of survival for groups 2 and 3 (considering the anastomosis that bled + those that did not bleed) with log-rank test determined that the level of statistical significance was p=0,022 (Figure 5).
For group 2 (980 nm), the average pressure at which there was a survival (the end point was the occurrence of bleeding) was 160,9 mmHg, which was lower than that for group 3 (196,4 mmHg), with statistical significance (p=0,022).
The probability of survival was greater for group 3 (1470 nm) than that for the group 2 (980nm) at all pressure levels (0 mmHg, 50 mmHg, 100 mmHg, 150 mmHg, 200 mmHg, and 250 mmHg) and was statistically significant (p=0,022).
Evaluating the probability of survival of the groups 2 and 3 (considering only the anastomosis that did not bleed), using log-rank test, there was no statistically significance (p=0,383) figure 6.
The risk of bleeding was calculated: 1- probability of survival.
For group 2 (980 nm), the risk of bleeding with blood pressures of 0 mmHg, 50 mmHg, 100 mmHg, 150 mmHg, 200 mmHg, and 250 mmHg was 55,3%, 55,3%, 67%, 80%, 100%, and 100%, respectively.
For group 3 (1470 nm), the risk of bleeding with blood pressures of 0 mmHg, 50 mmHg, 100 mmHg, 150 mmHg, 200 mmHg, and 250 mmHg was 31,2%, 31,2%, 31,2%, 56,2%, 87,5%, and 100%, respectively.
The bleeding risk was lower for group 3 (1470 nm) than that for the other groups at all pressure levels (0 mmHg, 50 mmHg, 100 mmHg, 150 mmHg, 200 mmHg and 250 mmHg), with statistical significance (p=0,022).
The spot size was 2,0mm (measured keeping the distance of 2mm between the output of the laser beam and the artery). The area of application of the laser (A= πr2) was A=0,031 cm2.
To calculate the energy density (D) or fluency (F), the following equation was used: t= D.A/P. For group 2 (980 nm), with t=26 s, A=0,031 cm2 and P= 5,0 W, the energy density was D=4193,5J/cm2. For group 3 (1470 nm), with t=26 s, A=0,031 cm2 and P= 5,1 W, the energy density was D=4277,4 J/cm2. The difference in the F between groups 2 and 3 (4277,4 J/cm2 - 4193,5 J/cm2) was 83,9 J/cm2; therefore, 83,9/4277,4= 0,019 or 1,9%.
The irradiance (I) was calculated using F= I x t. For group 2 (980 nm), t=26 s and I= 161,36 W/cm2. For group 3 (1470 nm), t=26 s and I= 164,51 W/cm2. The difference in the I of groups 2 and 3 (164,51 W/cm2 – 161,36 W/cm2) was 3,15; thus, 3.15/164,51= 0,019 or 1,9%.
Discussion
The walls of the blood vessels are composed largely of water (approximately 80%) and collagen fibers. As the composition of the arterial wall is mostly water, the optical absorption coefficient of the arterial wall is represented by the optical absorption coefficient for water [18].
The 808-nm diode laser does not present adequate absorption by water, whereas it presents adequate absorption by collagen. The 980-nm diode laser presents low absorption by water and great absorption by collagen. The 1470-nm and 1908-nm diode lasers present great absorption by water, but no collagen absorption19.
The 980-nm diode laser has a lower optical absorption coefficient for water (and thus less absorption of laser light by the arterial wall) than that of the 1470-nm diode laser [19].
As the laser effects on the tissue depends on its absorption by the tissue, the above discussion corroborates our findings of a lower risk of bleeding and greater probability of survival in anastomosis using the 1470-nm laser (p=0,022) than those values using the other lasers [19]. The 808-nm laser light has lower absorption by water and is minimally absorbed by the arterial tissue, justifying the non-welding of the arterial edges [19]. The 1980-nm laser light is highly absorbed by water; consequently, great absorption of laser light by the arterial wall leads to a rapid elimination of the water in the wall along with carbonization of the edges in the interval of time used in this study [19].
Another important differential factor is the optical penetration depth (OPD) among the wavelengths of 808 nm, 980 nm, 1470 nm and 1908 nm20. According to Cilip et al. [20], the OPD of the laser light at the wavelengths of 808 nm and 980 nm is 1,5 mm; the OPD of laser light at the wavelength of 1470 nm is 0,4 mm, and that of laser light at the wavelength of 1980 nm is 0,1 mm. As the swine carotid artery wall thickness is approximately 0,3 mm [21] and the OPD of the lasers at the wavelengths of 808 nm and 980 nm is 1,5 mm, there is insufficient deposition and absorption of optical energy in the vessel wall during irradiation at these wavelengths; most laser energy is scattered through the thickness of the vessel rather than being absorbed by the tissue20.The application of increased laser power and/or laser irradiation time could not compensate for the mismatch between the OPD and the tissue thickness [20]. The laser 1980 nm has a OPD of 0,1 mm, and most of its light is absorbed in the superficial layers of the artery, leading to the carbonization of the medium-sized arteries surface [20]. The 1470-nm laser has a OPD of 0,4 mm, allowing the laser light to be absorbed homogeneously in the arterial wall and promoting better laser effect to perform the anastomosis; this observation justifies the findings of a lower risk of bleeding and higher probability of survival (p= 0,022) [20].
The laser light scattering in the arterial wall decreases as the laser wavelength increases (in the spectral area where this study was carried out), thereby increasing the absorption [22]. The collagen fibers are mainly responsible for scattering [22]. Because of the higher scattering for light of lower wavelengths, it is possible to predict lower absorption for the laser energy at the wavelengths of 808-nm and 980-nm than that for the laser energy at the wavelengths of 1470-nm and 1908-nm, justifying the findings of lower welding of the arterial borders using laser light at the wavelength of 980 nm compared to 1470 nm [22].
Jonge et al. [9], reviewed the LAVA studies. Most of them used solder that gives better resistance to the anastomosis (because their chromophores improve laser absorption) and that does not allow excessive penetration that would result in injury of the intima. The problem with the use of solder is that most of them are liquid, and their liquefaction during the application of the laser reduces the reproducibility because the thickness and the area of the solder cannot be accurately controlled.
Leclere et al. [14], used LAVA for vascular reconstruction in emergency hand surgeries. Surgeries were performed in microvessels using laser diode at the wavelength of 1950 nm. This wavelength is considered to be ideal for microvascular anastomosis because of the penetration of light at that wavelength that covers the vascular wall in the adventitious and middle layers, allowing the suture to be achieved without the use of chromophores or solder. The resulting weld strength was comparable to the strength of suture repairs, and the occurrence of aneurysms was minimal when compared to that using CO2 and argon-ion lasers. The patency rate at the surgery was 100%, with immediate bleeding at the release of the clamping of approximately 27.2%.
Esposito et al. [23], performed vascular anastomosis (end-to-end) and vascular repair using an 800-nm diode laser and green indocyanine-infused chitosan patches in common carotid arteries of rabbits and evaluated their patency. There was no bleeding in the immediate postoperative period, and the vessels remained patent for up to 90 days.
Pabittei et al. [24], performed end-to-end anastomoses ex vivo in carotid arteries and porcine aortas using a 670-nm laser diode and application of albumin solder. The rupture pressure of the anastomoses and pulse pressure test were evaluated in 24 hours, with high rupture pressures, but with leaks in the 24-hour pulsatile pressure test.
Erdmann et al. [3], performed side-to-side arteriovenous anastomosis in femoral vessels of dogs using magnetic devices. The patency was 100% after 10 weeks, with no apparent aneurysm or other potentially catastrophic failure. However, the arteriovenous pressure gradient would produce forces exceeding the magnetic force, leading to failure of the anastomosis; compression of vascular tissue between the magnets would cause pressure necrosis of the vessels walls; direct contact between the blood and the magnet may cause thrombosis; a foreign body response or endothelial hyperplasia may lead to unacceptable narrowing of the anastomosis over time.
Liu et al. [4], compared magnetic compressive anastomosis and tradicional hand-suturing in canine femoral arteries. The anastomosis time was significantly shorter on the group of magnetic compressive anastomosis, though the bursting pressures were significantly lower on this group at times immediate and 4 weeks, but no significant difference at 12 weeks.
The mechanical resistance test was performed only on the immediate postoperative period because the objective of this study was evaluate the safety of these anastomosis (regarding percentual of immediate bleeding) and the best wavelength to achieve anastomosis with elevated leaking pressures. Thus, the resistance test was not performed on subsequent days.
The power of the 980-nm diode laser device, despite being of the same manufacturer, differed from the power of the other laser devices (808 nm, 1470 nm and 1908 nm) with a configuration that did not allow a power of 5.1 W to be chosen (as used in the other devices); the closest value was P= 5.0 W; therefore, this was the power used for this wavelength. The difference between the powers in groups 2 and 3 (5.1 W - 5.0 W) was 0.1 W, corresponding to 0.1 W / 5.1 W = 1.9%.
There was no statistical significance between the leakage pressures of the anastomosis with the 980-nm and 1470-nm laser diodes, possibly because of the exposure time used. As the objective of this study was to evaluate the best wavelength to perform arterial anastomosis in elastic arteries (it was observed that with the use of the 1470 nm laser, the results of bleeding risk and probability of survival were better), a next step would be to evaluate the ideal exposure time. With an adequate exposure time, it is possible that the anastomosis performed using a 1470-nm laser will have significantly higher leakage pressures than those performed using the 980-nm laser. Further studies are required to investigate the other laser dosimetry parameters.
Considering only the anastomosis that did not bleed on groups 2 (980nm) and 3 (1470nm), there was no statistical significance regarding the probability of survival. Possibly, it was due to the lower n after exclusion of the anastomosis that bled. With an increase of the n, statistical significance should be observed.
Conclusions
The wavelength that presented the best results for anastomosis in elastic arteries without solder, in the experimental swine model, was 1470 nm. The higher survival probability and lower bleeding risk was achieved on anastomosis using 1470nm.
The 1470-nm diode laser has optical parameters that allow it to be the best wavelength among those diode lasers evaluated for anastomosis of elastic arteries.
Further studies are required to investigate the other laser dosimetry parameters.
- Zeebregts CJ, Heijmen RH, Dungen Van den JJ, Schilfgaarde Van R (2003) Non-suture methods of vascular anastomosis. Br J Surg 3: 261–271. Link: https://goo.gl/ho4FG2
- Cole DH, Foley MJ (2003) Methods for forming magnetic vascular anastomosis. US Patent 6,652,540. Link: https://goo.gl/YNiJuA
- Erdmann D (2004) Side-to-side sutureless vascular anastomosis with magnets. J Vasc Surg 40: 505-511. Link: https://goo.gl/LDz7kv
- Liu S, Lei P, Lu Y, Guan Z, Gao R, et al. (2014) A comparative study on magnetic compressive anastomosis and traditional hand-suturing technology in canine femoral artery anastomosis. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 28: 885-890. Link: https://goo.gl/cNPgQM
- Quigley MR, Bailes JE, Kwaan HC, Cerullo LJ, Block S (1986) Comparison of myointimal hyperplasia in laser-assisted and suture anastomosed arteries. A preliminary report. J Vasc Surg 3: 217–219. Link: https://goo.gl/fK8SG1
- White RA, Kopchok GW, Donayre C (1986) Laser welding of larger diameter arteries and veins. Trans Am Artif Intern Organs 32: 181-183. Link: https://goo.gl/EnXz2j
- Quigley MR, Bailes JE, Kwaan HC, Cerullo LJ, Brown JT (1986) Aneurysm formation after low power carbon dioxide laser- assisted vascular anastomosis. Neurosurgery 3: 292–299. Link: https://goo.gl/6YhEp6
- Birch JF, Bell PR (2002) Methylene blue soldered microvascular anastomosis in vivo. Eur J Vasc Endovasc Surg 4: 325–330. Link: https://goo.gl/octgDV
- Jonge WICDYM, Beek JF, Balm R, Birch JF, Bell PR (2004) 25 years of laser assisted vascular anastomosis (LAVA): what have we learned? Eur J Vasc Endovasc Surg 27: 466–476. Link: https://goo.gl/cy6cHK
- Leclere F, Schoofs M, Buys B, Mordon S (2010) Outcomes after 1900nm Diode Laser-Assisted Anastomosis in Reconstructive Microsurgery: Results in 27 Patients. 125: 1167-1175. Link: https://goo.gl/m3f1W8
- Leclere F, Schoofs M, Florent A, Buys B, Mordon S (2011) Blood flow assessment with magnetic resonance imaging after 1900nm diode laser assisted arterial microanastomoses. Annales de Chirurgie Plastique et Esthetique 56: 540-547. Link: https://goo.gl/AKJKnk
- Giglio NC, Hutchens TC, Perkins WC, Letimer C, Ward A, et al. (2014) Rapid sealing and cutting of porcine blood vessels, ex-vivo, using a high power, 1470-nm diode laser. Journal of Biomedical Optics 19: 038002. Link: https://goo.gl/XaB7iF
- Ren Z, Xie H, Lagerquist KA, Burke A, Prahl S, et al. (2004) Optimal Dye Concentration and Irradiance for Laser-Assisted Vascular anastomosis. Journal of Clinical Laser Medicine & Surgery 22: 81- 86. Link: https://goo.gl/gKCDDv
- Leclere F, Schoofs M, Vogt P, Casoli V, Mordon S (2015) 1950-nm diode-assisted microanastomoses (LAMA): an innovative surgical tool for hand surgery emergencies. Lasers Med Sci 30: 1269-1273 Link: https://goo.gl/QbZy9S
- Almond GW (1996) Research applications using pigs. Veterinary clinics of North America. Food animal practice 12: 707-714. Link: https://goo.gl/Gp2zea
- Swindie MM (2002) Anesthesia, analgesia and perioperative techniques in suine. Available on the website: http//sinlairreserch 1-6.
- Klein SL, Israel JE, Kronengold RT (1995) New burst test method for comparing strengths of blood vessels repair. Microsurgery 16: 118-121. Link: https://goo.gl/bWW2mV
- Keijner M, Richards-Kortum RR, Jacques SL, Feld MS (1989) Fluorescence spectroscopy of turbid media: Autofluorescence of the human aorta. Applied Optics 28: 4286-4292. Link: https://goo.gl/hv7NxC
- Lakowicz JR (1999) Principles of Fluorescence Spectroscopy 25-61. Link: https://goo.gl/R3YYA4
- Cilip CM, Rosenbury SB, Giglio N, Hutchens TC, Schweinsberger GR, et al. (2013) Infrared laser thermal fusion of blood vessels: preliminary ex vivo tissue studies. Journal of Biomedical Optics 18: 058001. Link: https://goo.gl/iWF3xs
- Rocha EAV, Souza C (2007) (Hemodynamic evaluation of arterial anastomosis reinforced with fibrin sealant – Experimental study in swines) Avaliação hemodinâmica de anastomoses arteriais reforçadas com selante de fibrina- Estudo experimental em suínos. Rev Bras Cir Cardiovasc [online]. 22: 81-86. Link: https://goo.gl/PrfxZA
- Van Germet MJ, Welch AJ (1989) Time constants in thermal laser medicine. Lasers Surg Med 9: 405-421. Link: https://goo.gl/kZZsM1
- Esposito G, Rossi F, Matteini P, Scerrati A, Puca A, et al. (2013) In vivo laser assisted microvascular repair and end-to-end anastomosis by means of indocyanine green-infused chitosan patches: a pilot study. Lasers in Surgery and Medicine 45: 318-325. Link: https://goo.gl/weWFmS
- Pabittei DR, Herger M, Van Tujil S, Simonet M, de Boon W, et al. (2014) Ex- vivo proof-of-concept of end-toend scaffold-enhanced laser-assisted vascular anastomosis of porcine arteries. Journal of Vascular Surgery 62: 200-209. Link: https://goo.gl/tzHqYH
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
