International Journal of Vascular Surgery and Medicine
Acute on Chronic Renal Failure has Worse Postoperative Outcomes than End-Stage Renal Disease Following Cardiac Surgery
Barış Durgun1, Ahmet Yüksel2*, Gökhan Erol1, Mevlüt Kobuk1 and Suat Doğancı1
2Department of Cardiovascular Surgery, Bursa State Hospital, Bursa, Turkey
Cite this as
Durgun B, Yüksel A, Erol G, Kobuk M, Doğancı S (2017) Acute on Chronic Renal Failure has Worse Postoperative Outcomes than End-Stage Renal Disease Following Cardiac Surgery. Int J Vasc Surg Med 3(2): 026-032. DOI: 10.17352/2455-5452.000024Background: Renal failure is a systemic disorder and has destructive effects among all organs including cardiovascular system. The development of postoperative acute kidney injury has been recognized as one of the strongest risk factor for mortality in patients undergoing cardiac surgery.
Aim/Objective: To investigate postoperative course of acute on chronic renal failure after cardiac surgery and define perioperativerisk factors for predicting postoperative acute renal failure (ARF) development.
Materials and Methods: From January 2006 to December 2014, data of 3038 patients undergone cardiac surgery was retrospectively reviewed. Data of 42 chronic renal failure (CRF) patients who undergone dialysis at early postoperative period (≤30 days after cardiac surgery) were selected and evaluated. Group 1 (n=18) was consisted of patients who have preoperative dialysis dependent CRF and undergone dialysis after cardiac surgery, while Group 2 (n=24) was consisted of patients who have preoperative dialysis non-dependent CRF and undergone dialysis after cardiac surgery. Preoperative clinical characteristics and demographics, operational data of patients as well as postoperative outcomes of patient groups were analyzed, hereby a comparison of two groups was performed.
Results: There were not statistically difference between groups for neuropsychiatriccomplications, wound infectious complications, septiccomplications, reexploration, duration of ventilation, cardioversion, intraaortic balloon pump support, pleural effusion, pericardial effusion and total drainage amounts.
Positive inotropic necessity, significant electrocardiographicchanges, reintubation necessity and transfusion amounts were statistically higher in Group 2. Patients in Group 2 had a significant longer intensive care unit (ICU) stay (mean length of ICU stay was 4 days in Group 1, while 6 days in Group 2, p = 0.01) and total hospital stay (mean length of total hospital stay was 9 days in Group 1, while 15 days in Group 2, p = 0.006). Thirty-day mortality rate was statistically higher in Group 2 (p = 0.044).
Conclusion: End stage renal failure is one of the worst preoperative risk factor for cardiac surgery but postoperative ARF development results with worst outcomes even patients had preoperative dialysis non-dependent CRF. So preoperative estimated glomerular filtration rate should be calculated to estimate ARF development risk, instead of evaluating with only serum creatinine.
Introduction
Renal failure is a systemic disorder and has destructive effects among all organs including cardiovascular system. Many studies revealed chronic renal failure (CRF) is associated with cardiovascular diseases (CVD) [1,2]. The complex association of CRF with CVD is probably due to clustering of several cardiovascular risk factors including the “traditional factors” such as age, hypertension (HT), diabetes mellitus (DM), and hyperlipidemia (HL) and “nontraditional factors” that are specific to CRF such as anemia, volume overload, mineral metabolism abnormalities, proteinuria, malnutrition, oxidative stress and inflammation in CRF patients [3].
Acute or chronic renal failure which is characterised by elevated serum creatinine concentration or decreased glomerular filtration rate (GFR) level may be associated with an increased risk of mortality and morbidity for patients undergoing cardiac surgery. The development of postoperative acute kidney injury (AKI) has been recognized as one of the strongest risk factor for mortality following open heart surgery [4]. In cardiac surgery patients, an increase 25% at the serum creatinine level results with a 15 fold increase at the risk of hospital mortality [5,6]. Thus, deciding the treatment and operative strategy for cardiovascular patients with concomitant CRFis crucial. Despite dialysis-dependent CRF patients have an increased morbidity and mortality risk after cardiac surgery, end-stage renal disease (ESRD) should not be considered as a contraindication to cardiac surgery or cardiopulmonary bypass (CPB) [7]. One of the main problem faced by cardiac surgeons is a lack of consensus on the diagnostic criteria for acute renal failure (ARF) and the optimal time to initiate dialysis theraphy. Currently there is neither consensus nor a guideline to recommend the use of prediction models for acute kidney injury after cardiac surgery in non-dialysis dependent CRF patients.
ARF is a possible complication of cardiac surgery, and may develop in patients with CRF or normal renal functions preoperatively. Furthermore, ARF is associated with early and late mortality (up to 15-30% for dialysis non-dependent ARF and 60% for dialysis-dependent ARF), prolonged length of hospital and intensive care unit (ICU) stay, increased infective complications [8-10]. In the current literature, there are studies that compare ARF and dialysis dependent CRF patients or non renal failure and dialysis dependent CRF patients [11]. Nevertheless, we couldn’t find any study comparing dialysis dependent CRF and ARF on dialysis non-dependent CRF patients.
In this study, we aimed to investigate the postoperative course of acute on chronic renal failure (AonCRF) after cardiac surgery.Therefore, we evaluated the postoperative course by comparing mortality and morbidity parameters. Additionally, this study purposed to define perioperative risk factors for predicting postoperative ARF development.
Materials and Methods
Patient selection
This study was designed as retrospective study and approved by Gulhane Military Medical Academy Ethical Board. From January 2006 to December 2014, data of a total of 3038 patients who underwent cardiac surgery including isolated coronary artery bypass grafting (CABG), isolated aortic or mitral valve replacement, Bent hall procedure and combined cardiac surgery such as CABG with intra cardiac tumour surgery or valve replacement were reviewed. CPB was used for all type of surgery. From this database, data of 42 patients who had dialysis dependent or non-dependent CRF and underwent dialysis at early postoperative period (≤30 days after cardiac surgery) were analysed. After data collection, patients were divided into 2 groups. Group 1 was consisted of 18 patients who have preoperative dialysis dependent CRF and continued to have dialysis after cardiac surgery, while Group 2 was consisted of 24 patients who have preoperative dialysis non-dependent CRF and underwent hemodialysis after cardiac surgery. The exclusion criteria were as follows; emergent operations (operation within the first 24 hours of coronary angiography), low ejection fraction (EF) (EF<30%), temporary or permanent pacemaker and off-pump surgery.
Types of anaesthesia and CPB protocol
All patients in both groups received similar standardized anaesthesia management with use of propofol (1-2.5 mg/kg), remifentanil (1 mg/kg) bolus and infusion (0.4 μg/kg), and inhalational agents during CPB.Mild or modarate hypothermia (240-340) was used in all cases. Prime solution was contained 1100cc ringer lactate, 300cc mannitol, 100 mg unfractioned heparin, 250 mg methylprednisolone.Non-pulsatile perfusion techniques were used and flow rates of perfusion were1.8, 2.0, 2.2 and 2.4 L/m2/min at 240C, 300C, 340C and 370C, respectively. Crystalloid and blood cardiolegia were used for cardiac arrest. Dialysis administration criteria during CPB were based upon the institutional protocol and experience including excessive fluid volume overload (central venous pressure > 15 cmH2O), oliguria (urine output < 20 mL at the first hour of CPB), hyperkalemia (blood potassium level > 5.5 meq/L) and prolonged CPB time (> 180 min).
Renal failure definition
ARF was defined by using the Acute Kidney Injury Network (AKIN) stage 1-3 criteria – a x1.5 times or more increase in the serum creatinine and/or 25% or more decrease in estimated GFR, from preoperative to peak postoperative serum creatinine level. Preoperative and peak postoperative serum creatinine were defined as the creatinine values recorded within two week before the surgery and the highest creatinine level within 10 days after surgery, respectively [12].CRF was defined using the National Kidney Foundation Kidney Disease Outcome Quality Initative (NKF-K/DOQI) scale, which suggests estimated GFR below than 60 mL/min [13].
Study protocol
Preoperative demographic features and baseline clinical characteristics of patients including body mass index (BMI), body surface area (BSA), value of estimated glomerular filtration rate (eGFR) (calculated by using modification of diet renal disease formula), EF, cardiac rhythm, smoking, level of European System for Cardiac Operative Risk Evaluation (EuroSCORE), concomitant disorders such as HT, DM, HL and chronic obstructive pulmonary disease (COPD),presence of arteriovenous fistula, history of cardiac operation, and preoperative medications such as antihypertensive or lipid lowering therapies were recorded.In addition to these preoperative data, several laboratory tests such as creatinine, glycosylated hemoglobin, low density lipoprotein (LDL), total cholesterol and triglycerides were also included to our analyse. Being the value of LDL > 160 mg/dL, the value of total cholesterol> 240 mg/dL orthe value of triglycerid > 200 mg/dL is considered as diagnostic criteria of HL. Additionally, mean arterial pressures (MAP) and CRF stages of patients were also recorded. Operational data of patients such as type of surgery, total aortic cross-clamping time, total CPB time, number of proximal/distal anastomosis, lowest hematocrit ratio, MAP and body temparature were recorded. Necessity of ultrafiltration/hemodialysis during CPB and/or extracorporeal membran oxygenation (ECMO) were the other recorded variables. Postoperative data of patients such as mechanical ventilation time, length of ICU and hospital stay, drainage amount, necessity of transfusion of blood products, inotropic or mechanical support, cardiovertion and/or antiarrhythmic medication and postoperative reexploration rates were recorded. All recorded preoperative, intraoperative and postoperative data were analyzed, and then a comparison of two groups was performed. Moreover, a cardiologist who was unaware of patients’ groupdistribution,evaluated the postoperative daily electocardiograms of all patients. New developed branch blocks, disrythmias, conduction delays, ST depressions/elevations, pardee waves and pathologic Q waves, were considered as significant electrocardiographic(ECG) changes.Mortality prevalances were classified into 2 groups as early (in postoperative first 30 days), andlate (duration between postoperative 30thday and end of the follow up).Patients telephoned, mortality and medical status searched by questioning after discharged up to 5 years.
Statistical analysis
All statistical analyses were performed using Statistical Package for Social Sciences (SPSS) program (version 15.0, SPSS, Chicago, Illinois, USA).Continuous variables were expressed as mean ± standard deviation. Categorical variables were expressed as frequency and percentages. Differences between groups were analyzed by Fischer’s exact test and Pearson’s chi-square test for qualitative variables. Student T-test and Mann-Whitney U test werealso conducted out toanalyse the quantitative variables. Mortality rates were analyzed by using Kaplan Meier and log rank tests.A p value of <0.05 was considered as statistically significant with a 95% confidence interval.
Results
Baseline characteristics and demographics
Preoperative clinical characteristics and demographics of patient groups were summarized in table 1. Basic demographic variables were not statistically significantin both groups except gender, prevelance of DM and level of EuroSCORE. The proportion of female gender and prevelance of DM were higher in Group 2 than in Group 1.In addition to these differences, as expected, eGFR was also significantly higher in Group 2. (mean eGFR was 11.65 in Group 1, while 43.85 in Group 2; p = 0.01). Preoperative level of EuroSCORE of patients were used in order to determine the risk scores of patients. Group 1 had significantly higher risk score compared with Group 2 (p< 0.05). Preoperative medications were shown in table 2. The prevalence of use of preoperative oral antidiatebic and insulin therapy was higher in Group 2 (p=0.01).
Intra operative data
Intraoperative data of patient groups were listed in table 3. CABG was the most common surgery type for both groups. However, there was not any significant difference between the groups for neither surgery type nor surgical variables.
Post operative outcomes
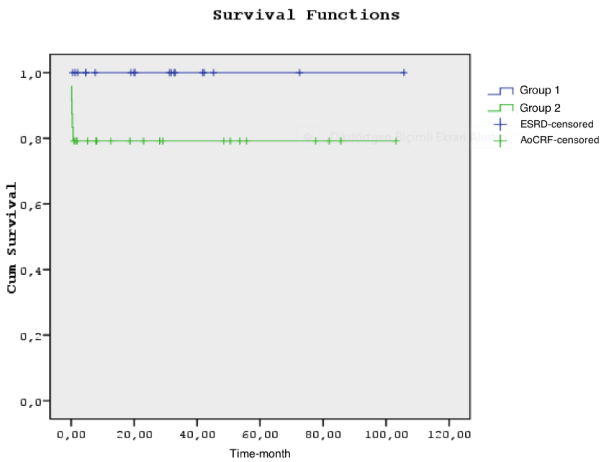
Postoperativedata of patient groups were listed in table 4. Various complication rates were found to be higher in Group 2 without statistical significancy (p >0.05). However, positive inotropic necessity, significant ECG changes, reintubation necessity and transfusion amounts were statistically higher in Group 2. Patients in Group 2 had a significant longer ICU stay (mean length of ICU stay was 4 days in Group 1, while 6 days in Group 2, p = 0.01) and total hospital stay (mean length of total hospital stay was 9 days in Group 1, while 15 days in Group 2, p = 0.006). All of patients in Group 1 continued to their dialysis programmes after discharge, while 5 patients in Group 2 continued to dialysis (by questioning up to 5 years). During a total follow-up period, there were 5 deaths (27.8%) in Group 1, and 9 deaths (37.5%) in Group 2. Cumulative survival rates of both groups were shown in figure 1. The survival rates at 6 month, 1 and 5 years were 100%, 81.6% and 67.3%in Group 1, and 66%, 66% and 59.4% in Group 2, respectively (by questioning up to 5 years). But these survival rates were not significantly different between the groups (p> 0.05). Thirty-day mortality rate was statistically higher in Group 2 (p = 0.044). There was no mortality in Group 1 patients, and there were 5 deaths in Group 2 patients during 30-day follow-up period. During 30-day follow-up period, the survival rates were 100% and 79.2% in Group 1 and 2, respectively. In Group 2, there were 6 patients (25%) who received dialysis during CPB, while 18 patients (75%) did not recieve dialysis. There was not statistically difference between groups in terms of neuro psychiatric complications, wound infectious complications, septic complications, reexploration, duration of ventilation, cardioversion, intraaortic balloon pump support, pleural effusion, pericardial effusion, total drainage amounts.
Discussion
Many authors suggest to use of the level of eGFR instead of creatinine level for estimating the preoperative renal reserve of the patients [14,15]. There are several creatinine-based formulas for the estimation of eGFR that are widely used in research and clinical practice. We used the formula which developed by the Modification of Diet in Renal Disease (MDRD) Study Group in 1999 [16]. It was simplified in 2002 by omitting albumin and blood urea nitrogen to a 4-variable MDRD which estimates eGFR using the variables of age, gender, race and serum creatinine [17]. MDRD formula was preferred for estimation of GFR since it is more precise in CRF patients. Other methods such as Cockcroft and Gault can lead to an overestimation of GFR in patients with ESRD [1,18]. Gong et al. concluded that postoperative elevation of AKI level was associated with a prolonged duration of mechanical ventilation, intensive care unit stay, postoperative hospital stay, delayed extubation, extubation failure and death [19]. Maguel-González et al. showed similar results in their study for patients especially stage 2 and stage 3 AKI. ARF had a longer hospital stay and a higher mortality [20]. Different from these studies, in our study, duration of mechanical ventilation found similar despite hospital stay and ICU time were found significantly longer and reintubation rates were found significantly higher for Group 2. Avoiding perioperative hypotension and volume overload, limitation of cardioplegic solutions, minimalization of CPB duration, detection of renal reserve precisely by calculating eGFR with MDRD formula, and performing dialysis during CPB period may prevent elevation of AKI stage in patients with dialysis non-dependent CRF after cardiac surgery. also renal protective treatment strategies such as perioperative statines, acetylsalicylic acid, tolvaptan, and dexmetedomidine use, choosing pulsatile flow technique during CPB period, maintaining hemoglobin level above 8 g/dL during surgery are some of the other relevant options.
In some studies, a positive correlation between postoperative blood transfusion and drainage amount was reported [21-23]. Postoperative blood transfusion usage was reported 17% for no ARF group while33% for ARF stage 1 and 67% for stage 2-3 ARF group [22]. Another study also found out mean: 3.3± 4.6 units for ARF group comparing to mean: 1.4 ± 2.5 for no ARF group (p< 0.05). As corralated to higher usage of blood transfusion, ARF group’s drainage were also reported higher for the first 12 hours postoperatively [21]. In our study despite similar drainage amounts for both groups, we found significantly higher blood transfusion rates in Group 2.
Electrolit imbalance such as hyperkalemia is one of the most important reason for mortal arrythmias. Hyperkalemia-induced accelerated idioventricular arrythmias reported before [22]. Some other studies showed the association between increased prevalance of arrythmias of patients with ARF and acute coronary syndromes [24]. In our study, despite atrial fibrillation, ventricular fibrillation, ventricular tachycardia and cardioversion rates were similar in both groups but significant ECG changes (bundle branch blocks, atrio-ventricular blocks, dysrhythmia, new onset ST segment elevation or depression, pardee wave, pathological Q waves accept as significant ECG changes) were higher in Group 2. The self-resistance of ESRD patients to electrolit imbalances may be the reason for decreased ECG changes. ARF on CRF patients do not have this resistance, thus performing dialysis to dialysis non-dependent CRF patients during CPB may avoid ECG changes due to decrease in electrolite imbalance. We offer the dialysis to all CRF patients undergoing cardiac surgery during CPB.
In contrast with our findings, Lopez-Delgado et al.have reported that sepsis is associated with AKI after cardiac surgery [21]. There is a lack of studies associating effusional complications and AKI after cardiac surgery. In our study neither pericardial nor pleural effusional complications weresignificantly differentbetween the groups. We hypothesized that our clinical strict dialysis protocol (in the events of persistent oligo-anuria, 20% increase in serum urea and creatinine levels, hyperkalemia, acidosis, and accumulation of total body fluid) [25], avoided volume overloads and effusional complications for both groups. From this point, we consideredthat there was not a consensus about timing of optimal renal replacement therapy (RTT) following cardiac surgery. Cresenzi et al. suggest that the starting of RRT after 12 hours follow-up of urine output less than 0.5 mL/kg/hour [26]. In our department, dialysis therapy has been starting after a nephrology consultation, without a strict value of urine output, volume overload, electrolit imbalances, etc.
As similar to our study, Bahar et al. have reported that the use of higher postoperative positive inotropic and IABP for ARF on CRF patients, comparing to dialysis non-dependent group (Group 1: 20% vs Group 2: 80%) [27]. Although we found that postoperative use of IABP was six times higher for group but it was not statistically significant. Larger studies with patient numbers are necessary to confirm this result.
In the literature there are many published studies which offers different preoperative medications for protect postoperative renal functions. There are also studies which suggest perioperative prophylactic dialysisfor CRF patients whether dialysis dependent or not- dependent [25,28]. Beside this, CPB strategy may be another important factor. Two large studies have shown better postoperative renal outcomes by using off-pump techiniques [15,29]. Conversely, some other authors reported different results such as no difference between the off-pump and on-pump groups [30]. It is contraversial that CPB durationis a risk factor for postoperative ARF development. Some studies reported as limitation of CPB duration for ARF development is 115 minutes [31] and above. Suen et al. reported as limitation of CPB duration for ARF development 140 minutes [32] and above. Cut-off level for total aortic occlusion time for postoperative ARF development was reported by several studies between 79-87 minutes and above [33]. In our study, mean aortic occlusion time of Group 2 was 77 minutes.
In current literature, there are many papers revealed no relationship between MAP during CPB and postoperative ARF development [34-36]. However in another study, this correlation have been detected for anemic patients [37]. All these studies used only MAP during CPB whereas Kanji et al. used also preoperative MAP for predicting postoperative ARF development.In their study, they reported that difference between preoperative and intraoperative MAP above 26mmHg is associated with ARF development [38]. In our study, preoperative and intraoperative mean MAP difference was 35,46 mmHg. Association between hypothermia and ARF development is still a debate for cardiac surgery. Kourliouros et al. reported thathypothermia at 27 0C and below is a risk factor for postoperative ARF development [39]. In our study, there were 8 patientswho have 27 0C and below hypothermia at group.
Kaplan-Meier plots, shown in figure 1, illustrated that patients in Group 2 had worse short-term survival during the follow-up period. Our findings are consistent with Ivert et al. and Filsofui et al. who suggest the highest mortality during postoperative first month [6,40]. We think that not after the 30 days but the first 30 days of the heart surgery is the most critical period for the patient’s physiological balance and compansatuar reactions against surgical stress-induced imbalance status couldn’t performed by the patients who encounter firstly. On the other hand preoperative dialysis dependent patients are already used to live with this imbalance situation. Because their compensatorr mechanisms which developed in years let them to live with this abnormalities. For example, a low hematocrit level can be one of the reason of tachycardia or a high potassium level can be one of the reason of fibrillation for a preoperative non- dialysis dependent patient while the same levels can cause no symptoms for a preoperative dialysis dependent one. Because a patient who is dialysis dependent preoperatively has self resistance status among these abnormalities. For all this reasons, there is significant difference between the groups for the 30 mortality but no difference after 30 day.
Our study had several limitations. Perhaps the most major limitation of this study was the relatively small sample size of study groups.
Conclusion
To the best of our knowledge this study is the first one which compares dialysis dependent CRF and ARF on dialysis non dependent CRF. According to our findings, despite mean preoperative level of Euro SCORE of Group 1 were significantly higher than Group 2, postoperative outcomes such as ICU and hospital stays were longer, and positive inotropic support, significant ECG changes, transfusion and early mortality were higher for Group 2 (ARF on CRF group).
Bleeding diathezis, electolit imbalances, volume overloads due to acute renal insufficeny may be reason for these morbidities. This can be explained by lack of the adaptation period for ESRD in ARF on CRF patients. ESRD is one of the worst preoperative risk factor for cardiac surgery but postoperative ARF development results with even worse outcomes in patients with preoperative dialysis non-dependent CRF. So preoperative eGFR should be calculated to estimate ARF development risk, instead of evaluating with only serum creatinine. If eGFR calculated below 60 mL/min, planned dialysis during CPB can be used to reduce postoperative complications. A model that accurately estimates a patient’s risk for ARF after cardiac surgery can optimize clinical decision-making, preoperative and operative treatment strategies to minimize the risk of morbidity and mortality.
- Dhanani J, Mullany DV, Fraser JF (2013) Effect of preoperative renal function on long-term survival after cardiac surgery. J Thorac Cardiovasc Surg 146: 90-95. Link: https://goo.gl/wdKPrN
- Tong B, Stevenson C (2007) Comorbidity of cardiovascular disease, diabetes and chronic kidney disease in Australia. Canberra, A. C. T. Australian Institute of Health and Welfare. Link: https://goo.gl/ASm8Fx
- Liu M, Li XC, Lu L, Cao Y, Sun RR, et al. (2014) Cardiovascular disease and its relationship with chronic kidney disease. Eur Rev Med Pharmacol Sci 18: 2918-2926. Link: https://goo.gl/7zYfOy
- Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J (1998) Independent association between acute renal failure and mortality following cardiac surgery. Am J Med 104: 343-348. Link: https://goo.gl/9dg65N
- Loef BG, Epema AH, Smilde TD, Henning RH, Ebels T, et al. (2005) immediate postoperative renal function deterioration in cardiac surgical patients predicts in-hospital mortality and long-term survival. J Am Soc Nephrol 16: 195-200. Link: https://goo.gl/Ohavif
- Ivert T, Holzmann MJ, Sartipy U (2014) Survival in patients with acute kidney injury requiring dialysis after coronary artery bypass grafting. Eur J Cardiothorac Surg 45: 312-317. Link: https://goo.gl/UHZlTi
- Unić Stojanović D, Milicić M, Vuković P, Babić S, Jović M (2013) Heart surgery in patients on chronic dialysis. Med Pregl 66: 64-69. Link: https://goo.gl/u6TrQ1
- Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, et al. (2004) Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol 15: 1597-1605. Link: https://goo.gl/VVWS5m
- Moguel-González B, Wasung-de-Lay M, Tella-Vega P, Riquelme-Mc-Loughlin C, Villa AR, et al. (2013) Acute kidney injury in cardiac surgery. Rev Invest Clin 65: 467-475.
- Newland RF, Tully PJ, Baker RA (2013) Hyperthermic perfusion during cardiopulmonary bypass and postoperative temperature are independent predictors of acute kidney injury following cardiac surgery. Perfusion 28: 223-231. Link: https://goo.gl/DrLGlH
- Tabary SZ, Fazli M (2013) Clinical outcome of coronary artery bypass grafting (CABG) in hemodialysis-dependent patients and comparison with non-renal failure patients. Eur Rev Med Pharmacol Sci 19: 2628-2631. Link: https://goo.gl/WM6j6j
- Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, et al. (2007) Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: 31. Link: https://goo.gl/LkPWM9
- Eknoyan G, Levin NW, Eschbach JW (2008) National Kidney Foundation–Kidney Disease Outcomes Quality Initiative (KDOQI) Guidelines.
- Bardakci H, Cheema FH, Topkara VK, Dang NC, Martens TP, et al. (2007) Discharge to home rates are significantly lower for octogenarians undergoing coronary artery bypass graft surgery. Ann Thorac Surg 83: 483-489. Link: https://goo.gl/dQXcEk
- Sajja LR, Mannam G, Chakravarthi RM, Sompalli S, Naidu SK, et al. (2007) Coronary artery bypass grafting with or without cardiopulmonary bypass in patients with preoperative non-dialysis dependent renal insufficiency: a randomized study. J Thorac Cardiovasc Surg 133: 378-388. Link: https://goo.gl/rxQzMS
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, et al. (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461-470. Link: https://goo.gl/zEwq8z
- National Kidney Foundation (2002) K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis 39: 1-266. Link: https://goo.gl/KF4ngz
- Domoto S, Tagusari O, Nakamura Y, Takai H, Seike Y, et al. (2014) Preoperative estimated glomerular filtration rate as a significant predictor of long-term outcomes after coronary artery bypass grafting in Japanese patients. Gen Thorac Cardiovasc Surg 62: 95-102. Link: https://goo.gl/ttaMq6
- Gong ZY, Gao CQ, Li BJ, Jiang SL, Xiao CS, et al. (2012) acute kidney injury early after cardiac surgery with cardiopulmonary bypass: clinical analysis. Zhonghua Yi Xue Za Zhi 92: 3283-3287. Link: https://goo.gl/LDkyDq
- Moguel-González B, Wasung-de-Lay M, Tella-Vega P, Riquelme-Mc-Loughlin C, Villa AR, et al. (2013) Acute kidney injury in cardiac surgery. Rev Invest Clin 65: 467-475.
- Lopez-Delgado JC, Esteve F, Torrado H, Rodríguez-Castro D, Carrio ML, et al. (2013) Influence of acute kidney injury on short- and long-term outcomes in patients undergoing cardiac surgery: risk factors and prognostic value of a modified RIFLE classification. Crit Care 17: R293. Link: https://goo.gl/MxPysF
- Kandler K, Jensen ME, Nilsson JC, Møller CH, Steinbrüchel DA (2014) Acute kidney injury is independently associated with higher mortality after cardiac surgery. J Cardiothorac Vasc Anesth 28: 1448-1452. Link: https://goo.gl/F2BIlr
- Gul EE, Erdogan HI, Yıldırım O, Soylu A, Nikus KC (2012) Hyperkalemia-induced accelerated idioventricular rhythm in a patient with acute renal failure. Ren Fail 34: 543-544. Link: https://goo.gl/ZtcVkD
- Lisowska A, Tycińska A, Knapp M, Lisowski P, Musiał WJ (2011) The incidence and prognostic significance of cardiac arrhythmias and conduction abnormalities in patients with acute coronary syndromes and renal dysfunction. Kardiol Pol 69: 1242-1247. Link: https://goo.gl/vMmyqm
- Bingol H, Akay HT, Iyem H, Bolcal C, Oz K, et al. (2007) Prophylactic dialysis in elderly patients undergoing coronary bypass surgery. Ther Apher Dial 11: 30-35. Link: https://goo.gl/y74PX2
- Crescenzi G, Torracca L, Pierri MD, Rosica C, Munch C, et al. (2015) ’Early’ and ’late’ timing for renal replacement therapy in acute kidney injury after cardiac surgery: a prospective, interventional, controlled, single-centre trial. Interact Cardiovasc Thorac Surg 20: 616-621. Link: https://goo.gl/a4xvKA
- Bahar I, Akgul A, Ozatik MA, Vural KM, Demirbag AE, et al. (2005) Acute renal failure following open heart surgery: risk factors and prognosis. Perfusion 20: 317-322. Link: https://goo.gl/TpFFeT
- Durmaz I, Yagdi T, Calkavur T, Mahmudov R, Apaydin AZ, et al. (2003) Prophylactic dialysis in patients with renal dysfunction undergoing on-pump coronary artery bypass surgery. Ann Thorac Surg 75: 859-864. Link: https://goo.gl/i5TvKX
- Bucerius J, Gummert JF, Walther T, Schmitt DV, Doll N, et al. (2004) On-pump versus off-pump coronary artery bypass grafting: impact on postoperative renal failure requiring renal replacement therapy. Ann Thorac Surg 77: 1250-1256. Link: https://goo.gl/PfsDoa
- Schopka S, Diez C, Camboni D, Floerchinger B, Schmid C, et al. (2014) Impact of cardiopulmonary bypass on acute kidney injury following coronary artery bypass grafting: a matched pair analysis. J Cardiothorac Surg 18: 20. Link: https://goo.gl/2azqHO
- Brito DJ, Nina VJ, Nina RV, Figueiredo Neto JA, Oliveira MI, et al. (2009) Prevalence and risk factors for acute renal failure in the postoperative of coronary artery bypass grafting. Rev Bras Cir Cardiovasc 24: 297-304. Link: https://goo.gl/dnNjIR
- Suen WS, Mok CK, Chiu SW, Cheung KL, Lee WT, et al. (1998) Risk factors for development of acute renal failure (ARF) requiring dialysis in patients undergoing cardiac surgery. Angiology 49: 789-800. Link: https://goo.gl/hZHWBd
- Parolari A, Pesce LL, Pacini D, Mazzanti V, Salis S, et al. Monzino Research Group on Cardiac Surgery Outcomes (2012) Risk factors for perioperative acute kidney injury after adult cardiac surgery: role of perioperative management. Ann Thorac Surg 93: 584-591. Link: https://goo.gl/6Pe1Np
- Sirvinskas E, Benetis R, Raliene L, Andrejaitiene J (2012) the influence of mean arterial blood pressure during cardiopulmonary bypass on postoperative renal dysfunction in elderly patients. Perfusion 27: 193-198. Link: https://goo.gl/iZvuIw
- Azau A, Markowicz P, Corbeau JJ, Cottineau C, Moreau X, et al. (2014) Increasing mean arterial pressure during cardiac surgery does not reduce the rate of postoperative acute kidney injury. Perfusion 29: 496-504. Link: https://goo.gl/USVqGV
- Kandler K, Jensen ME, Nilsson JC, Møller CH, Steinbrüchel DA (2015) Arterial pressure during cardiopulmonary bypass is not associated with acute kidney injury. Acta Anaesthesiol Scand 59: 625-631. Link: https://goo.gl/XXLW2c
- Haase M,Bellomo R, Story D, Letis A, Klemz K, et al. (2012) Effect of mean arterial pressure, haemoglobin and blood transfusion during cardiopulmonary bypass on post-operative acute kidney injury. Nephrol Dial Transplant 27: 153-160. Link: https://goo.gl/vyWlNP
- Kanji HD, Schulze CJ, Hervas-Malo M, Wang P, Ross DB, et al. (2010) Difference between preoperative and cardiopulmonary bypass mean arterial pressure is independently associated with early cardiac surgery-associated acute kidney injury. J Cardiothorac Surg 5: 71. Link: https://goo.gl/91eX5Y
- Kourliouros A, Valencia O, Phillips SD, Collinson PO, van Besouw JP, et al. (2010) Low cardiopulmonary bypass perfusion temperatures are associated with acute kidney injury following coronary artery bypass surgery. Eur J Cardiothorac Surg37: 704-709. Link: https://goo.gl/KUACxG
- Filsoufi F, Rahmanian PB, Castillo JG, Silvay G, Carpentier A, et al. (2008) Predictors and early and late outcomes of dialysis-dependent patients in contemporary cardiac surgery. J Cardiothorac Vasc Anesth 22: 522-529. Link: https://goo.gl/ZXepA5
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
