International Journal of Vascular Surgery and Medicine
Growth and Vascular Remodeling Factors during a Basketball Season in Adolescent Boys
Elias Armenis1, Anastassios Philippou2*, Maria Maridaki1, Panayiotis Baltopoulos1 and Maria Tsironi3
2Medical School, National and Kapodistrian University of Athens, Athens, Greece
3Department of Nursing, University of Peloponnese, Sparta, Greece
Cite this as
Armenis E, Philippou A, Maridaki M, Baltopoulos P, Tsironi M (2017) Growth and Vascular Remodeling Factors during a Basketball Season in Adolescent Boys. Int J Vasc Surg Med 3(1): 001-007. DOI: 10.17352/2455-5452.000018Background: Circulating factors during exercise training are implicated in the adaptive mechanisms of physical conditioning. However, there is lack of information regarding the effect of basketball training on circulating growth and vascular remodeling factors in adolescents. The purpose of this study was to characterize the serum insulin-like growth factor 1 (IGF-1), vascular endothelial growth factor (VEGF), matrix metalloproteinase 3 (MMP-3), albumin (ALB) and aldolase (ALD) responses in healthy adolescent boys over the course of a regular basketball season, and compared them with age- and sex-matched participants not involved systematically in any physical activity.
Materials and Methods: We evaluated systemic and body composition changes in basketball athletes (n=34; mean age 17.1±0.7 yrs) and controls (n=21; mean age 17.2±0.9 yrs) before training, at peak season (8 wks), and at the end of the season (32 wks).
Results: Changes in % body fat and body mass index (BMI) were observed in the training group (TG) over time (P<0.05-0.001), while these parameters remained unchanged in the control group (CG). TG exhibited higher serum ALB and ALD levels compared with CG (P<0.001) while they did not change over time (P>0.05). Circulating IGF-1, VEGF and MMP-3 levels increased in TG over time (P<0.01) and were higher compared to CG (P<0.001), in which there were not any changes.
Conclusion: The elevated levels of growth, metabolic and vascular remodeling factors in the adolescent basketball athletes potentially indicate beneficial, anabolic and angiogenic, processes in response to this mode of exercise training.
Introduction
Athletic training to improve physical performance involves periods of high physical stress followed by a reduction in the stress levels, thus including repetitive phases of normal training, high training load, overload, and recovery [1,2]. A model of biphasic adaptive responses to training overload has been proposed, involving peripheral mechanisms in the early phases of training overload and more central mechanisms in the intensive phases of training [1,3,4].Specifically, muscular damage and increased metabolic needs are mainly involved in the acute response, while the chronic training response results in changes not only in tissue metabolism, body composition, and somatic growth, but also to central regulatory adaptations [1].
Assessment of circulating factors during prolonged exercise training has received considerable attention due to their implication in the adaptive mechanisms of physical conditioning [1,2]. Thus, hormonal measurements of the insulin-like growth factor 1 (IGF-1) axis have been extensively used to assess training status and possible development of overtraining in athletes [2] and particularly in adolescents [3,5]. Furthermore, it has been proposed that the variable responses of circulating IGF-1 to acute and chronic exercise reflect the influence of other determining factors, such as variables of the exercise program or subjects’ metabolic status [6]. In addition, serum albumin (ALB) has been used as a biochemical indicator of protein status of the body [7] and has been associated with changes in skeletal muscle mass, with lower values indicating a diminished protein reserve and stimulated catabolic processes that lead to muscle breakdown [8].
On the other hand, exercise training-induced muscle adaptations include the development of new capillaries, known as angiogenesis [9]. Specifically, exercise-induced metabolic stress has been shown to stimulate pro-angiogenic factors, such as vascular endothelial growth factor (VEGF) [10], which is essential for embryonic angiogenesis [11] and exercise-induced increases in skeletal muscle vascular density [12]. Moreover, there is evidence suggesting that VEGF-induced angiogenesis is influenced by matrix metalloproteinases (MMPs) which are a family of enzymes contribute to both normal and pathological tissue remodeling [13]. In particular, circulating MMPs facilitate physiological responses to exercise such as angiogenesis [14] and are thought to modulate the activation of growth factors through degradation of their precursors, inhibitors, or binding proteins [13,15,16]. Thus, MMPs have been proposed to enhance VEGF-induced vascular cell proliferation and survival via proteolytically cleaving and modulating molecules in VEGF signaling [13,17]. Interestingly, MMP-3 possesses the broadest substrate specificity among all MMPs [13] and a relationship has been found between serum levels of MMP-3 and VEGF in angiogenesis [17].
Nevertheless, the effects of acute and chronic exercise on circulating serum VEGF have been reported equivocal [10, 18]. Moreover, although the effects of an acute bout of exercise on MMPs levels in the circulation have been explored [19], there is little information regarding the effects of exercise training on circulating MMP concentrations [16], suggesting, though, that the mode of exercise training influences the MMP responses and, thus, indicating a possible role of MMPs in training-specific adaptations.
In particular, there is lack of longitudinal studies examining the potentially beneficial or detrimental effect of the intensive basketball training on circulating growth and vascular remodeling factors in adolescents. Therefore, the purpose of this study was to characterize the serum IGF-1, VEGF, MMP-3, ALB and aldolase (ALD) responses in adolescent boys during and at the end of a regular basketball season, and compared them with those of age- and sex-matched participants not involved systematically in any physical activity. We hypothesized that long term basketball training would influence circulating levels of those factors in the adolescent basketball athletes, suggesting their role as mediators or indicators of adaptation to physical stress caused by this mode of athletic conditioning.
Methods
Subjects
Power analysis was performed using the G*Power statistical power analysis program [20], to calculate the minimum number of participants needed for a statistical power of 0.95. Thus, fifty five healthy male adolescents participated in the study after a medical examination by a pediatrician, divided in the training group (TG) and the control group (CG). The TG consisted of 34 male adolescent basketball players (17.1±0.7 years old), having an average basketball athletic experience of 8 years and participated in a systematic exercise program at least 5 times per week. The CG comprised of 21 adolescent boys (17.2±0.9 years old) not involved systematically in any physical activity or participating in any systematic exercise program during, at least, the last 5 years. The participants were free from any musculoskeletal injuries, did not have a history of any infectious, metabolic or chronic pathologic condition, and had not presented any fever episode at least four weeks before the initiation of the experimental procedures. These individuals also were not smokers and refrained from consuming any alcohol or taking any medications or nutritional supplementations at least two weeks before and throughout the experimental period. No attempt was made to change the physical activities of daily living in either control or the training group, however the participants were asked not to change their activity patterns from those before the study. Further, all volunteers were instructed to maintain their normal dietary habits during the experimental period, nevertheless, on the day prior to and the day of each blood draw, they were asked to have similar meals.
Anthropometric measurements
Participants’ body weight (W) and height (H) were measured using standard techniques and their body mass index (BMI) was calculated using the formula BMI = W (kg)/[H (m) x H (m)]. Body fat was assessed by skinfold thickness measurements (i.e. subscapular, triceps, pectoral, suprailiac, abdomen, mid-alar, thigh, gastrocnemius and hamstring) using the Harpenden Skinfold Caliper (Baty International, UK) and the body fat percentage (%) was calculated according to the Durnin and Rahaman [21] equations. All skinfolds were measured by the same technician throughout the experimental period using the same skinfold caliper.
Blood sampling and serum measurement
Blood samples were collected from both groups before the start of the pre-season training (baseline), on its completion (Peak-season - 8 wks into training) and at the end of the regular (competitive) season (Post-season - 32 wks into training), at the same time of the day (i.e. between 07.30 and 8.30 am) and two days after the last training session. The subjects were at rest for at least 30 min prior to collection of 10 mL of blood obtained from an antecubital vein. Blood samples were allowed to clot at room temperature for 30 min. Serum was collected following centrifugation at 2,000 g at 40 C for 10 min, stored frozen in 0.5 mL aliquots at –800C, and thawed only once at the time of analysis.
Serum levels of total IGF-1, VEGF and MMP-3 were determined by standard sandwich enzyme-linked immunosorbent assay (ELISA) using commercially available kits (IGF-1: R&D Systems Inc., Minneapolis, USA; VEGF and MMP-3: Invitrogen, Camarillo, CA, USA). Optical density measurements were performed with a microplate reader (Versamax, Molecular Devices, Sunnyvale, CA, USA) at 450 nm, and calculations were performed using a SoftMax Pro software (Molecular Devices, Sunnyvale, CA, USA). According to manufacturers, the minimal detection limits of the assays were 0.5 ng . mL–1 , 0.5 pg . mL–1 , 0.1 ng . mL–1 , for IGF-1, VEGF and MMP-3, respectively. The intra- and inter-assay coefficients of variation (CV) were as follows: 4.3% and 8.3% for IGF-1, 4.8% and 8.2% for VEGF, and 4.9% and 5.6% for MMP-3, respectively.
Serum ALD and ALB levels were determined with automated standard assays using commercially available kits (ALD: Hitachi 717, Mannheim, Germany/Randox, Crumlin, UK; ALB: Versamax, Molecular Devices, Sunnyvale, CA, USA/BioVision, Inc. Milpitas, CA, USA). All samples for each target analyte were analyzed simultaneously in duplicate and the results averaged.
Exercise training program
The subjects of the TG underwent a typical basketball training program during a full basketball season (August to April).Training involved basketball technical and tactical drills, speed, power and endurance drills, aerobic and strength training. Briefly, it included two sessions per day for 6 days each week during the pre-season and one session per day during the competitive season, while typically each training session lasted 90 min. Training intensity was graded according to 50–80% of the individual maximum heart rate (HRmax) calculated using the general formula 220 – age. HR was continuously tracked using a Polar chest-belt HR monitor (Polar Electro Oy, Kempele, Finland). The total exercise time in each session and the training intensity, as determined by the target HR (% of the HRmax), varied during the study and were adapted according to the training-induced improvement in the subjects’ submaximal HR. During the experimental period the subjects of the CG were physically active but without following any systematic training program.
Statistical analysis
Analysis of variance (ANOVA) with repeated measures over time was employed to evaluate changes in all serum measurements (SPPS v. 18 statistical package; SPSS Inc., Chicago, IL, USA). Kolmogorov-Smirnov test was used to test the normality of the data distribution. Where significant main effects or interactions were noted (P<0.05), the means were compared using Tukey’s post-hoc tests. Pearson’s correlation coefficient (r) was used to determine correlations between variables. Results are presented as mean + SD and the statistical significance was set at P<0.05.
Results
Anthropometric measurements
Anthropometric features of the basketball athletes and the control group are shown in Table 1. Height and weight did not change significantly throughout the experimental period. The average body weight and %fat in the TG were less compared with the CG (P<0.05-0.001). Significant changes in the % body fat as well as BMI were observed in the TG compared with baseline (PRE), while these parameters were unchanged in the CG throughout the experimental period (P<0.05-0.001). Specifically, there were significant decreases in % body fat, while BMI also decreased at Peak-season and exhibited an increase at Post-season compared with baseline (Table 1).
Circulating growth and vascular remodeling factors
The TG exhibited higher values of serum ALB and ALD compared with the CG at each time point of measurement (P<0.001). However, no changes were revealed in the ALB or ALD values during the experimental period in either the TG or CG (P>0.05; Table 1).
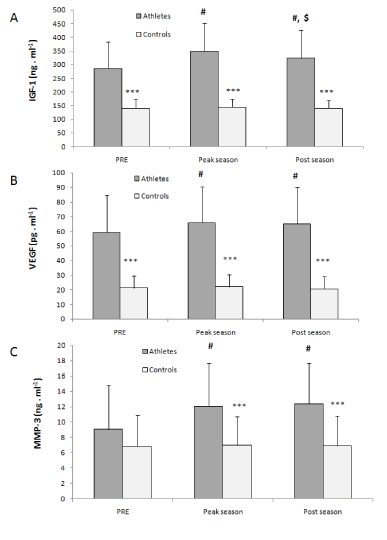
The coefficient r2 for standard curves of all the ELISA analyses was 0.994 - 1. Circulating levels of the IGF-1 in the TG were higher compared with the CG throughout the experimental period (Figure 1A; P<0.001). In particular, serum IGF-1 levels peaked by the completion of the pre-season training, exhibiting an increase compared to the baseline values (P<0.01). Interestingly, there was a decrease in the IGF-1 levels at the end of the competitive season (Post-season, P<0.01) compared with the Peak-season, though its levels remained significantly elevated compared with baseline (P<0.01). Circulating IGF-1 levels remained unchanged in the CG over the experimental period (P>0.05).
Higher levels of VEGF were observed in the TG compared with the CG throughout the experimental period (Figure 1B; P<0.001). Specifically, basketball training resulted in increased serum VEGF both at the completion of the pre-season training and at the end of the competitive season compared with baseline (P<0.01). In the control group, circulating VEGF levels did not change throughout the experimental period (P>0.05).
No differences were observed in the serum MMP-3 levels between the TG and the CG at baseline. However, on the Peak-season and Post-season measurement, the TG exhibited increased MMP-3 levels compared to the CG (P<0.001; Figure 1C). Moreover, as a result of the basketball training, increased serum MMP-3 levels were observed both at the Peak-season and at the Post-season measurement compared with baseline (P<0.001). Again, no changes were observed in the MMP-3 levels of the CG throughout the experimental period.
Correlational analyses in the TG revealed highly positive associations over the experimental period between IGF-1 and VEGF (r=0.599-0.646; P<0.01), VEGF and MMP-3 (r=0.498-0.699; P<0.01), and also between MMP-3 and IGF-1 (r=0.375-0.493; P<0.05-0.01), (Figure 2A-C). Moreover, a significant negative correlation was found between the baseline IGF-1 levels and the individual percent changes of this factor after the completion of the pre-season training (Peak-season, r= - 0.414; P<0.02), but not after the end of the competitive season (Post-season, r= - 0.213; P>0.05), (Figure 2D).
Discussion
Our study investigated the possible beneficial or detrimental effects of the long-term exercise training and intensive physical and metabolic stress, induced by the chronic basketball training, in the context of potential anabolic/angiogenic or catabolic (e.g., overtraining) responses of the adolescent athlete during the somatic growth and development. Our findings demonstrated that the responses to a full period of basketball training in adolescence are characterized by remarkable increases in the circulating levels of the growth and vascular remodeling factors IGF-1, VEGF and MMP-3. Interestingly, the serum levels of these factors as well as of the indicators of metabolic status, ALB and ALD, were higher in the group of basketball athletes compared with those of the untrained adolescents, which remained unchanged throughout the experimental period.
Similarly to the findings of the present study, previous cross-sectional studies have demonstrated that circulating IGF-1 levels are higher in fitter adolescents and adults [4,22], indicating that exercise training is associated with the enhancement of anabolic activity through the function of IGF-1. This anabolic factor is mainly produced in the liver and enters into the circulation as a hormone, but also it is expressed in skeletal muscle and other tissues. IGF-1 has long been recognized as a crucial factor which regulates a wide range of cellular responses in multiple biological systems [23,24]. Moreover, it exerts important effects on the hormonal regulation in response to exercise and training [1] and is particularly a critical factor for increasing muscle mass and enhancing muscle repair [25].
Several studies have suggested that the imposition of an exercise training program leads initially to decreases in IGF-1 levels, as a consequence of increase in pro-inflammatory cytokines and stress proteins, characterized by a catabolic-type response [1,5]. As the training adaptation progresses, an anabolic rebound in circulating growth factors may ensue, and IGF-1 exceed the pre-training levels, although it remains unknown how and when exactly this switch takes place [3,4]. Consistent with the two phases hypothesis, our findings suggest that longer periods of training are associated with stable or increased circulating IGF-1 levels [4]. Specifically, we measured the first adaptational response to training at the completion of a 2-week relative tapering period following a long term (6 wks) intensive pre-season training, apparently exceeding the first phase of the systemic IGF-1 down-regulation [4]. Tapering down the training intensity prior to the competition has been associated with a parallel increase in circulating IGF-1 levels [1,26]. Moreover, we found that lower individual pre-training IGF-1 levels were associated with more substantial increases in IGF-1 at the end of the pre-season/tapering period. This finding expands in basketball training the duration but also the pattern of the inverse relationship previously observed between individual pre-training IGF-1 levels and its changes after training [27]. Moreover, the relative IGF-1 decline at the end of the competitive season may reflect a time-dependent systemic adaptation of this anabolic mediator to training [6], and particularly during the competitive period in the adolescent basketball athletes.
The increased levels of IGF-1 were not accompanied by changes in body weight in the basketball athletes. Although we did not perform energy intake analyses, the fact that those athletes remained weight-stable during the training period with simultaneous significant increases in the IGF-1 levels, and in conjunction with previous findings [28], may challenge the hypothesis that energy balance by itself, e.g. weight stability, regulates circulating IGF-1 [29]. Thus, our findings further support the notion that exercise-associated mechanisms such as increased energy flux rather than negative energy balance may regulate the serum concentration of this anabolic mediator after exercise training [6,27,28].
Nevertheless, we observed changes in body composition in the TG particularly at Post-season measurement, i.e. an increase in BMI and decrease in % body fat, which indicate an increase in fat-free mass and may be associated with the IGF-1 responses to basketball training. A relationship between circulating IGF-1 and body composition alterations has been proposed, as well as the possibility this growth factor to reflect metabolic stress and anabolic adaptations [30], such as increases in fat-free mass during exercise training [6] and particularly in young adults [30]. Exercise training may result in increased IGF-1 production both in skeletal muscle and liver, if the substantial increase in tissue metabolism and protein demands associated with training are met [31]. Thus, high levels of circulating IGF-1 may have particular hypertrophic importance in a training period when muscles are primed for anabolism [32].
Moreover, increased levels of ALB and ALD were also revealed throughout the basketball season. Serum ALB has been associated with the protein balance of the body [7] and a strong relationship between low serum ALB and skeletal muscle loss, not associated with a low protein intake, has been found in older persons during a 5-year follow up, suggesting a specific role of ALB in changes of skeletal muscle mass [8]. Furthermore, higher levels of serum ALD activity at rest were reported in trained athletes compared to non-athletes due to the higher levels and proportion of the predominant in skeletal muscle ALD isoenzyme A [33]. In addition, significant increases in ALD activity were found in hind leg muscles after long-term exercise training in young and intermediate age mice [34]. Thus, we speculate that the elevated levels of ALB and ALD in the TG may imply increase anabolic processes in the muscles. However, in the interpretation of these data it is critical to consider the fact that they represent systemic responses and may not reflect well the local skeletal muscle adaptation.
On the other hand, exercise training-induced adaptations include angiogenesis, which is a multiple process of vascular remodeling that involves several factors such as VEGF and MMPs [9]. In this study, we found significant increases in circulating VEGF and MMP-3 in the TG during the basketball training. Vascular formation and remodeling include recruitment, proliferation and migration of vascular cells such as endothelial cells (ECs) and vascular smooth muscle cells (VSMCs). VEGF stimulates ECs proliferation, differentiation and migration, while prevents endothelial apoptosis and promotes survival of the newly formed blood vessels [35]. Moreover, decreased levels of VEGF have been associated with endothelial dysfunction and increased rate of ECs apoptosis, while this factor also regulates vascular permeability and vasodilatation [36]. Furthermore, enhanced proliferation and survival of ECs by differential expression of anti-apoptotic genes and in part by activation of MMPs has been proposed as a possible mechanism of VEGF regulation of angiogenesis [17].
Similarly, in skeletal muscle MMPs function to process extracellular matrix (ECM) proteins [16,37], while the circulating MMPs facilitate angiogenesis [38]. Moreover, increasing evidence suggests that MMPs, and particularly MMP-3, play a critical role in vascular formation and remodeling through degrading vascular basement membrane and ECM proteins [13,14], to facilitate the progression of cell migration and invasion in vascular tissues. Indeed, studies in animal models have shown that MMP-3 knockout significantly reduces VSMCs migration in vitro and neointima formation in vivo [39]. Since serum concentrations of VEGF [18] and MMPs [16], peak within a short period of time following a single bout of exercise, their increased levels found in our study represent the effects of training that may impact specific remodeling cascades in the vasculature, inducing angiogenic adaptations. Interestingly, VEGF is positively controlled by cytokines and growth factors while MMPs also modify angiogenic growth factors, including IGF-1, facilitating their bioavailability in vascular remodeling [13,15,16]. Thus, the significant correlations found in this study between VEGF, MMP-3 and IGF-1 may indicate their interactions in vascular remodeling and/or common responses to the exercise training.
The utilization of age- and sex-matched untrained adolescents as a control group in this study and their unchanged values in all the measured variables during the 32-week experimental period documented that the physiological body growth and development of the adolescent athletes did not influence their responses to basketball training. Particularly at baseline, the higher serum concentrations of IGF-1, VEGF, ALB and ALD in the basketball athletes apparently reflect their metabolic status due to an expected higher level of physical activity prior to the training period compared to the untrained adolescents.
Conclusions
In conclusion, this study described systemic components of the adaptation profile of adolescent athletes during a full basketball season. These adaptational responses included elevated serum levels of growth, metabolic and vascular remodeling factors, potentially indicating beneficial, anabolic and angiogenic, processes in the adolescent athlete, in response to the long-term basketball training. Our findings are consistent with previous research regarding the beneficial effect of long-term exercise training on growth and tissue remodeling factors in adolescence [3,22], emphasizing the potential significance and impact of exercise in the developing body of the adolescent athlete [40]. However, due to the systemic nature of our data, further studies to examine the local responses of those anabolic and angiogenic mediators in skeletal muscle and/or bone tissue of the adolescent athlete would be of great importance.
The authors are greatly indebted to the study participants for their invaluable contribution to this work.
Ethical approval
A written informed assent was obtained by all the volunteers as well as informed consent from their parents or legal guardians to participate in this study, which was approved by the Ethics Committee of the University, and all experimental procedures conformed to the Declaration of Helsinki.
- Steinacker JM, Lormes W, Reissnecker S, Liu Y (2004) New aspects of the hormone and cytokine response to training. Eur J Appl Physiol 91: 382-91. Link: https://goo.gl/FXVWvc
- Jurimae J, Maestu J, Jurimae T, Mangus B, von Duvillard SP (2011) Peripheral signals of energy homeostasis as possible markers of training stress in athletes: a review. Metabolism 60: 335-50. Link: https://goo.gl/lT0U6f
- Nemet D, Pontello AM, Rose-Gottron C, Cooper DM (2004) Cytokines and growth factors during and after a wrestling season in adolescent boys. Med Sci Sports Exerc 36: 794-800. Link: https://goo.gl/pzYzuk
- Eliakim A, Nemet D (2010) Exercise training, physical fitness and the growth hormone-insulin-like growth factor-1 axis and cytokine balance. Med Sport Sci 55: 128-40. Link: https://goo.gl/Wx0Xtk
- Scheett TP, Nemet D, Stoppani J, Maresh CM, Newcomb R, et al. (2002) The effect of endurance-type exercise training on growth mediators and inflammatory cytokines in pre-pubertal and early pubertal males. Pediatr Res 52: 491-497. Link: https://goo.gl/QJSJ9i
- Nindl BC, Pierce JR (2010) Insulin-like growth factor I as a biomarker of health, fitness, and training status. Med Sci Sports Exerc 42: 39-49. Link: https://goo.gl/fwJrvu
- Koskelo EK, Saarinen UM, Siimes MA (1990) Skeletal muscle wasting and protein-energy malnutrition in children with a newly diagnosed acute leukemia. Cancer 66: 373-376. Link: https://goo.gl/AE56ib
- Visser M, Kritchevsky SB, Newman AB, Goodpaster BH, Tylavsky FA, et al. (2005) Lower serum albumin concentration and change in muscle mass: the Health, Aging and Body Composition Study. Am J Clin Nutr 82: 531-537. Link: https://goo.gl/kwZfQB
- Prior BM, Yang HT, Terjung RL (2004) What makes vessels grow with exercise training? J Appl Physiol (1985) 97: 1119-1128. Link: https://goo.gl/ewBJN4
- Wahl P, Schmidt A, Demarees M, Achtzehn S, Bloch W, et al. (2013) Responses of angiogenic growth factors to exercise, to hypoxia and to exercise under hypoxic conditions. Int J Sports Med 34: 95-100. Link: https://goo.gl/TOVVtY
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, et al. (1996): Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 380: 439-442. Link: https://goo.gl/bOejTf
- Amaral SL, Papanek PE, Greene AS (2001) Angiotensin II and VEGF are involved in angiogenesis induced by short-term exercise training. Am J Physiol Heart Circ Physiol 281: H1163-H1169. Link: https://goo.gl/4zEHdd
- Chen Q, Jin M, Yang F, Zhu J, Xiao Q, et al. (2013) Matrix metalloproteinases: inflammatory regulators of cell behaviors in vascular formation and remodeling. Mediators Inflamm 2013: 928315. Link: https://goo.gl/IgSUuG
- Kjaer M (2004) Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev 84: 649-698. Link: https://goo.gl/idrICp
- Nakamura M, Miyamoto S, Maeda H, Ishii G, Hasebe T, et al. (2005) Matrix metalloproteinase-7 degrades all insulin-like growth factor binding proteins and facilitates insulin-like growth factor bioavailability. Biochem Biophys Res Commun 333: 1011-1016. Link: https://goo.gl/O8DtsG
- Urso ML, Pierce JR, Alemany JA, Harman EA, Nindl BC (2009) Effects of exercise training on the matrix metalloprotease response to acute exercise. Eur J Appl Physiol 106: 655-663. Link: https://goo.gl/2PXIyt
- Tas F, Duranyildiz D, Oguz H, Camlica H, Yasasever V, et al. (2008) Circulating levels of vascular endothelial growth factor (VEGF), matrix metalloproteinase-3 (MMP-3), and BCL-2 in malignant melanoma. Med Oncol 25: 431-436. Link: https://goo.gl/hFdARq
- Kraus RM, Stallings HW 3rd, Yeager RC, Gavin TP (2004) Circulating plasma VEGF response to exercise in sedentary and endurance-trained men. J Appl Physiol (1985) 96: 1445-1450. Link: https://goo.gl/xFzAzy
- Tayebjee MH, Lip GY, Blann AD, Macfadyen RJ (2005) Effects of age, gender, ethnicity, diurnal variation and exercise on circulating levels of matrix metalloproteinases (MMP)-2 and -9, and their inhibitors, tissue inhibitors of matrix metalloproteinases (TIMP)-1 and -2. Thromb Res 115: 205-210. Link: https://goo.gl/mqhVgs
- Faul F, Erdfelder E, Lang AG, Buchner A (2007): G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39: 175-191. Link: https://goo.gl/YigW6P
- Durnin JV, Rahaman MM (1967) The assessment of the amount of fat in the human body from measurements of skinfold thickness. Br J Nutr 21: 681-689. Link: https://goo.gl/O3iGuI
- Pareja-Galeano H, Brioche T, Sanchis-Gomar F, Montal A, Jovani C, et al. (2013) Impact of exercise training on neuroplasticity-related growth factors in adolescents. J Musculoskelet Neuronal Interact 13: 368-371. Link: https://goo.gl/1d9fI5
- Barton ER, Park S, James JK, Makarewich CA, Philippou A, et al. (2012) Deletion of muscle GRP94 impairs both muscle and body growth by inhibiting local IGF production. FASEB J 26: 3691-3702. Link: https://goo.gl/2zjFVJ
- Philippou A, Maridaki M, Pneumaticos S, Koutsilieris M (2014) The complexity of the IGF1 gene splicing, posttranslational modification and bioactivity. Mol Med 20: 202-214. Link: https://goo.gl/lB2qJL
- Philippou A, Barton ER (2014) Optimizing IGF-I for skeletal muscle therapeutics. Growth Horm IGF Res 24: 157-163. Link: https://goo.gl/9KNoiC
- Eliakim A, Nemet D, Bar-Sela S, Higer Y, Falk B (2002) Changes in circulating IGF-I and their correlation with self-assessment and fitness among elite athletes. Int J Sports Med 23: 600-603. Link: https://goo.gl/n9AAgD
- Nishida Y, Matsubara T, Tobina T, Shindo M, Tokuyama K, et al. (2010) Effect of low-intensity aerobic exercise on insulin-like growth factor-I and insulin-like growth factor-binding proteins in healthy men. Int J Endocrinol Link: https://goo.gl/K2HAv4
- Rarick KR, Pikosky MA, Grediagin A, Smith TJ, Glickman EL, et al. (1985) Energy flux, more so than energy balance, protein intake, or fitness level, influences insulin-like growth factor-I system responses during 7 days of increased physical activity. J Appl Physiol 103: 1613-1621. Link: https://goo.gl/ar7GgE
- Nemet D, Connolly PH, Pontello-Pescatello AM, Rose-Gottron C, Larson JK, et al. (1985) Negative energy balance plays a major role in the IGF-I response to exercise training. J Appl Physiol 96: 276-282. Link: https://goo.gl/gXFSma
- Nindl BC, Alemany JA, Kellogg MD, Rood J, Allison SA, et al. (2007) Utility of circulating IGF-I as a biomarker for assessing body composition changes in men during periods of high physical activity superimposed upon energy and sleep restriction. J Appl Physiol (1985) 103: 340-346. Link: https://goo.gl/0SzGTR
- Berg U, Bang P (2004) Exercise and circulating insulin-like growth factor I. Horm Res 62 Suppl 1: 50-58. Link: https://goo.gl/R6FoDf
- Schoenfeld BJ (2013) Postexercise hypertrophic adaptations: a reexamination of the hormone hypothesis and its applicability to resistance training program design. J Strength Cond Res 27: 1720-1730. Link: https://goo.gl/OY0OMm
- Haralambie G (1981) Serum aldolase isoenzymes in athletes at rest and after long-lasting exercise. Int J Sports Med 2: 31-36. Link: https://goo.gl/HgI7Pe
- Reznick AZ, Steinhagen-Thiessen E, Gellersen B, Gershon D (1983) The effect of short- and long-term exercise on aldolase activity in muscles of CW-1 and C57/BL mice of various ages. Mech Ageing Dev 23: 253-258. Link: https://goo.gl/MUlCxH
- Ferrara N, Davis-Smyth T (1997) The biology of vascular endothelial growth factor. Endocr Rev 18: 4-25. Link: https://goo.gl/KczN0G
- Czarkowska-Paczek B, Bartlomiejczyk I, Przybylski J (2006) The serum levels of growth factors: PDGF, TGF-beta and VEGF are increased after strenuous physical exercise. J Physiol Pharmacol 57: 189-197. Link: https://goo.gl/ALGuxK
- Philippou A, Maridaki M, Koutsilieris M (2008) The role of urokinase-type plasminogen activator (uPA) and transforming growth factor beta 1 (TGFbeta1) in muscle regeneration. In Vivo 22: 735-750. Link: https://goo.gl/JKgp26
- Haas TL, Milkiewicz M, Davis SJ, Zhou AL, Egginton S, et al. (2000) Matrix metalloproteinase activity is required for activity-induced angiogenesis in rat skeletal muscle. Am J Physiol Heart Circ Physiol 279: H1540-H1547. Link: https://goo.gl/I5IVPH
- Johnson JL, Dwivedi A, Somerville M, George SJ, Newby AC (2011) Matrix metalloproteinase (MMP)-3 activates MMP-9 mediated vascular smooth muscle cell migration and neointima formation in mice. Arterioscler Thromb Vasc Biol 31: e35-44. Link: https://goo.gl/OhjOsC
- Barnekow-Bergkvist M, Hedberg G, Pettersson U, Lorentzon R (2006) Relationships between physical activity and physical capacity in adolescent females and bone mass in adulthood. Scand J Med Sci Sports 16: 447-455. Link: https://goo.gl/EV17gi
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
