International Journal of Sexual and Reproductive Health Care
Increase in the number of consultations for early telarche and the subsequent diagnosis of early or early puberty in pediatric endocrinology in Alava, after the confinement suffered during the COVID-19 pandemic
Leyre Aurora Vilella San Martin1, Ignacio Díez López2*, Amaia San Martín Orayen1, Sandra Maeso Méndez1 and Ainhoa Sarasua Miranda3
2Assistant of Pediatric Endocrinology at HU Araba and Lecturer of the UPV, Vitoria, Spain
3Adjunct of Pediatric Endocrinology of the HU Araba, Vitoria, Spain
Cite this as
San Martin LAV, López ID, Martín Orayen AS, Méndez SM, Miranda AS (2023) Increase in the number of consultations for early telarche and the subsequent diagnosis of early or early puberty in pediatric endocrinology in Alava, after the confinement suffered during the COVID-19 pandemic. Int J Sex Reprod Health Care 6(1): 004-010. DOI: 10.17352/ijsrhc.000040Copyright Licence
© 2023 San Martin LAV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Introduction: Due to the COVID-19 pandemic and home confinement measures, many of our routines and daily habits have been both affected and modified. Have these factors conditioned a real increase in precocious (PP) and accelerated puberty (AP)?
Objectives: This is an observational and retrospective study in which the incidence of medical consultations due to premature thelarche is compared between March to December 2019 and 2020. The medical consultations occurred in the Pediatric Endocrinology (PE) consultation of our hospital.
Patients and methods: The analysis involved 75 cases of young girls consulted with premature thelarche in 2019 and 97 girls which were consulted in 2020. From each patient, different variables were analyzed, such as somatometry, hormones, eco, and treatment.
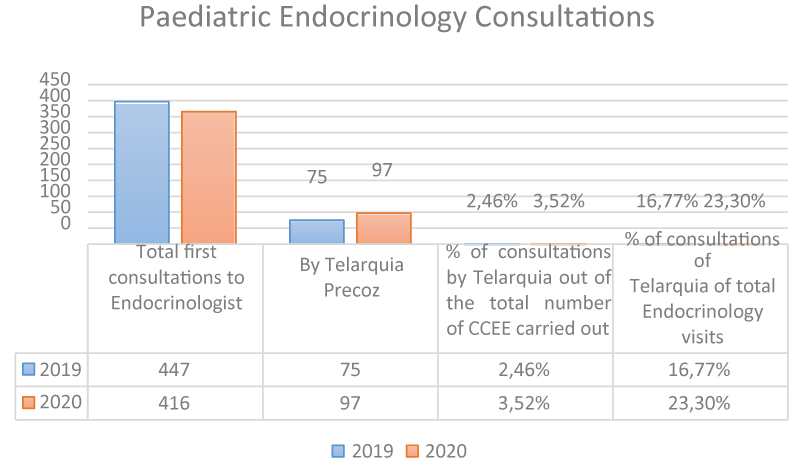
Results: In 2019, 75 first visits of PE were due to premature thelarche in young girls (accounting for 2.46% of the total number of first visits carried out in Pediatrics Outpatient Clinics (POC) and 16.77% of PE first visits). However, in 2020, 97 first visits were due to PE (3.52% of the total first-time visits of OC and 23.3% in PE). It was found that in 2020 the number of first-time visits due to premature thelarche increased by 28% compared to 2019 (p: 0.0001 mean comparison).
In 2019, out of the 75 consultations due to premature thelarche, 40% were diagnosed with an earlier physiological puberty. Conversely in 2020, out of the 97 consultations, 61.77% had normal physiological puberty. These figures represent an OR of 1.54 (p: 0.02 t Student for independent samples). It was evaluated whether the patients had experienced > 3kg weight gain above 6 months prior to the medical visit. In 2020, 31.57% of patients experienced weight gain, whereas 68.42% did not. In 2019 patients who experienced weight gain accounted for 12.16% and 87.83% did not experience significant weight gain (p: 0.01 t Student for independent samples).
Conclusion: Our data demonstrates there has been an increase in the incidence of referrals from Primary Attention to Pediatric Endocrinology to examine advanced thelarche during the period of lockdown measures (2020), in addition to an increase in the number of diagnostics of PP and AP in young girls in our hospital when compared to figures for 2019.
Furthermore, in 2020 there was a greater number of patients who experienced a weight gain increase 6 months prior to the medical consultations.
We hypothesize that the lockdown inflicted sedentary lifestyles together with changes in dietary habits, promoting weight gain in patients. This effect could have caused a body fat mass increase in girls, suggesting a “trigger effect” in the activation of the gonadal axis, causing a rise in the number of early puberty cases.
IRB: CIEC 01/2017.
Abbreviations
PP: Precocious Puberty; PA: Advanced Puberty; TP: Early Telarche; EP: Pediatric Endocrinology; CCEE: Outpatient Clinics; OR: Odds Ratio; EO: Bone Age
Introduction
Puberty is a complex biological process in which children undergo a series of hormonal changes leading to the maturation of secondary sexual characteristics, reaching adult size and reproductive capacity [1,2]. All these hormonal changes are due to the activation of the hypothalamic-pituitary-gonadal axis, which is regulated by a large number of elements, including genetic and environmental determinants [3,4].
According to the Spanish Society of Paediatric Endocrinology, we understand Precocious Puberty (PP) as the onset of the appearance of secondary sexual characteristics before the age of 8 years in girls and 9 years in boys; and as advanced puberty (AP) when the onset occurs between 8 and 9 years in girls and between 9 and 10 years in boys [5,6]. However, for some years now, it has been observed and described that the age of pubertal onset is advancing, especially in girls [2,7-9]. Although some of these cases may be explained by genetic/familial factors, most of them lack a family history of precocious puberty and it is believed that such an increase may be due to nutritional, lifestyle, and/or environmental changes, although these are not well known [10-13].
During the COVID-19 pandemic and the confinement experienced as a result of it, many routines and habits of our lives have been affected and modified. That is why, at the beginning of this study and in relation to the onset of puberty, this question was asked: “Could all the restriction measures applied during COVID-19 have led to an increase in early and early puberty in the children of our Autonomous Community?
As a background to our study, we find the article “Increased incidence of precocious and accelerated puberty in females during and after the Italian lockdown for the coronavirus 2019 (COVID-19) pandemic” (published in 2020 by Stagi, et al. in the Italian Journal of Pediatrics) [14], in which they describe a higher incidence of CPP in Italy, as well as a more rapid progression of puberty in girls who had previously been diagnosed with CPP.
This study is a retrospective observational analytical study comparing the incidences of first consultations for early telarche between the months of March to December 2019 and between the same period of time in 2020 at the Paediatric Endocrinology (PE) consultation of our hospital. In addition, we compare the increase in the diagnosis of early puberty (EP) and early puberty (AP) in the cases assessed for this reason, with the aim of demonstrating that the feeling that there has been in Primary Care as well as in the PD service about the increase in the incidence of these pathologies during confinement and the COVID period is real.
Other studies make reference to this [15,16].
Patients and methods
We first requested the total number of first consultations that had been made to the Paediatric ECC from March to December 2019 and in the same period of time in 2020. Subsequently, we retrospectively evaluated the first consultations made to the Paediatric Endocrinology Service, and how many of them the reason for referral was due to early telarche (PT).
Patients referred for this reason who were male and those who had dropped out of follow-up were excluded from the study. Patients younger than 24 months were also excluded. Therefore, the patients included in our study were all female and between 6 and 9 years of age at the time of the first consultation.
Study design
The study included 77 patients who consulted for early telarche at 2019 and 99 in 2020.
Once the girls who had first consulted for PT in one of the previously described periods had been selected, different characteristics recorded during the first consultation were retrospectively reviewed.
For each of them, we collected: age at the first visit, origin, family history of precocious puberty, weight, height, body mass index (BMI), whether or not they had adrenarche, and whether it had been prior to the onset of telarche, month in which they consulted, bone age (OA) at the first visit, the difference between bone age and chronological age, and whether they had experienced weight gain in the last 6 months. If available, the existence of disruptors and the results of the abdominal ultrasound were noted. Baseline FSH, LH, and E2 analytical values were also recorded.
Once all these data had been collected, the successive consultations of those who had had data on early puberty were reviewed, assessing which had undergone the Procrin test and which had not, and from all of them the final diagnosis was recorded, the options being: asymptomatic telarche, precocious puberty, early puberty, early puberty and physiological puberty (normal physiological development).
Aspects of the different variables assessed
- BMI was calculated by dividing the patient’s weight in kilograms by the square of height in meters.
For the calculation of the EO, an X-ray of the left hand was performed at the first consultation and the Greulich and Pyle method was used.
To calculate the difference between bone age (OA) and chronological age (CE), first, the decimal age to which OA and CE corresponded was calculated, taking into account the years and months that the girls were at the time of consultation, and then OA minus CE was subtracted.
- Ultrasonography: girls with an abdominal ultrasound were classified as prepubertal or pubertal, the latter group including those with an ovarian volume greater than 4 ml - 4.5 ml, uterine corpus length > 3 cm, and/or corpus/uterine cervix ratio of 2:1.
- Laboratory tests: all laboratory tests were performed by the same nursing staff and on an empty stomach.
The gonadal axis stimulation test performed was PROCRIN (leuprolide acetate: GnRH analog): a single dose of 500 ugs subcutaneously. This test was performed after explaining to the parents what the procedure consisted of, the purpose for which it was being performed, and after signing informed consent. All of them were performed in a day hospital, collecting FSH and LH values on admission, 3 hours, and 24 hours after administering PROCRIN. E2 values were also collected on admission and 24 hours after administration.
- Weight gain in the last 6 months: In this study, this concept was defined as an increase of > 3 kg during this period of time. It was decided to consider as weight gain if there was an increase of more than 3 kg in the last six months, since, physiologically, between one and one and a half years of life there is a slowing down of the growth rate until it increases considerably before puberty and menarche. Therefore, considering the age of our patients, the growth rate should be low and therefore the weight gain was attributed to an increase in fat mass and consequently in BMI. Furthermore, an increase of more than 3 kg in 6 months was, in most cases, a percentile increase in weight.
Concepts and Definitions
- Physiological puberty was defined as when pubertal changes had begun after the age of 9 years.
- Isolated telarche was defined as an increase in breast size without other associated hypothalamic-pituitary-gonadal axis activation.
- Precocious puberty was defined as the development of pubertal changes before the age of 8 years with an EO> 2DE for their age, with a stimulated LH > 6 IU/L or a baseline LH value of > 1.1 IU/L in the presence of pubertal signs and a stimulated LH/FSH ratio > 0.6 with associated clinical data of secondary sexual characteristics development.
- Advanced puberty was defined with the same characteristics as PP. But being in those girls between 8 and 9 years old.
Statistical analysis
Statistical analyses were carried out using SPSS software. Some of the characteristics analyzed in this study were continuous quantitative and others nominal categorical. To compare the latter between the two years, a legend was created in such a way that a number corresponded to one of the options of the variable; for example, in the case of the ultrasound variable, O: if it was not done, 1: if it had pre-pubertal characteristics and 2: if it had pubertal characteristics.
The categorical variables studied were described with frequencies while the quantitative variables were described with means and ranges.
Student’s t-test and Chi-Square were used to evaluate and compare the variables.
All statistical tests were two-tailed and a p < 0.05 was considered statistically significant.
Results
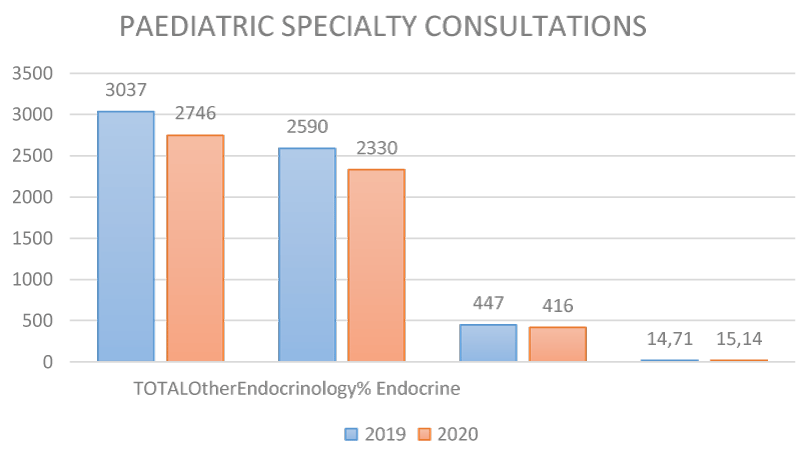
In 2019, 3037 first consultations were made to the different subspecialties of Paediatrics, of these 447 were to Paediatric Endocrinology (PE) which accounted for 14.71% of the CCEE. In 2020, a total of 2746 first consultations were performed, 416 of which were to Paediatric Endocrinology, representing 15.14%, i.e. 0.43% more than in 2019.
If we focus on the first consultations made to EP for early telarchies in girls, we observe that in 2019 there were 75, which represents 2.46% of the first consultations to CCEE and 16.77% of the first consultations to EP. However, in 2020, there were 97 first consultations for this reason, which represents 3.52% of the visits to CCEE and 23.3% in PE. Comparing both years, in 2020 there were 28% more first consultations for early telarchies than in 2019 (p: 0.0001 mean comparisons). We, therefore, understand that there is a significant difference, with a higher frequency of first consultations for early telarchies in 2020 compared to 2019 [16,17].
When studying the correlation between the related samples we observed that there might be a difference between what happened in 2019 and 2020 in the gender distribution (in 2019, 2.6% males and 97.4 females; and in 2020, 2% males and 97.4 females; and in 2020, 2% males and 97.4 females). 98% of women; p < 0.05), in the presence of family history (in 2019 the 84% had no family history compared to 15.6% who did, and in 2020, the 76% had no family history and 24% did; p: 0.011) and in the month in which they consulted (although looking at the months in both samples, we can observe that the months with the most consultations are between August and December, with August and October in 2019 with 13 consultations and October in 2020 with 19 consultations). In the rest of the variables studied, no significant differences were observed, so we can deduce that the children who consulted in both years had similar characteristics, except for the frequency of consultations, which were higher in 2020 [18,19].
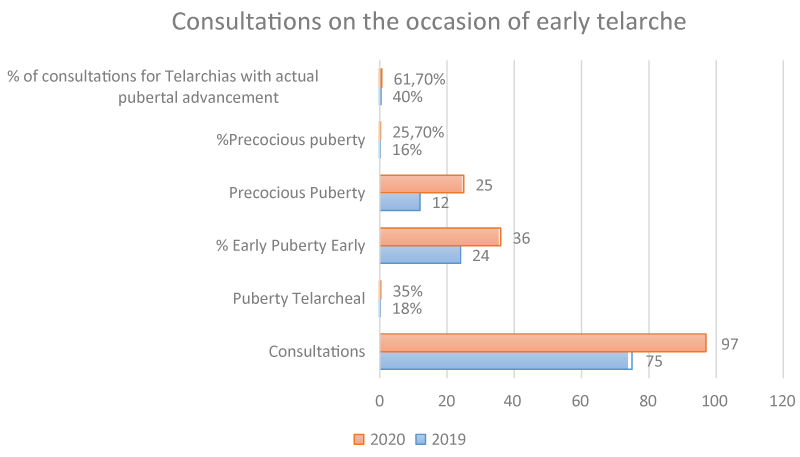
In 2019 out of 75 consultations for precocious puberty, 18 (24%) were advanced puberty and 12 (16%) were precocious puberty, i.e. 40% of consultations for precocious puberty were diagnosed with physiological pubertal advancement; while in 2020 out of 97 consultations for this diagnosis, 35 (36%) were advanced puberty and 25 (25.77%) were precocious puberty, i.e., of the total number of patients with precocious puberty, 61.77% had normal pubertal advancement, giving an OR of 1.54 (p < 0.05. Student’s t-test for independent samples).
On the other hand, assessing the average BMI of the girls who consulted in both years, we observe that it is around the 50th percentile; however in 2020 19.58 % (19 cases) had a BMI > 19.5 (p85, overweight) and 7.2% (7 cases) had a BMI >21.5 (p95, obese), compared to 17.33% (13 cases) with BMI > 19.5 in 2019 and 9.3% (7 cases) with BMI >21.5. (p < 0.05 Student’s t for independent samples).
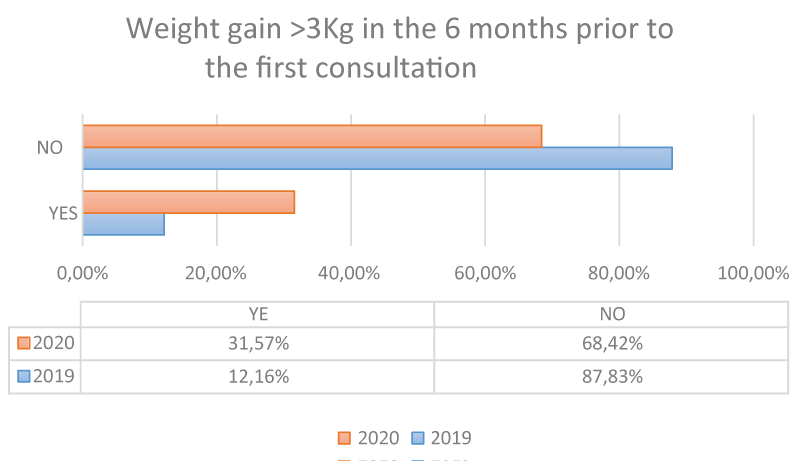
When assessing whether there has been a weight increase > 3 kg in the 6 months prior to the consultation, we see that in 2020 31.57% experienced such an increase compared to 68.42% who did not; and in 2019 12.16% did see their weight change compared to 87.83% who did not (P: 0.0001 Student’s t for independent samples) Figures 1-4.
Our data show that there has been an increase in the first consultations made to EP for advanced telarchies during the confinement period, as well as an increase in the diagnostic incidence of PP and PA in our hospital compared to the same period in 2019. Furthermore, the diagnostic increase in 2020 of both pathologies has been similar (OR of PA in 2020 vs. 2019: 1.5 and OR of PP in 2020 vs. 2019: 1.6).
Comparing 2019 vs. 2020 of total visits.
✓ Telarche advanced 24% vs. 36% (P: 0.0032 chi-squares for independent samples); 18/75 vs. 35/97
✓ Early telarchies 16% vs. 25.77% (P: 0.0049 chi-squares for independent samples); 12/75 vs. 25/97
In conclusion, we could say that in 2020 we have seen an age-independent acceleration of puberty, with the incidence increasing statistically significantly and almost equally in both PP and PA.
The rest of the girls studied were given other diagnoses: isolated telarchia, lipomastia, or normal puberty.
We can also observe that in 2020 there are more girls consulting with BMI > p85, i.e. overweight or obese, as well as a higher frequency of patients with an increase in weight gain in the last 6 months.
Discussion
The main objective of this study was to assess whether the subjective feeling in Paediatric Endocrinology in Alava regarding the increase in referrals for early telarche was real, as well as the increase in definitive diagnoses of precocious and early puberty in girls in that region. The results show a statistically significant increase in both the incidence of referrals and definitive diagnoses of pubertal advancement during the pandemic, which is in line with other studies [11,14,15,20-22]. These findings are probably related to the changes observed in children’s daily routines during this time [23-27]
It is known that pubertal development is largely dependent on genetic factors, but the role of environmental factors is also well known [17] and it is this latter point that has probably conditioned the increased incidence of AP and PP during confinement.
The virulence and lethality of coronavirus disease 2019, caused governments to take very restrictive and limiting decisions for today’s society that led to a lot of changes in the daily routines of all people, including children. Specifically, Spain entered into a state of alarm on 14 March 2020, establishing a few days later the confinement of the population which, among other things, prevented children from leaving the house, i.e. they could not go to school, to sports activities, or to the park to play. This meant a halt in children’s lives, leading to a decrease in physical exercise and changes in eating schedules and probably in diet due to spending more time at home and more time in front of digital screens [21-23,24-26,28].
All this increase in sedentary behaviors can lead to weight gain and an increase in body mass index. This, in turn, may lead to an increase in fat mass, which could be an inducer of puberty, favoring the development of precocious puberty in girls [5,26,29-32]. In this sample, it was observed that the weight gain of more than 3 kg in the last 6 months prior to the consultation for early telaquia was statistically significant; however, this data has its limitations, as it would have been correct to take into account BMI as well as growth rate and body composition. Therefore these data suggest that there was an increase in weight in the girls who consulted, but it would not be correct to conclude that this increase was due to an increase in body fat. Although it is true, and according to other studies, confinement favored sedentary lifestyles and sedentary lifestyles favor an increase in body fat which in turn favors the onset of puberty [11,15,21,22,28].
These results seem to suggest a correlation between environmental factors and the early onset and progression of puberty.
On the other hand, one could believe that the anxiety generated by this pandemic on a social level may have caused parents to analyze their children’s health more closely because of the risk of illness and to be more aware of physical changes.
The girls have consulted for early telarche at a younger age and the number of consultations has been higher [11,19,21,22,33]. We see this reflected in our study, where we observed a clear increase in the number of consultations for suspected precocious or precocious puberty during confinement.
Therefore, our study may hypothesize that a sedentary lifestyle that favors screen use, decreased physical exercise, increased intake, and dietary changes may account for the timing and pace of pubertal development [11,34-45].
Perhaps if healthier lifestyle habits had been adopted during confinement, we would not have seen an increase in pubertal disorders during the pandemic.
Nevertheless, it would be advisable for parents to pay attention to pubertal changes in their children’s bodies and to consult if any abnormalities are suspected.
On the other hand, we must not forget that this study has several limitations, in addition to the one already discussed in the previous paragraphs on the weight gain variable, another one would be the small sample size.
Conclusion
In conclusion, despite its limitations, this study shows some of the collateral effects that confinement may have had and that could have gone unnoticed initially. Our data suggest a significant increase in the incidence of PE consultations for precocious telarche as well as new cases of both precocious and early puberty in girls in Alava during confinement due to the COVID-19 pandemic, and the increase in both pathologies has been similar.
Nevertheless, we believe that further multi-center research involving all the variables that may be involved in early pubertal development is needed to confirm this phenomenon on a larger scale and to establish the causal relationship with specific pathogenic factors.
- Brito VN, Latronico AC. Puberty: when is it normal? Arch Endocrinol Metab. 2015 Apr;59(2):93-4. doi: 10.1590/2359-3997000000018. Epub 2015 Apr 1. PMID: 25993668.
- Brito VN, Spinola-Castro AM, Kochi C, Kopacek C, Silva PC, Guerra-Júnior G. Central precocious puberty: revisiting the diagnosis and therapeutic management. Arch Endocrinol Metab. 2016 Apr;60(2):163-72. doi: 10.1590/2359-3997000000144. Erratum in: Arch Endocrinol Metab. 2016 Aug;60(4):407. PMID: 27191050.
- Reinehr T, Roth CL. Is there a causal relationship between obesity and puberty? Lancet Child Adolesc Health. 2019 Jan;3(1):44-54. doi: 10.1016/S2352-4642(18)30306-7. Epub 2018 Nov 14. PMID: 30446301.
- Kim YJ, Kwon A, Jung MK, Kim KE, Suh J, Chae HW, Kim DH, Ha S, Seo GH, Kim HS. Incidence and Prevalence of Central Precocious Puberty in Korea: An Epidemiologic Study Based on a National Database. J Pediatr. 2019 May;208:221-228. doi: 10.1016/j.jpeds.2018.12.022. Epub 2019 Mar 8. PMID: 30857777.
- Bräuner EV, Busch AS, Eckert-Lind C, Koch T, Hickey M, Juul A. Trends in the Incidence of Central Precocious Puberty and Normal Variant Puberty Among Children in Denmark, 1998 to 2017. JAMA Netw Open. 2020 Oct 1;3(10):e2015665. doi: 10.1001/jamanetworkopen.2020.15665. PMID: 33044548; PMCID: PMC7550972.
- Eckert-Lind C, Busch AS, Petersen JH, Biro FM, Butler G, Bräuner EV, Juul A. Worldwide Secular Trends in Age at Pubertal Onset Assessed by Breast Development Among Girls: A Systematic Review and Meta-analysis. JAMA Pediatr. 2020 Apr 1;174(4):e195881. doi: 10.1001/jamapediatrics.2019.5881. Epub 2020 Apr 6. PMID: 32040143; PMCID: PMC7042934.
- Feibelmann TC, Silva AP, Resende DC, Resende EA, Scatena LM, Borges Mde F. Puberty in a sample of Brazilian schoolgirls: timing and anthropometric characteristics. Arch Endocrinol Metab. 2015 Apr;59(2):105-11. doi: 10.1590/2359-3997000000021. Epub 2015 Apr 1. PMID: 25993671.
- Abreu AP, Macedo DB, Brito VN, Kaiser UB, Latronico AC. A new pathway in the control of the initiation of puberty: the MKRN3 gene. J Mol Endocrinol. 2015 Jun;54(3):R131-9. doi: 10.1530/JME-14-0315. PMID: 25957321; PMCID: PMC4573396.
- Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020 Feb 15;395(10223):470-473. doi: 10.1016/S0140-6736(20)30185-9. Epub 2020 Jan 24. Erratum in: Lancet. 2020 Jan 29;: PMID: 31986257; PMCID: PMC7135038.
- Martins WP, Nastri CO. Ultrasonographic measurement of ovarian volume in the diagnosis of central precocious puberty. Ultrasound Obstet Gynecol. 2009 Oct;34(4):484-5. doi: 10.1002/uog.7355. PMID: 19790095.
- Verzani M, Bizzarri C, Chioma L, Bottaro G, Pedicelli S, Cappa M. "Impact of COVID-19 pandemic lockdown on early onset of puberty: experience of an Italian tertiary center". Ital J Pediatr. 2021 Mar 5;47(1):52. doi: 10.1186/s13052-021-01015-6. PMID: 33673836; PMCID: PMC7935003.
- Bradley SH, Lawrence N, Steele C, Mohamed Z. Precocious Puberty. BMJ. 2020; 368: l6597.
- Dorn LD, Susman EJ, Nottelmann ED, Chrousos GP. Perceptions of Puberty: Adolescent and Parent Ratings of Pubertal Stage. Pediatric Res. 1987; 21: 173.
- Stagi S, De Masi S, Bencini E, Losi S, Paci S, Parpagnoli M, Ricci F, Ciofi D, Azzari C. Increased incidence of precocious and accelerated puberty in females during and after the Italian lockdown for the coronavirus 2019 (COVID-19) pandemic. Ital J Pediatr. 2020 Nov 4;46(1):165. doi: 10.1186/s13052-020-00931-3. PMID: 33148304; PMCID: PMC7609833.
- Street ME, Sartori C, Catellani C, Righi B. Precocious Puberty and Covid-19 Into Perspective: Potential Increased Frequency, Possible Causes, and a Potential Emergency to Be Addressed. Front Pediatr. 2021 Sep 20;9:734899. doi: 10.3389/fped.2021.734899. PMID: 34616700; PMCID: PMC8488256.
- Kang MJ, Oh YJ, Shim YS, Baek JW, Yang S, Hwang IT. The usefulness of circulating levels of leptin, kisspeptin, and neurokinin B in obese girls with precocious puberty. Gynecol Endocrinol. 2018 Jul;34(7):627-630. doi: 10.1080/09513590.2017.1423467. Epub 2018 Jan 5. PMID: 29303010.
- Fudvoye J, Lopez-Rodriguez D, Franssen D, Parent AS. Endocrine disrupters and possible contribution to pubertal changes. Best Pract Res Clin Endocrinol Metab. 2019 Jun;33(3):101300. doi: 10.1016/j.beem.2019.101300. Epub 2019 Jul 27. PMID: 31401055.
- Nagu P, Parashar A, Behl T, Mehta V. CNS implications of COVID-19: a comprehensive review. Rev Neurosci. 2020 Dec 7;32(2):219-234. doi: 10.1515/revneuro-2020-0070. PMID: 33550782.
- Stagi S, De Masi S, Bencini E, Losi S, Paci S, Parpagnoli M, Ricci F, Ciofi D, Azzari C. Increased incidence of precocious and accelerated puberty in females during and after the Italian lockdown for the coronavirus 2019 (COVID-19) pandemic. Ital J Pediatr. 2020 Nov 4;46(1):165. doi: 10.1186/s13052-020-00931-3. PMID: 33148304; PMCID: PMC7609833.
- Chen Y, Chen J, Tang Y, Zhang Q, Wang Y, Li Q, Li X, Weng Z, Huang J, Wang X, Liu S. Difference of Precocious Puberty Between Before and During the COVID-19 Pandemic: A Cross-Sectional Study Among Shanghai School-Aged Girls. Front Endocrinol (Lausanne). 2022 Mar 21;13:839895. doi: 10.3389/fendo.2022.839895. PMID: 35392135; PMCID: PMC8979840.
- Maria Gabriela Di B. Precocious puberty in girls: potential impact of pandemic and COVID-19 confinement. Evidence, update in outpatient practice. 2021:
- Ariza Jimenez AB. Probable impact of COVID-19 on referrals to paediatric endocrinology: increased incidence of precocious puberty in a tertiary hospital. Endocrinology, Diabetes and Nutrition. 2021; 69: 542-544.
- Huber BC, Steffen J, Schlichtiger J, Brunner S. Altered nutrition behavior during COVID-19 pandemic lockdown in young adults. Eur J Nutr. 2021 Aug;60(5):2593-2602. doi: 10.1007/s00394-020-02435-6. Epub 2020 Dec 1. PMID: 33258996; PMCID: PMC7705857.
- Teixeira MT, Vitorino RS, da Silva JH, Raposo LM, Aquino LA, Ribas SA. Eating habits of children and adolescents during the COVID-19 pandemic: The impact of social isolation. J Hum Nutr Diet. 2021 Aug;34(4):670-678. doi: 10.1111/jhn.12901. Epub 2021 Apr 26. PMID: 33811690; PMCID: PMC8251498.
- Füzéki E, Schröder J, Carraro N, Merlo L, Reer R, Groneberg DA, Banzer W. Physical Activity during the First COVID-19-Related Lockdown in Italy. Int J Environ Res Public Health. 2021 Mar 3;18(5):2511. doi: 10.3390/ijerph18052511. PMID: 33802549; PMCID: PMC7967499.
- Chioma L, Bizzarri C, Verzani M, Fava D, Salerno M, Capalbo D, Guzzetti C, Penta L, Di Luigi L, di Iorgi N, Maghnie M, Loche S, Cappa M. Sedentary lifestyle and precocious puberty in girls during the COVID-19 pandemic: an Italian experience. Endocr Connect. 2022 Feb 14;11(2):e210650. doi: 10.1530/EC-21-0650. PMID: 35029543; PMCID: PMC8859940.
- Li W, Liu Q, Deng X, Chen Y, Liu S, Story M. Association between Obesity and Puberty Timing: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health. 2017 Oct 24;14(10):1266. doi: 10.3390/ijerph14101266. PMID: 29064384; PMCID: PMC5664767.
- Gallè F, Sabella EA, Ferracuti S, De Giglio O, Caggiano G, Protano C, Valeriani F, Parisi EA, Valerio G, Liguori G, Montagna MT, Romano Spica V, Da Molin G, Orsi GB, Napoli C. Sedentary Behaviors and Physical Activity of Italian Undergraduate Students during Lockdown at the Time of CoViD-19 Pandemic. Int J Environ Res Public Health. 2020 Aug 25;17(17):6171. doi: 10.3390/ijerph17176171. PMID: 32854414; PMCID: PMC7504707.
- Yackobovitch-Gavan M, Fisch Shvalb N, Bhutta ZA. Malnutrition and Catch-Up Growth during Childhood and Puperty. In World Review of Nutrition and Dietetics; Karger: Basel, Switzerland, 2019; 120.
- Sedlak P, Pařízková J, Samešová D, Musálek M, Dvořáková H, Novák J. Secular Changes in Body Build and Body Composition in Czech Preschool Children in the Context of Latent Obesity. Children (Basel). 2020 Dec 31;8(1):18. doi: 10.3390/children8010018. PMID: 33396305; PMCID: PMC7823761.
- Wagner IV, Sabin MA, Pfäffle RW, Hiemisch A, Sergeyev E, Körner A, Kiess W. Effects of obesity on human sexual development. Nat Rev Endocrinol. 2012 Jan 31;8(4):246-54. doi: 10.1038/nrendo.2011.241. PMID: 22290357.
- Jasik CB, Lustig RH. Adolescent obesity and puberty: the "perfect storm". Ann N Y Acad Sci. 2008;1135:265-79. doi: 10.1196/annals.1429.009. PMID: 18574233.
- Papadimitriou A, Papadimitriou DT. Endocrine-Disrupting Chemicals and Early Puberty in Girls. Children (Basel). 2021 Jun 10;8(6):492. doi: 10.3390/children8060492. PMID: 34200537; PMCID: PMC8226958.
- Karpati AM, Rubin CH, Kieszak SM, Marcus M, Troiano RP. Stature and pubertal stage assessment in American boys: the 1988-1994 Third National Health and Nutrition Examination Survey. J Adolesc Health. 2002 Mar;30(3):205-12. doi: 10.1016/s1054-139x(01)00320-2. PMID: 11869928.
- Khan L. Puberty: Onset and Progression. Pediatr Ann. 2019 Apr 1;48(4):e141-e145. doi: 10.3928/19382359-20190322-01. PMID: 30986314.
- Durand A, Bashamboo A, McElreavey K, Brauner R. Familial early puberty: presentation and inheritance pattern in 139 families. BMC Endocr Disord. 2016 Sep 13;16(1):50. doi: 10.1186/s12902-016-0130-x. PMID: 27624871; PMCID: PMC5022170.
- Sultan C, Gaspari L, Maimoun L, Kalfa N, Paris F. Disorders of puberty. Best Pract Res Clin Obstet Gynaecol. 2018 Apr;48:62-89. doi: 10.1016/j.bpobgyn.2017.11.004. Epub 2017 Nov 14. PMID: 29422239.
- Kaplowitz P, Bloch C; Section on Endocrinology, American Academy of Pediatrics. Evaluation and Referral of Children With Signs of Early Puberty. Pediatrics. 2016 Jan;137(1). doi: 10.1542/peds.2015-3732. Epub 2015 Dec 14. PMID: 26668298.
- Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020 Mar 26;382(13):1199-1207. doi: 10.1056/NEJMoa2001316. Epub 2020 Jan 29. PMID: 31995857; PMCID: PMC7121484.
- WHO Multicentre Growth Reference Study Group; De Onis, M. WHO Child Growth Standards Based on Length/Height, Weight and Age. Acta Paediatr. 2006; 95: 76-85.
- Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969 Jun;44(235):291-303. doi: 10.1136/adc.44.235.291. PMID: 5785179; PMCID: PMC2020314.
- Reynolds E. Radiographic Atlas of Skeletal Development of the Hand and Wrist Greulich WW, Pyle, SI, Eds.; Stanford University Press: Redwood City, CA, USA, 1950; 8: 518-520.
- El-Eshmawy MM, Abdel Aal IA, El Hawary AK. Association of ghrelin and leptin with reproductive hormones in constitutional delay of growth and puberty. Reprod Biol Endocrinol. 2010 Dec 22;8:153. doi: 10.1186/1477-7827-8-153. PMID: 21176234; PMCID: PMC3022842.
- Chen C, Zhang Y, Sun W, Chen Y, Jiang Y, Song Y, Lin Q, Zhu L, Zhu Q, Wang X, Liu S, Jiang F. Investigating the relationship between precocious puberty and obesity: a cross-sectional study in Shanghai, China. BMJ Open. 2017 Apr 11;7(4):e014004. doi: 10.1136/bmjopen-2016-014004. Erratum in: BMJ Open. 2017 Aug 23;7(8):e014004corr1. PMID: 28400459; PMCID: PMC5566589.
- Naulé L, Maione L, Kaiser UB. Puberty, A Sensitive Window of Hypothalamic Development and Plasticity. Endocrinology. 2021 Jan 1;162(1):bqaa209. doi: 10.1210/endocr/bqaa209. PMID: 33175140; PMCID: PMC7733306.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
