International Journal of Spine Research
Body composition assessment in patients with spinal cord injury by bioimpedance
Mariana Buratti Mascarenhas1*, Nicolli S Scarabelli2, Cinthia Bittar2, Felipe R Mascarenhas2, Orcizo F Silvestre2 and Alberto Cliquet2
2Universidade Estadual de Campinas, Brazil
Cite this as
Mascarenhas MB, Scarabelli NS, Bittar C, Mascarenhas FR, Silvestre OF, et al. (2022) Body composition assessment in patients with spinal cord injury by bioimpedance. Int J Spine Res 4(1): 013-018. DOI: 10.17352/ijsr.000023Copyright License
© 2022 Mascarenhas MB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Background: Individuals with spinal cord injury develop alterations in the metabolism of carbohydrates and lipids, chronic inflammation, abnormal control of glycemia, as well as loss of lean mass, and increased adiposity, these being some risk factors for the development of diseases and decreased quality of life. This research aimed to investigate the body composition during a treatment protocol with NMES in a spinal cord injured population.
Methods: An initial bioimpedance evaluation using Biodynamics 310e was performed on 19 spinal cord injury patients. During the following 4 months, they received neuromuscular stimulation. The individuals ended the clinic once a week and performed NMES for 45 min, 20 min on quadriceps, and 15 min on peroneal nerve, on both legs with a frequency in the range of 18 to 25 Hz. A new bioimpedance test was performed after this period.
Results: For data analysis, a 5% significance level was considered. There were statistically significant gains in lean mass with p= 0.0001 and a decrease in bio-strength with p= 0.0457. There was a loss of body fat, but not significant.
Conclusion: Individuals with spinal cord injury showed measurable body composition changes during a four-month neuromuscular electrical stimulation treatment.
Introduction
Spinal Cord Injury (SCI) is defined as an injury to the spinal cord that causes, consequently, a disruption in the conduction of motor, sensory, and autonomic nervous system signals extending from the level of injury to below it [1,2]. It is estimated that the worldwide incidence of new cases of LM is around 40 to 80 new cases per million people, totaling around 250,000 to 500,000 new cases per year [2,3].
Among the consequences of irreversible motor and sensory impairment caused by SCI, these individuals have difficulty in moving and develop changes in carbohydrate and lipid metabolism, chronic inflammation, abnormal blood glucose control, as well as muscle atrophy, and increased adiposity, which are some risk factors for the development of Cardiovascular Disease (CVD) [4]. A study conducted in 2013 with 354 patients with SCI, showed a 2.72 times greater chance of people in this group having a CVD than those of the same age and sex, but without SCI, in addition, the prevalence of CVD was 17.1% among spinal cord injured against 4.9% among individuals without injury [5].
Individuals with SCI have more difficulties performing physical activities, causing muscle atrophy, increased adiposity, and reduced bone density, among other metabolic consequences already mentioned [4,6]. The practice of physical activity in individuals with SCI has shown to have a positive, important, and strong relationship to the quality of life of these individuals [7], besides improving several physical parameters such as wheelchair skills and an increase in resistance and muscle strength [8,9].
Body composition is known to be an important factor for the development of diseases such as type 2 diabetes, metabolic syndrome, cardiovascular disease, and other conditions secondary to the increase in body fat to the detriment of lean mass [10,11].
According to the American Heart Association (AHA), metabolic syndrome is defined by the presence of at least three of the following criteria: increased blood glucose, decreased HDL cholesterol, increased triglycerides, large abdominal circumferences (according to pre-established values), and hypertension.
Still on metabolic syndrome, in 2018 Gater et al. pointed out that the prevalence of metabolic syndrome in veterans with MS was 57.5%, while obesity alone affected 76% of this group [23]. According to this study, this occurs in this group mainly due to the decrease in lean mass and bone mineral density concomitantly with the increase in fat mass, such change in body composition of these individuals is justified mainly by decreased mobility resulting from the sequelae of the injury [12,13,14-18].
In order to evaluate the body composition evolution of individuals with SCI, as well as to follow up on whether dietary and physical exercise measures are having an effect on this parameter, it is necessary to have a valid and reliable tool to help monitor the body composition of these individuals. For this, some of the techniques available are abdominal circumference and fold thickness, dual-energy x-ray absorptiometry (DXA), magnetic resonance imaging (MRI), and bioelectrical impedance analysis (BIA) [12,13,19,20].
BIA uses the principle that body tissues have different resistances to the passage of electric current, it is known that in lean tissues the conduction of electric current is faster due to the greater amount of water and electrolytes present in these, so it has a lower resistance to electric current. Unlike fatty tissues, which in turn provide greater resistance to electric current conduction [21-23].
Bioimpedance history or other data on
One of the BIA body composition monitors is the Biodynamics model 310e. It emits a low-frequency current (80µA - 50 kHz) that travels throughout the body measuring the resistance imposed by the tissues. The variables that this device provides are water, lean mass, fat, and bioresorbent [13].
These changes in body composition can occur by leading individuals to a healthier situation through physical activity, physical therapy, and dietary re-education. But they can also happen unfavorably when there is a sedentary lifestyle and bad eating habits leading to comorbidities. These improvements in body composition through physical activity can be observed with a minimum of four months of training and with a frequency between one and three times a week [24].
In the search for an improvement in body composition, there is also neuromuscular electrical stimulation (NMES), which is a therapeutic resource used for the rehabilitation of individuals with SCI. It is a technique that has the function of stimulating the muscles through electrical impulses, in which the electrical currents are generated by a device and passed to the body through electrodes fixed on the skin. The use of NMES activates the musculoskeletal system producing muscle responses that promote various gains in sensory and motor skills leading to new adjustments in the organization and functionality of individuals. Successive training promotes optimization of intramuscular collagen distribution and also a gain in muscle tissue, movement, and neuroplasticity [25].
Understanding the physical limitation that individuals with SCI present, as well as highlighting the importance of body composition in these individuals due to their higher risk of developing CVD, this study proposed to investigate the change in body composition during a treatment protocol with NMES in a population of spinal cord injured individuals.
Materials and methods
This research was prospective and observational. The research was approved by the Ethics and Research Committee of the University. Ethics approval number: 4.834.349.
The research happened during the covid-19 pandemic. It was carried out in the outpatient clinic for spinal cord injuries where everyone does electrostimulation for clinical improvement and in times of a pandemic the laboratory was maintained so as not to disturb their clinical protocol, and consequently, we did not use a control group at this time so that no individual would stop receiving treatment. It started with forty individuals, but because of the pandemic, many had to move away.
Nineteen individuals with SCI were selected, 5 women and 14 men, aged between 18 and 75 years. Regarding quadriplegia and paraplegia, we worked with seven quadriplegics and twelve paraplegics individuals (Table 1).
The NMES protocol has the following characteristics: electrical stimulator - four channels - 25 Hz, monophasic - rectangular pulses with 300μs - the intensity of 70-150 V and a maximum of 300 V (1kΩ load).
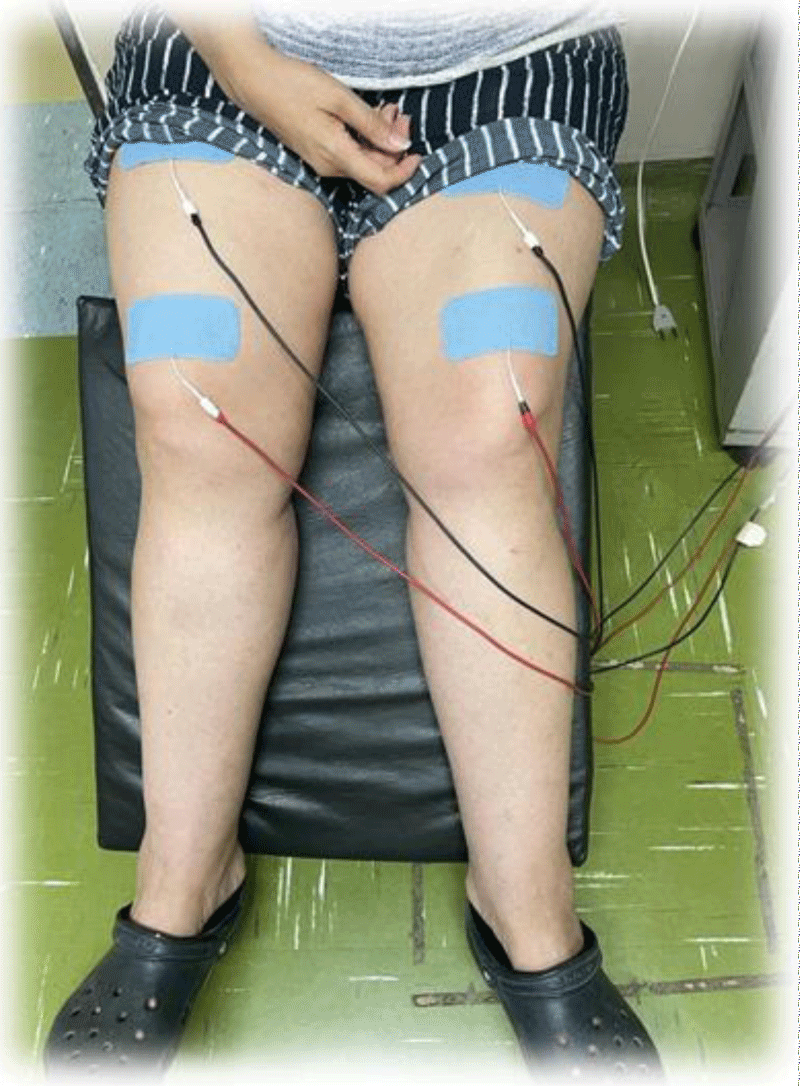
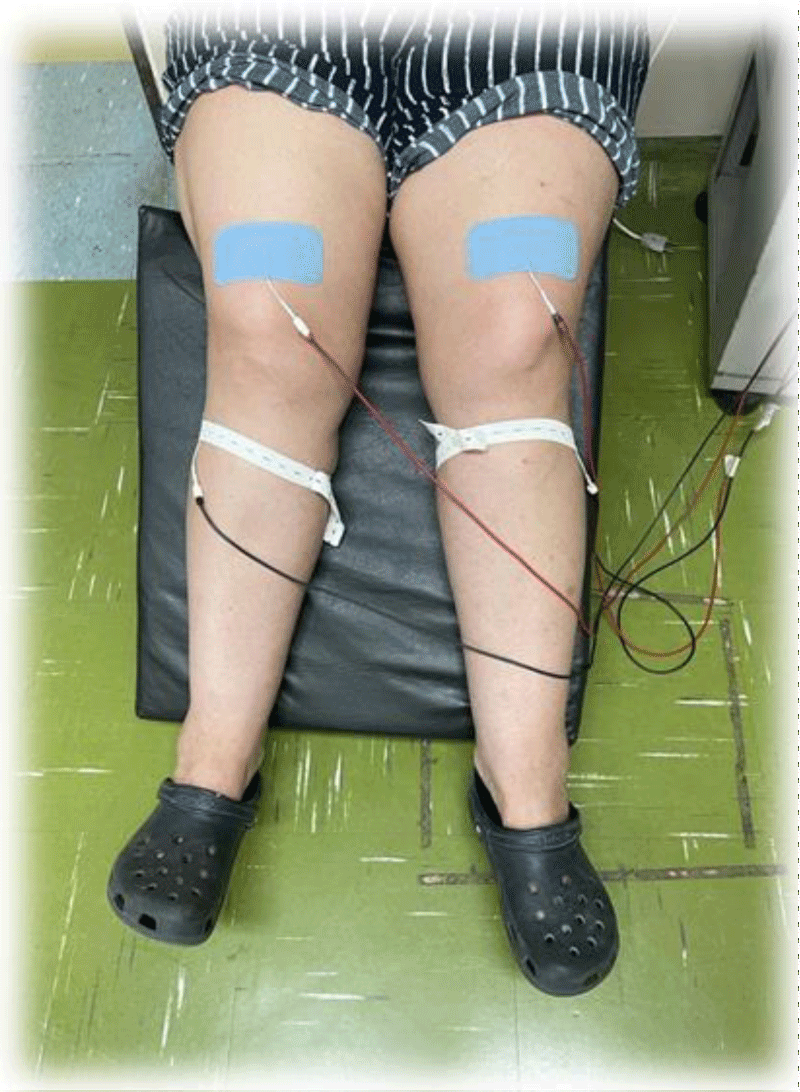
Valutrode brand self-adhesive surface electrodes were used to perform NMES. For the NMES protocol of the quadriceps femoris muscle (QDs), two adhesive surface electrodes measuring 5x9 cm were used on each thigh (quadriceps femoris muscle). For the NMES of the tibialis anterior (TA) muscle, a 5x9 cm self-adhesive surface electrode was used on the distal thigh (QDs) and a smaller circular diameter electrode, 3.0 cm, 60 positioned near the fibula head. All electrodes used were new, and each subject had his or her own electrodes.
The individuals ended the clinic once a week and performed NMES for 45 min, 20 min on quadriceps, and 15 min on peroneal nerve, on both legs with a frequency in the range of 18 to 25 Hz (Figures 1,2).
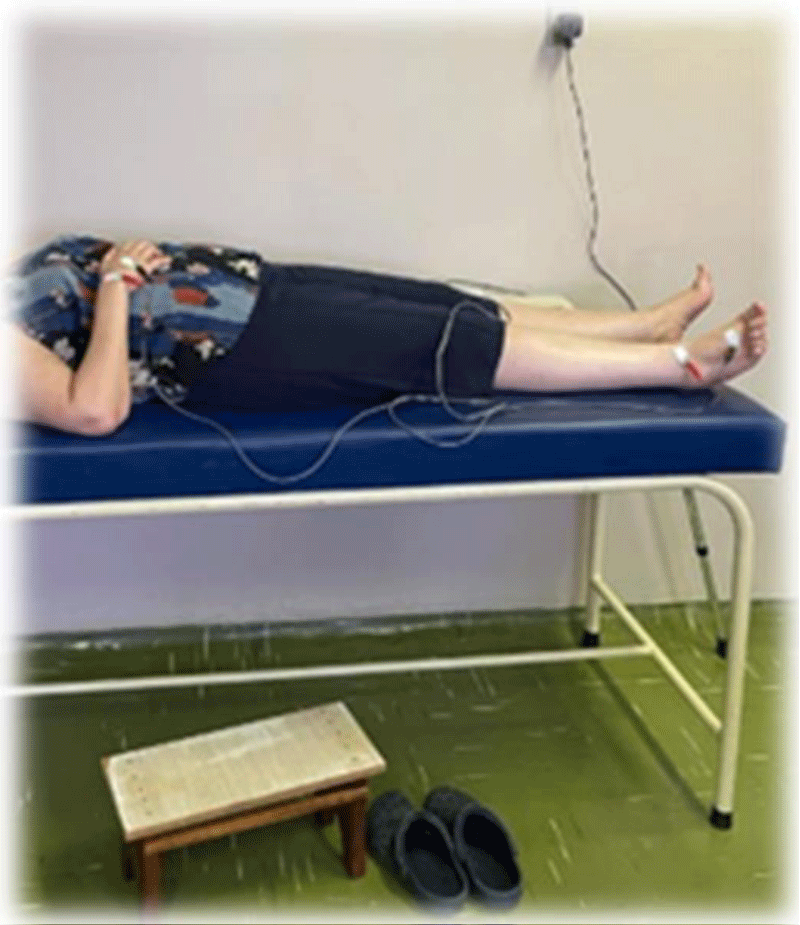
An initial bioimpedance evaluation was performed using the Biodynamics body composition monitor model 310e. It is a model tested and approved in the United States and Europe and sold on the internet on several websites.
For the exam, the electrodes were positioned on the right side of the subjects, at the base of the III metacarpal and between the styloid process of the radius and the ulna, at the base of the III metatarsal, and between the lateral and medial malleoli (Figure 3).
For the BIA, subjects avoided alcohol and caffeine consumption 24 hours before the test, did not perform intense physical activity 4 hours before the test, avoided heavy meals 4 hours before the test, and discontinued diuretic medication 24 hours before the test (recommendations in the device manual). We performed an identical final evaluation after four months and compared the data. Between collection and one and two patients followed the training routine and the diet guidelines proposed by the research.
Results
To describe the sample profile, we produced frequency tables of categorical variables with values of absolute frequency (n) and percentage (%) and descriptive measures (mean, standard deviation, minimum, median, and maximum) of quantitative variables. Comparison between initial and final measurements was assessed with paired Wilcoxon test. ANOVA for repeated measures was used to evaluate the group and measure the effects. The significance level adopted for the study was 5%.
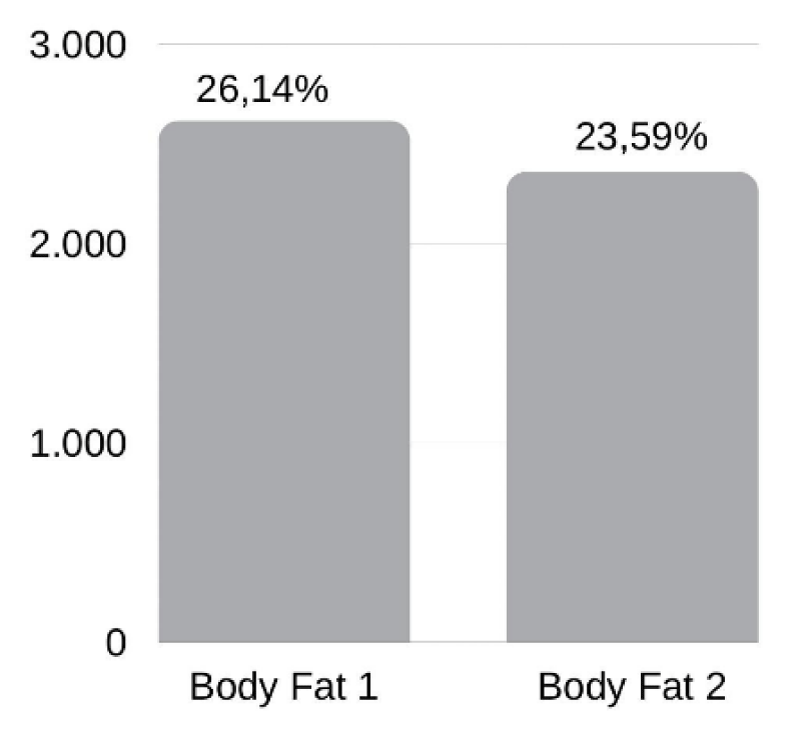
As shown in the graph below, there was a decrease in body fat, but it was not statistically significant, with p = 0.0506 (Graph 1).
We can see that the loss of body fat is well proportional between paraplegic and quadriplegic individuals. Of the 12 paraplegic individuals, 10 lost body fat, while of the 7 quadriplegic individuals, 4 lost fat.
As for the lean mass gain, we could observe an extremely statistically significant gain, with p = 0.0001 (Graph 2).
Of the three individuals who did not gain lean mass, we observed that one is paraplegic and two are quadriplegic.
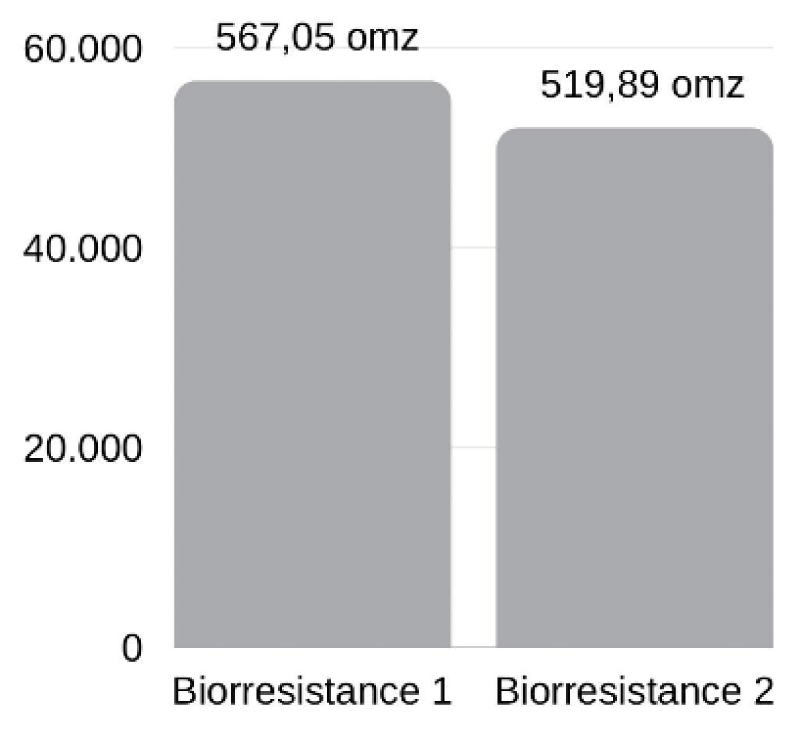
Regarding the decrease in bio resistance, the result was statistically significant, with p = 0.0457 (Graph 3).
Among the paraplegic individuals, only one did not have a reduction in bioresistance, while eleven did. Of the quadriplegics, there was almost a ratio between those who reduced and did not reduce bioresistance, with 4 reducing and 3 not.
Discussion
This research analyzed whether the interventions performed in favor of increasing the mobility of individuals with SCI impacted an increase in lean mass and decrease in body fat, thus decreasing morbidity secondary to spinal cord injury arising from increased fat mass and the development of obesity [20,18,26,27].
It was observed that in only four months of treatment the recruited individuals presented results regarding body composition. Among the 19 individuals, seven were quadriplegic, and twelve were paraplegic, and there was no statistically significant difference between these two groups for any of the parameters evaluated in this research.
There was a decrease in body fat, but since some individuals gained weight, the result on average was not significant, and even so it was not by much. These few individuals who gained body fat reported that they did not have healthy eating due to various emotional, social, and environmental issues and even because of the covid-19 pandemic.
Just as the literature shows that NMES has great benefits in gaining lean mass and muscle hypertrophy in individuals with LM who do it [28,29], this study evidenced the increase in lean mass. We associate that such gain happened due to the NMES and that it was not necessary to add weight or load to gain lean mass.
It is known that the NMES presents several benefits to the individual with SCI, such as improved quality of life through the improvement of physical functioning and independence [30], and it has been shown that those who participate in some rehabilitation programs have the better functional capacity and are more motivated [31,32].
In addition to NMES, individuals with SCI can gain lean mass with physical activity. A study conducted in 2016 with quadriplegic high-performance wheelchair rugby players showed that the practice of this sport promoted in this group an increase in lean mass and bone mineral content in the upper limbs, in addition to fat reduction throughout the body, supporting the practice of this activity to promote a better metabolic profile in patients with SCI [33].
The practice of physical activity promotes lean mass gain that, besides being excellent for systemic health, is of extreme importance for the quality of life of these individuals since it provides more independence for changes in decubitus and activities of daily living [7,8,34].
The practice of regular physical activity by individuals with SCI has also shown to be associated with improved left ventricular diastolic function in this group [35.36], it is known that people with SCI [37], bedridden, or with low mobility have a greater chance of developing such cardiac dysfunction and that this is associated with increased cardiovascular risk [38,39].
Furthermore, physical activity has shown to improve the carotid intima layer thickness in patients with SCI [40,41], such measurement becoming not only significantly lower than the one presented by sedentary individuals with SCI, but also with values comparable to those of individuals without injury [42]. Such data is relevant because it has been demonstrated in the literature that people with LM, even if they present low cardiovascular risk, have higher carotid intima layer values [43,44] and such measurement has been associated with cardiovascular risk [45-48].
It is known that in lean tissues the conduction of electric current is faster due to the higher amount of water and electrolytes present in these tissues, and therefore it has a lower resistance to the electric current. This is different from fatty tissues, which in turn provide greater resistance to electric current conduction [21,23]. Therefore, the decrease in bioresistance seen in this study corroborates the large increase in lean mass seen, even without the significant loss of fat, being a parameter indicative of improvement in the body composition of the individuals studied.
As for the comparison between individuals with paraplegia and tetraplegia, we observed that the paraplegic individuals obtained better results than the tetraplegic individuals in all parameters, as happened in a study conducted in 2016 [13]. This is probably due to the greater use that paraplegics have of their upper limbs and trunk, as well as the greater number of activities they are able to perform on a daily basis.
Conclusion
The individuals with LM showed changes in body composition with lean mass gain and decreased. For better results, clinical studies with a greater number of patients and longer treatment time and collections are necessary.
- Rupp R, Biering-Sørensen F, Burns SP, Graves DE, Guest J, Jones L, Read MS, Rodriguez GM, Schuld C, Tansey-Md KE, Walden K, Kirshblum S. International Standards for Neurological Classification of Spinal Cord Injury: Revised 2019. Top Spinal Cord Inj Rehabil. 2021 Spring;27(2):1-22. doi: 10.46292/sci2702-1. PMID: 34108832; PMCID: PMC8152171.
- Biering-Sørensen F, Kirshblum S. International perspectives on spinal cord injury care. Spinal Cord Medicine: Third Edition. 2018; 1007–1022.
- Perrouin-Verbe B, Lefevre C, Kieny P, Gross R, Reiss B, Le Fort M. Spinal cord injury: A multisystem physiological impairment/dysfunction. Rev Neurol (Paris). 2021; https://linkinghub.elsevier.com/retrieve/pii/S0035378721005087
- Bauman WA, Korsten MA, Radulovic M, Schilero GJ, Wecht JM, Spungen AM. 31st g. Heiner sell lectureship: secondary medical consequences of spinal cord injury. Top Spinal Cord Inj Rehabil. 2012;18(4):354-78. doi: 10.1310/sci1804-354. PMID: 23459498; PMCID: PMC3584784.
- Cragg JJ, Noonan VK, Dvorak M, Krassioukov A, Mancini GB, Borisoff JF. Spinal cord injury and type 2 diabetes: results from a population health survey. Neurology. 2013 Nov 19;81(21):1864-8. doi: 10.1212/01.wnl.0000436074.98534.6e. Epub 2013 Oct 23. PMID: 24153440; PMCID: PMC3821709.
- Hospital TW. Neurosurgery Focus Issue : – Traumatic Spinal Cord Injury Repair and Pathophysiology.Availablefrom:https://core.ac.uk/reader/81680040?utm_source=linkot 2017.
- Stevens SL, Caputo JL, Fuller DK, Morgan DW. Physical activity and quality of life in adults with spinal cord injury. J Spinal Cord Med. 2008;31(4):373-8. doi: 10.1080/10790268.2008.11760739. PMID: 18959354; PMCID: PMC2582427.
- Durán FS, Lugo L, Ramírez L, Eusse E. Effects of an exercise program on the rehabilitation of patients with spinal cord injury. Arch Phys Med Rehabil. 2001 Oct;82(10):1349-54. doi: 10.1053/apmr.2001.26066. PMID: 11588736.
- Jacobs PL, Nash MS. Exercise recommendations for individuals with spinal cord injury. Sports Med. 2004;34(11):727-51. doi: 10.2165/00007256-200434110-00003. PMID: 15456347.
- Meldrum DR, Morris MA, Gambone JC. Obesity pandemic: causes, consequences, and solutions—but do we have the will? Fertil Steril. 2017; 107(4):833–9. Available from: http://dx.doi.org/10.1016/j.fertnstert.2017.02.104
- Paim LR, Schreiber R, de Rossi G, Matos-Souza JR, Costa E Silva AA, Calegari DR, Cheng S, Marques FZ, Sposito AC, Gorla JI, Cliquet A Jr, Nadruz W Jr. Circulating microRNAs, Vascular Risk, and Physical Activity in Spinal Cord-Injured Subjects. J Neurotrauma. 2019 Mar 19;36(6):845-852. doi: 10.1089/neu.2018.5880. Epub 2018 Sep 27. PMID: 30122113.
- van der Scheer JW, Totosy de Zepetnek JO, Blauwet C, Brooke-Wavell K, Graham-Paulson T, Leonard AN, Webborn N, Goosey-Tolfrey VL. Assessment of body composition in spinal cord injury: A scoping review. PLoS One. 2021 May 7;16(5):e0251142. doi: 10.1371/journal.pone.0251142. PMID: 33961647; PMCID: PMC8104368.
- Azevedo ERFBM, Alonso KC, Cliquet A. Body composition assessment by bioelectrical impedance analysis and body mass index in individuals with chronic spinal cord injury. J Electr Bioimpedance. 2016; 7(1):2–5.
- McMillan DW, Maher JL, Jacobs KA, Nash MS, Gater DR Jr. Exercise Interventions Targeting Obesity in Persons With Spinal Cord Injury. Top Spinal Cord Inj Rehabil. 2021;27(1):109-120. doi: 10.46292/sci20-00058. PMID: 33814889; PMCID: PMC7983638.
- Raguindin PF, Bertolo A, Zeh RM, Fränkl G, Itodo OA, Capossela S. Clinical Medicine Body Composition According to Spinal Cord Injury Level: A Systematic Review and Meta-Analysis. J Clin Med. 2021;10. Available from: https://doi.org/10.3390/jcm10173911
- Castro MJ, Apple DF Jr, Rogers S, Dudley GA. Influence of complete spinal cord injury on skeletal muscle mechanics within the first 6 months of injury. Eur J Appl Physiol. 2000 Jan;81(1-2):128-31. doi: 10.1007/PL00013785. PMID: 10552277.
- Frey-Rindova P, de Bruin ED, Stüssi E, Dambacher MA, Dietz V. Bone mineral density in upper and lower extremities during 12 months after spinal cord injury measured by peripheral quantitative computed tomography. Spinal Cord. 2000 Jan;38(1):26-32. doi: 10.1038/sj.sc.3100905. PMID: 10762194.
- Fisher JA, McNelis MA, Gorgey AS, Dolbow DR, Goetz LL. Does Upper Extremity Training Influence Body Composition after Spinal Cord Injury? Aging Dis. 2015 Aug 1;6(4):271-81. doi: 10.14336/AD.2014.0912. PMID: 26236549; PMCID: PMC4509476.
- Cirnigliaro CM, La Fountaine MF, Emmons R, Kirshblum SC, Asselin P, Spungen AM, Bauman WA. Prediction of limb lean tissue mass from bioimpedance spectroscopy in persons with chronic spinal cord injury. J Spinal Cord Med. 2013 Sep;36(5):443-53. doi: 10.1179/2045772313Y.0000000108. PMID: 23941792; PMCID: PMC3739894.
- Desneves KJ, Panisset MG, Galea MP, Kiss N, Daly RM, Ward LC. Comparison of segmental lean tissue mass in individuals with spinal cord injury measured by dual energy X-ray absorptiometry and predicted by bioimpedance spectroscopy. Spinal Cord. 2021;59(7):730–7. http://dx.doi.org/10.1038/s41393-020-00568-3
- Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, Heitmann BL, Kent-Smith L, Melchior JC, Pirlich M, Scharfetter H, Schols AM, Pichard C; Composition of the ESPEN Working Group. Bioelectrical impedance analysis--part I: review of principles and methods. Clin Nutr. 2004 Oct;23(5):1226-43. doi: 10.1016/j.clnu.2004.06.004. PMID: 15380917.
- Britto EP De, Mesquita ET. Bioimpedância Elétrica Aplicada à Insuficiência Cardíaca. 2008; 21(3):178–83.
- Kamimura MA, Draibe SA, Sigulem DM, Cuppari L. Methods of body composition assessment in patients undergoing hemodialysis. Rev Nutr. 2004; 17(1):97–105.
- Schoenfeld BJ, Ogborn D, Krieger JW. Effects of Resistance Training Frequency on Measures of Muscle Hypertrophy: A Systematic Review and Meta-Analysis. Sports Med. 2016 Nov;46(11):1689-1697. doi: 10.1007/s40279-016-0543-8. PMID: 27102172.
- Varoto R, Cliquet A Jr. Experiencing Functional Electrical Stimulation Roots on Education, and Clinical Developments in Paraplegia and Tetraplegia With Technological Innovation. Artif Organs. 2015 Oct;39(10):E187-201. doi: 10.1111/aor.12620. Epub 2015 Oct 6. PMID: 26437800.
- Wahman K, Nash MS, Lewis JE, Seiger A, Levi R. Cardiovascular disease risk and the need for prevention after paraplegia determined by conventional multifactorial risk models: the Stockholm spinal cord injury study. J Rehabil Med. 2011 Feb;43(3):237-42. doi: 10.2340/16501977-0658. PMID: 21305240.
- Fitzgerald SG, Kelleher AR. Mobility Challenges in Individuals with a Spinal Cord Injury with Increased Body Weight. Top Spinal Cord Inj Rehabil. 2007; 12(4):54–63. Available from: www.thomasland.com
- Chandrasekaran S, Davis J, Bersch I, Goldberg G, Gorgey AS. Electrical stimulation and denervated muscles after spinal cord injury. Neural Regeneration Research. 2020; 1397-407. Available from: www.nrronline.org
- Gorgey AS, Dolbow DR, Dolbow JD, Khalil RK, Gater DR. The effects of electrical stimulation on body composition and metabolic profile after spinal cord injury--Part II. J Spinal Cord Med. 2015 Jan;38(1):23-37. doi: 10.1179/2045772314Y.0000000244. Epub 2014 Jul 8. PMID: 25001669; PMCID: PMC4293531.
- Teeter L, Gassaway J, Taylor S, LaBarbera J, McDowell S, Backus D, Zanca JM, Natale A, Cabrera J, Smout RJ, Kreider SE, Whiteneck G. Relationship of physical therapy inpatient rehabilitation interventions and patient characteristics to outcomes following spinal cord injury: the SCIRehab project. J Spinal Cord Med. 2012 Nov;35(6):503-26. doi: 10.1179/2045772312Y.0000000058. PMID: 23318034; PMCID: PMC3522894.
- Lindberg J, Kreuter M, Taft C, Person L-O. Patient participation in care and rehabilitation from the perspective of patients with spinal cord injury. Spinal Cord. 2013; 51:834-7. www.nature.com/sc
- AlHuthaifi F, Krzak J, Hanke T, Vogel LC. Predictors of functional outcomes in adults with traumatic spinal cord injury following inpatient rehabilitation: A systematic review. J Spinal Cord Med. 2017 May;40(3):282-294. doi: 10.1080/10790268.2016.1238184. Epub 2016 Nov 17. PMID: 27852160; PMCID: PMC5472016.
- Gorla JI, Costae Silva ADA, Borges M, Tanhoffer RA, Godoy PS, Calegari DR. Impact of wheelchair rugby on body composition of subjects with tetraplegia: A pilot study. Arch Phys Med Rehabil. 2016; 97(1):92–6. >http://dx.doi.org/10.1016/j.apmr.2015.09.007
- Sandrow-Feinberg HR, Houlé JD. Exercise after spinal cord injury as an agent for neuroprotection, regeneration and rehabilitation. Brain Res. 2015 Sep 4;1619:12-21. doi: 10.1016/j.brainres.2015.03.052. Epub 2015 Apr 9. PMID: 25866284; PMCID: PMC4540698.
- DE Rossi G, Matos-Souza JR, Costa E Silva AD, Campos LF, Santos LG, Azevedo ER, Alonso KC, Paim LR, Schreiber R, Gorla JI, Cliquet A Jr, Nadruz W Jr. Physical activity and improved diastolic function in spinal cord-injured subjects. Med Sci Sports Exerc. 2014;46(5):887-92. doi: 10.1249/MSS.0000000000000187. PMID: 24126969.
- Schreiber R, Paim LR, de Rossi G, Matos-Souza JR, Costa E Silva Ade A, Souza CM, Borges M, Azevedo ER, Alonso KC, Gorla JI, Cliquet A Jr, Nadruz W Jr. Matrix metalloproteinases and left ventricular function and structure in spinal cord injured subjects. Clin Chim Acta. 2014 Nov 1;437:136-40. doi: 10.1016/j.cca.2014.07.018. Epub 2014 Jul 23. PMID: 25064800.
- Matos-Souza JR, Pithon KR, Oliveira R, Téo FH, Blotta M, Cliquet A. Altered left ventricular diastolic function in subjects with spinal cord injury. Spinal Cord. 2011; 49:65–9. www.nature.com/sc
- Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbély A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007 Oct;28(20):2539-50. doi: 10.1093/eurheartj/ehm037. Epub 2007 Apr 11. PMID: 17428822.
- Perhonen MA, Zuckerman JH, Levine BD. Deterioration of left ventricular chamber performance after bed rest : "cardiovascular deconditioning" or hypovolemia? Circulation. 2001 Apr 10;103(14):1851-7. doi: 10.1161/01.cir.103.14.1851. PMID: 11294802.
- Matos-Souza JR, de Rossi G, Costa E Silva AA, Azevedo ER, Pithon KR, Schreiber R, Sposito AC, Gorla JI, Cliquet A Jr, Nadruz W Jr. Impact of Adapted Sports Activities on the Progression of Carotid Atherosclerosis in Subjects With Spinal Cord Injury. Arch Phys Med Rehabil. 2016 Jun;97(6):1034-7. doi: 10.1016/j.apmr.2015.11.002. Epub 2015 Nov 25. PMID: 26625710.
- Paim LR, Schreiber R, Matos-Souza JR, Silva AA, Campos LF, Azevedo ER, Alonso K, de Rossi G, Etchebehere M, Gorla JI, Cliquet A Jr, Nadruz W Jr. Oxidized low-density lipoprotein, matrix-metalloproteinase-8 and carotid atherosclerosis in spinal cord injured subjects. Atherosclerosis. 2013 Dec;231(2):341-5. doi: 10.1016/j.atherosclerosis.2013.10.005. Epub 2013 Oct 15. PMID: 24267248.
- Matos-Souza JR, Silva AA, Campos LF, Goulart D, Schreiber R, De Rossi G. Physical activity is associated with improved subclinical atherosclerosis in spinal cord injury subjects independent of variation in traditional risk factors. Int J Cardiol. 2013; 167(2):592–3. http://dx.doi.org/10.1016/j.ijcard.2012.09.222
- Matos-Souza JR, Pithon KR, Ozahata TM, Gemignani T, Cliquet A Jr, Nadruz W Jr. Carotid intima-media thickness is increased in patients with spinal cord injury independent of traditional cardiovascular risk factors. Atherosclerosis. 2009 Jan;202(1):29-31. doi: 10.1016/j.atherosclerosis.2008.04.013. Epub 2008 Apr 22. PMID: 18511054.
- Matos-Souza JR, Pithon KR, Ozahata TM, Oliveira RT, Téo FH, Blotta MH. Subclinical atherosclerosis is related to injury level but not to inflammatory parameters in spinal cord injury subjects. Spinal Cord. 2010;48:740–4. Available from: www.nature.com/sc
- Willeit P, Tschiderer L, Allara E, Reuber K, Seekircher L, Gao L. Carotid intima-media thickness progression as surrogate marker for cardiovascular risk: Meta-Analysis of 119 clinical trials involving 100 667 patients. Circulation. 2020; 621-42. Available from: http://ahajournals.org
- King JE. What is metabolic syndrome? Nursing. 2006 Jun;36(6):31. doi: 10.1097/00152193-200606000-00022. PMID: 16741405.
- Tune JD, Goodwill AG, Sassoon DJ, Mather KJ. Cardiovascular consequences of metabolic syndrome. Transl Res. 2017; 183:57–70. Available from: http://dx.doi.org/10.1016/j.trsl.2017.01.001
- Gater DR Jr, Farkas GJ, Berg AS, Castillo C. Prevalence of metabolic syndrome in veterans with spinal cord injury. J Spinal Cord Med. 2019 Jan;42(1):86-93. doi: 10.1080/10790268.2017.1423266. Epub 2018 Jan 11. PMID: 29323633; PMCID: PMC6340269.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
