International Journal of Spine Research
Occipital cervical fusion with a vascularized free fibular graft
Myron L Rolle1,2, Rosemary E Henn1,2, David W Low1,2 and Phillip B Storm1,2*
2Department of Neurosurgery, Hospital of the University of Pennsylvania, Philadelphia, PA, USA
Cite this as
Rolle ML, Henn RE, Low DW, Storm PB (2019) Occipital cervical fusion with a vascularized free fibular graft. Int J Spine Res 1(1): 023-025. DOI: 10.17352/ijsr.000005Obtaining a solid bony fusion after an extensive skull base and upper cervical spine resection in a patient who has previously received radiation therapy is extremely difficult and usually results in a pseudoarthrosis. We report a technique that uses a free fibular graft with an arteriovenous loop for a posterior occipital cervical fusion.
A retrospective review was performed on a case of a 13-year-old boy who had a malignant nerve sheath tumor (MPNST) of the occipital skull base and C1. He underwent an aggressive resection of his tumor, placement of occiput to C4 rods and screws, and placement of a microvascular fibular flap from occiput to C4.
The microvascular anastomosis was successfully performed using an arteriovenous loop of the saphenous vein graft to the anterior neck vessels. The length of the fibula harvested was 24cm and the ischemic time was 30 minutes.
The free fibula flap is effective and has low morbidity making it an excellent option for obtaining a bony fusion in patients who have been previously irradiated. The arteriovenous saphenous loop facilitates the microvascular anastomosis in a posterior approach where there are few suitable vessels.
Introduction
Treatment of residual or recurrent spinal neoplasms is difficult because many of the patients have undergone radiation therapy. These tumors often require a gross total resection to offer the patient any hope for a cure. In order to completely remove these lesions, the surgeon must perform a radical resection that destabilizes the spine. Standard techniques for arthrodesis of the occipital cervical junction involve inserting rods and screws into the spine and occiput and using autologous or allograft bone to achieve a bony fusion. These techniques are extremely successful in trauma and tumor patients who have not received radiation therapy [1,2]; However, they often result in a pseudoarthrosis when the patient has been irradiated.
Vascularized bone grafts offer the opportunity for a solid fusion in the previously irradiated spine because cell viability is maintained in the graft. The living bone graft is able to grow into the spine because the primary bone healing occurs at the fusion site rather than the creeping substitution into the scaffold of necrotic nonvascularized bone graft. Structural viability and strength is maintained throughout the healing process [3-7]. Osseous union rates with vascularized bone grafts have been reported as high as 82-90 percent after tumor resection [8-10].
Vascularized free fibula grafts have been described with good results from an anterior approach [11-17]. Using these grafts in a posterior location is less well described and often adds the difficulty of finding adequate donor vessels [18]. In this case we used a vascularized fibula bone graft with an arteriovenous reverse saphenous vein loop.
Case Report
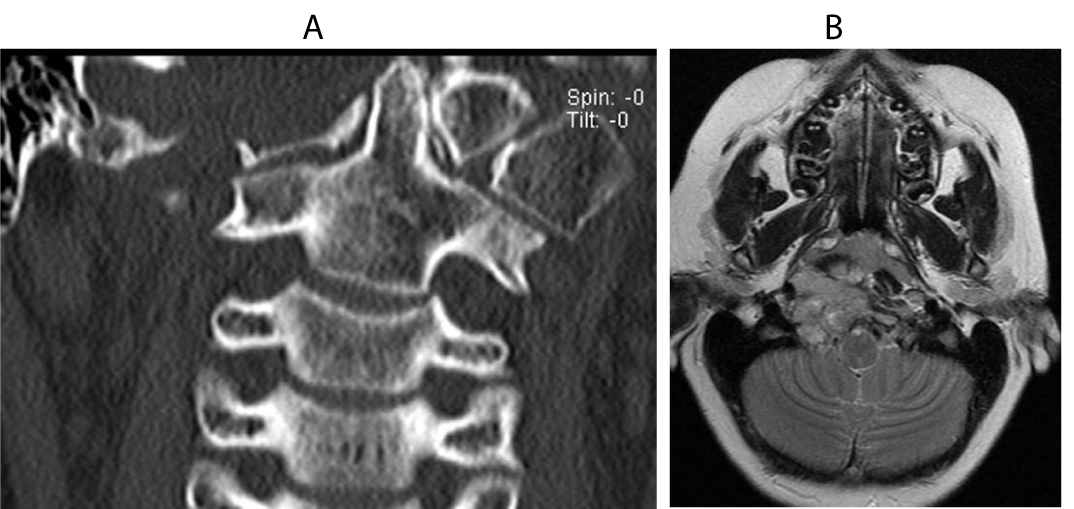
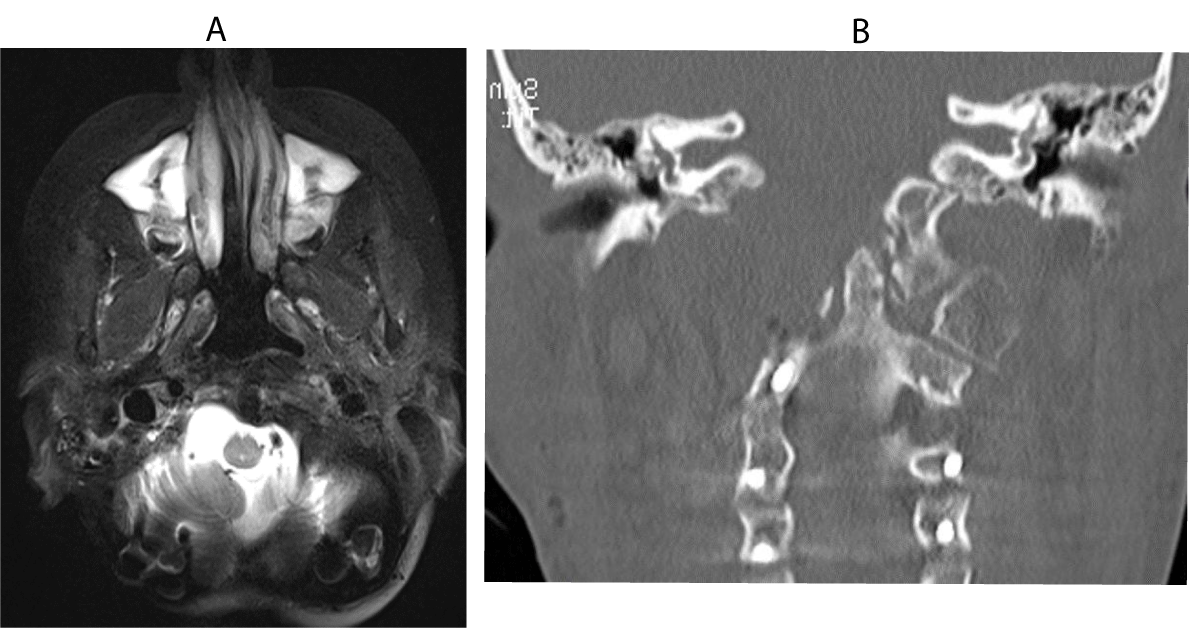
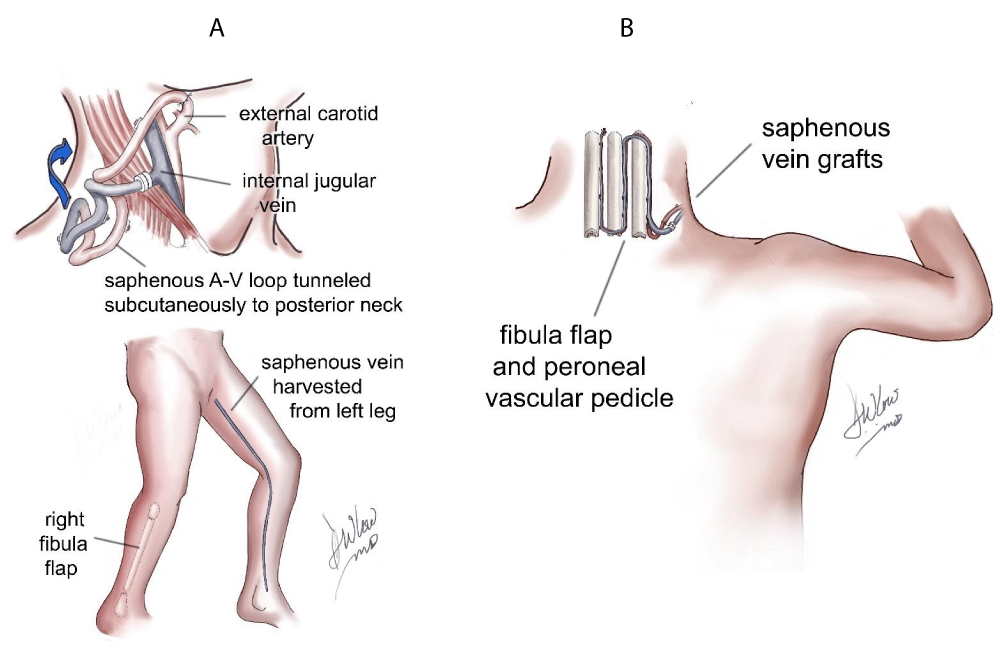
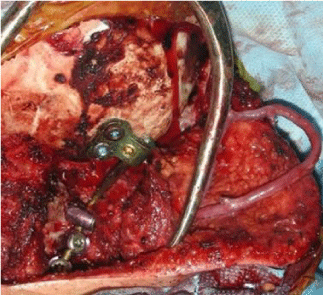
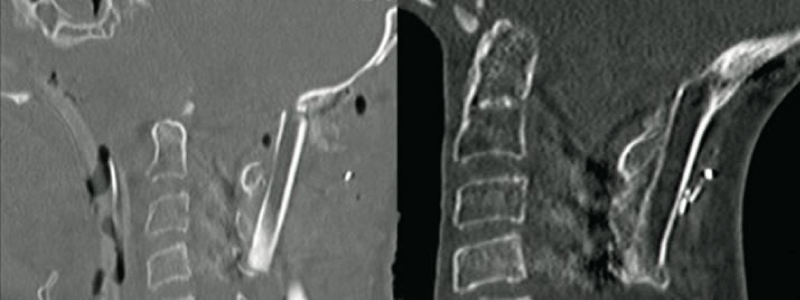
A 13-year-old boy with a MPNST of the skull base and upper cervical spine presented to our institution with residual disease after a needle biopsy and treatment with chemotherapy and radiation therapy (Figure 1). The decision was made to attempt a radical resection to make him disease free prior to any further oncological therapy. He was taken to the operating room for a far lateral approach and a C1 laminectomy for removal of the involved lateral mass of C1 and the involved occipital condyle with neuromonitoring. The right vertebral artery was completely identified from C2 to its intradural entry and cranial nerves 9-11 were isolated at the right jugular foramen. Cranial nerve 12 was identified as the right condyle was removed. Negative margins were confirmed on frozen section except where the tumor was stripped from the petrous carotid artery. The patient was then placed in a halo vest and taken for an MRI prior to hardware placement. Postoperatively he had a right cranial nerve paresis that had completely resolved at his 1 month follow up visit. The postoperative MRI and CT showed a gross total resection (Figure 2), and he was taken back to the operating room the next day with plastic surgery for instrumentation and a free fibula graft. The patient was placed in the supine position and a saphenous vein graft was harvested from the left leg and anastomosed as an arteriovenous loop to a branch of the internal jugular vein and the external carotid artery. The loop was tunneled subcutaneously to the posterior neck and used for the anastomosis to the free fibula pedicle, which was harvested from the right leg (Figure 3). The fibula flap was 24cm in length. The fibula was freed and the sagittal saw was used to cut the fibula proximally (just below the common peroneal nerve) and distally (approximately 2cm above the ankle to allow muscle insertion), but the final artery and vein were not divided. The vein and artery were left intact to keep the fibula viable while the patient was repositioned and the hardware was inserted. The incisions in the legs and the neck were closed and the patient was flipped to the prone position. The previous posterior incision was opened and the hardware was inserted from the occiput to C4. Once the rods and screws were placed the plastic surgery team reentered the case, identified the reverse saphenous fistula, pulled it into the field (Figure 4), and reopened the right leg incision and removed the fibula by ligating the last artery and vein. Two osteotomies were made in the fibula to form 3 struts for stabilization and bony fusion of the occipital cervical junction. The anastomosis was performed between the reverse saphenous graft and the fibula without difficulty, and the total ischemia time was approximately 30 minutes. Prior to positioning the fibula on the spine, the periosteum of the lateral fibula was elevated and the underlying cortex was removed with a burr. The patient did well postoperatively. He remained in the halo vest for 3 weeks and CT scans were performed on postoperative day 1 and then again 6 months after surgery (Figure 5A,B). There were no signs or symptoms of pseudoarthrosis; however, 15 months after surgery the occipital hardware eroded through the previously irradiated skin. He was taken back to the operating room for removal of the right-sided hardware. The fibula was viable and there was a solid bony fusion.
Discussion
Bony fusion after spine surgery can usually be achieved with instrumentation and nonvascularized autograft or allograft, especially in pediatric patients [1,2]. The exception is when the patient has previously received radiation therapy. The radiated spine does not provide the osteogenic factors needed to form a solid bony fusion because of poor osseous and soft tissue healing. Vascularized grafting allows stabilization and fusion in an irradiated spine. Our indications for vascularized fibula grafting to the spine include prior radiation or pseudoarthrosis secondary to an infection. All vascularized bone grafting is accompanied by instrumentation with rods and screws.
The reverse saphenous arteriovenous vascularized fibula flap works well because it provides a strong, long segment of bone that can easily be osteotomized at multiple levels to conform to the curvature of the spine [19]. The saphenous vein loop facilitates the microvascular anastomosis because this location lacks suitable recipient vessels. The osteotomies do not weaken the bone or risk devascularization as long as they are not too close together and do not jeopardize the vascular pedicle on the medial side of the bone. Postoperative monitoring of the pedicle is done by placing a skin suture over the saphenous pedicle in the subcutaneous tunnel so that the nursing staff can use the handheld Doppler to assess patency.
Because of the high rate of pseudoarthrosis with allograft and nonvascularized autograft, and the low morbidity of the free fibula flap, we now advocate using the vascularized fibula graft at the time of initial arthrodesis in patients with extensive resections and a history of prior radiation therapy.
Conclusions
The free fibula flap with a reverse saphenous graft provides a solid bony fusion for posterior spinal tumor resection after a history of radiation therapy. Because of the low morbidity of the free fibula flap we now advocate using the vascularized fibula onlay graft at the time of the tumor resection and instrumentation in patients who have previously received radiation.
- Baumann JA, Hardesty DA, Heuer GG, Storm JP (2011) Use of occipital bone graft in pediatric posterior cervical fusion: an alternative paramedian technique and review of the literature. J Neurological Surgery 7: 475-481. Link: http://bit.ly/2OJDURV
- Heuer GG, Hardesty DA, Bhowmick DA, Bailey R, Magge SN, et al. (2009) Treatment of pediatric atlantoaxial instability with traditional and modified Goel-Harms fusion constructs. Eur Spine J 18: 884-892. Link: http://bit.ly/2KXq90S
- Arata MA, Wood MB, Cooney WP III (1984) Revascularized segmental diaphyseal bone transfers in the canine: An analysis of viability. J Reconstr Microsurg 1:11-19. Link: http://bit.ly/33gnZz
- Cutting CB, McCarthy JG (1983) Comparison of residual osseous mass between vascularized and nonvascularized onlay bone transfers. Plast Reconstr Surg 72: 672-675. Link: http://bit.ly/37N70si
- Han CS, Wood MB, Bishop AT, Cooney WP III (1922) Vascularized bone transfer. J Bone Joint Surg Am 74: 1441-1449. Link: http://bit.ly/2DhMVfK
- Lonstein JE, Winter RB (2002) Long multiple struts for severe kyphosis. Clin Orthop Relat Res 394: 130-138. Link: http://bit.ly/33lpLQ2
- Shin AY, Dekutoski MB (2007) The role of vascularized bone grafts in spine surgery. Orthop Clin North Am 38: 61-72. Link: http://bit.ly/2DiDa0I
- de Boer HH, Wood MB, Hermans J (1990) Reconstruction of large skeletal defects by vascularized fibula transfer: Factors that influenced the outcome of union in 62 cases. Int Orthop 14: 121-128. Link: http://bit.ly/2Olf4sE
- Erdmann D, Meade RA, Lins RE, McCann RL, Richardson WJ, et al. (2006) Use of the microvascular free fibula transfer as a salvage reconstruction for failed anterior spine surgery due to chronic osteomyelitis. Plast Reconstr Surg 117: 2438-2445, discussion 2446–2447. Link: http://bit.ly/37AgEye
- Wood MB (1986) Free vascularized bone transfers for nonunions, segmental gaps, and following tumor resection. Orthopedics 9: 810-816. Link: http://bit.ly/37FUF8Y
- Asazuma T, Yamagishi M, Nemoto K, Amako M, Osada M, et al. (1997) Spinal fusion using a vascularized fibular bone graft for a patient with cervical kyphosis due to neurofibromatosis. J Spinal Disord 10: 537-540. Link: http://bit.ly/34xxPOQ
- Hu H, Winters HA, Paul RM, Wuisman PI (2007) Internal thoracic vessels used as pedicle graft for anastomosis with vascularized bone graft to reconstruct C7-T3 spinal defects. Spine (Phila Pa 1976) 32: 601-605. Link: http://bit.ly/2rovCqs
- Kaneda K, Kurakami C, Minami A (1998) Free vascularized fibular strut graft in the treatment of kyphosis. Spine (Phila Pa 1976) 13: 1273-1277. Link: http://bit.ly/2saNFAL
- Minami A, Kaneda K, Satoh S, Abumi K, Kutsumi K (1997) Free vascularised fibular strut graft for anterior spinal fusion. J Bone Joint Surg Br 79: 43-47. Link: http://bit.ly/33myvFq
- Nijland EA, van den Berg MP, Wuisman PL, van Royen BJ, Winters HA, et al. (1998) Correction of a dystrophic cervicothoracic spine deformity in Recklinghausen’s disease. Clin Orthop Relat Res 349: 149-155. Link: http://bit.ly/2sdDEml
- Saraph VJ, Bach CM, Krismer M, Wimmer C (2005) Evaluation of spinal fusion using autologous anterior strut grafts and posterior instrumentation for thoracic/thoracolumbar kyphosis. Spine (Phila Pa 1976) 30: 1594-1601. Link: http://bit.ly/37CE0mW
- Winters HA, van Engeland AE, Jiya TU, van Royen BJ (2010) The use of free vascularised bone grafts in spinal reconstruction. J Plast Reconstr Aesthet Surg 63: 516-523. Link: http://bit.ly/2DjePYt
- Moran SL, Bakri K, Mardini S, Shin AY, Bishop AT (2009) The use of vascularized fibular grafts for the reconstruction of spinal and sacral defects. Microsurgery 29: 393-400. Link: http://bit.ly/2KQIs7K
- McLaughlin EJ, Heuer GG, Whitmore RG, Birknes JK, Belasco J, et al. (2011) Treatment of a malignant peripheral nerve sheath tumor and its complications through a multidisciplinary approach. J Neurosurg Pediatr 7: 543-548. Link: http://bit.ly/33nK7YS
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
