International Journal of Radiology and Radiation Oncology
Behavioral impairments and biochemical alterations in brain following exposure to WiFi radiation and aluminum in rats
Haifa Othman1*, Mariem Tanazefti1, Mohsen Sakly1, Hafedh Abdelmelek1 and Mohamed Ammari1,2
2Higher Institute of Applied Biological Sciences of Tunis, University of Tunis El Manar, Tunis, Tunisia
Cite this as
Othman H, Tanazefti M, Sakly M, Abdelmelek H, Ammari M (2021) Behavioral impairments and biochemical alterations in brain following exposure to WiFi radiation and aluminum in rats. Int J Radiol Radiat Oncol 7(1): 006-013. DOI: 10.17352/ijrro.000044Today, WiFi radiofrequencies exposure is becoming almost unavoidable. Besides, aluminum is widely used in daily life despite its involvement in neurodegenerative diseases onset.
In this study, we investigated the effects of a 14 day exposure to WiFi radiation for 2 hours a day and aluminum chloride (AlCl3) at 200mg/Kg/day alone and associated on behavior, oxidative stress, oligoelements homeostasis and metals accumulation in the brain of rats.
Results showed that WiFi radiation alone induced anxiety. Aluminum administration triggered anxiety, locomotor deficits and exploratory behavior impairments. WiFi radiation and aluminum association impaired emotional and exploratory behavior. At biochemical level, WiFi and aluminum co-exposure induced cerebral oxidative stress compared to other experimental groups. Moreover, aluminum intake increased cerebral aluminum content. WiFi radiation coupled with aluminum increased cerebral aluminum, iron and cadmium contents compared to control, WiFi and Aluminum groups, and lead content compared to WiFi and Aluminum groups. Our results reveal that WiFi radiation and aluminum, especially when associated, are harmful for the brain. Thus, it is prominent to limit the exposure to WiFi radiation and aluminum for healthy nervous system.
Abbreviations
Al: Auminum; AlCl3: Aluminum Chloride; ANOVA: Analysis of Variance; °C: Celsius Degree; Ca: Calcium; CAT: Catalase; Cd: Cadmium; Cm: Centimeter; Cu: Copper; DNA: Deoxyribonucleicacid; EEG: Electroencephalogram; EDTA: Ethylenediaminetetraacetic Acid; Fe: Iron; GHz: Giga Hertz; GSH-Px: Glutathioneperoxidase; GST: Glutathione S-Transferase; h: hour; IARC: International Agency for Research in Cancer; Kg: Kilogram; LTE: Long-Term Evolution; MDA: Malondialdehyde; Mg: Magnesium; mg: milli gram; ml: milliliter; NO: Nitric Oxide; PLSD: Protected Least Significant Difference; Pb: Lead; rpm: rotation per minute; s: second; SOD: Superoxide Dismutase; U: Unit; UMTS: Universal Mobile Telecommunications System; WiFi: Wireless Fidelity; Zn: Zinc
Introduction
The recent widespresad use of wireless devices and the emerging of the fifth technology generation are continuously rising worries about potential adverse health effects of the radiofrequencies emissions [1-3]. Wireless Fidelity (WiFi) technology operating at 2.45GHz is among the most important sources of these non ionizing electromagnetic radiation [4,5].
Radiofrequency radiation are reported harmful for immune [6-8], reproductive [9-11], cardiovascular [12], endocrine [13] and other body systems. Although the brain is recognized among the most vulnerable organs to radiofrequencies [14,15], there are many controversies regarding neurological outcomes of these radiation in both laboratory animals [16] and humans [17]. Behavioral changes associated with radiofrequency radiation were due to several neural and molecular mechanisms [18] including changes in hippocampal lipidome and transcriptome [19] and an increase in vacuolization in brain tissues [13]. WiFi radiation are reported to be an important threat to human health [20]. They induced electrohypersensitivity and demyelinating syndrome leading to focal seizures, ataxia, vertigo and headaches in humans [21]. Radiofrequencies are classified as 2B agents (possibly carcinogenic to humans) by the International Agency for Research in Cancer (IARC) [22]. However, according to other studies, radiofrequency radiation do not increase brain tumor risk [23] and can be even used as a treatment of brain metastasis [24] and Alzheimer’s disease [25-28].
Aluminum (Al) is among the most enriched metals in our biosphere. Despite its extensive use in daily life (adjuvanted vaccins [29] and other pharmaceuticals [30], food additives [31,32], cosmetics…), Al has unknown biological function [33]. This heavy metal is able to cross biological barriers, reach various body fluids, enter cells and accumulate in different organs, mainly in the central nervous system [33]. Al is associated with Alzheimer’s disease [34], Down’s syndrome [34], dialysis dementia syndrome [34], dementia [35] and epilepsy [36] in humans. Daily oral administration of aluminum chloride AlCl3 (100mg/Kg) elicited cognitive impairments, anxiety and motor deficits in rats following a treatment of 15 days [37] and 42 days [38,39]. These aluminum-induced behavioral changes are due to several mechanisms including hippocampal and cortical oxidative stress [40], in vitro and in vivo neuronal and glial cell death [41], neurotransmission disruption [37,39,40], cerebral Al-catecholamine complexes generation [42] and amyloid plaques deposition [43].
In the present investigation, we were interested in neurological effects of concomitant exposure to WiFi radiation and aluminum for several reasons. Indeed, the brain is the target of both WiFi radiofrequencies and aluminum. Then, the daily and high exposure to WiFi and aluminum increases the risk of concomitant exposure to these two factors. Besides, coeexposure effects of WiFi and aluminum have not been yet studied which emphasizes the interest of our investigation. Thus, the objective of this study is to evaluate the neurological effects of exposure to WiFi radiation (2 h/day) and aluminum chloride (AlCl3) (200mg/Kg/day) alone and associated during 14 consecutive days in rats. For this purpose, we focused on emotional, locomotor and exploratory behavior (open field test), oxidative stress and oligoelements (iron, zinc and calcium) and metals (aluminum, lead and cadmium) contents in the brain.
Materials and methods
Animals and experimental design
Male Wistar rats weighing about 120 g were procured from SIPHAT, Tunisia. The animals were kept under 12h/12h light-dark cycle conditions in a temperature-and humidity-controlled animal facility with free access to standard pellet diet and water. All procedures were approved by the Faculty Ethics Committee (Faculty of Sciences of Bizerte, Tunisia).
After an adaptive phase, the rats were randomised into four groups of six animals each:
- Control group: Rats received daily distillated water and were kept away from WiFi radiation sources during 14 days.
- WiFi group: Rats were daily exposed to 2.45GHz WiFi radiation for 2h/day and received distillated water (vehicle) along 14 days.
- Chloride aluminum group (AlCl3): Rats were administrated daily AlCl3 (200mg/Kg/day) without exposure to WiFi radiation for 14 days.
- WiFi+AlCl3 group: Rats were exposed daily to both WiFi radiation (2h/day) and AlCl3 (200mg/Kg/day) during 14 days.
WiFi radiation are emitted for 2h/day from a D-Link® modem at 25cm away from exposure cage [44]. Chloride aluminum was dissolved in distillated water and administered to the animals per os at a dose of 200mg/Kg of body weight /day [45].
Behavioral assessment: Open field test
The next day (15th day), open field test was performed. The apparatus was a clear plastic circular area (1m in diameter) with a 50cm wall and divided into 1 central and 6 peripheral parts of equal surface [46]. Three identical objects were located in three peripheral parts of the apparatus. The rat is placed in the open field for one session of 5 minutes. Centre entries and time reflected anxious behavior. Locomotor abilities were evaluated through the locomotion time and the number of peripheral crossed quadrants. Objects exploration number and time serve for exploratory behavior assessment. Object exploration was defined as physical contact with an object with snout, vibrissae or forepaws. The open-field apparatus and the objects were wiped out using a 10% alcohol solution after every trial to eliminate olfactive clues.
Biochemical determinations
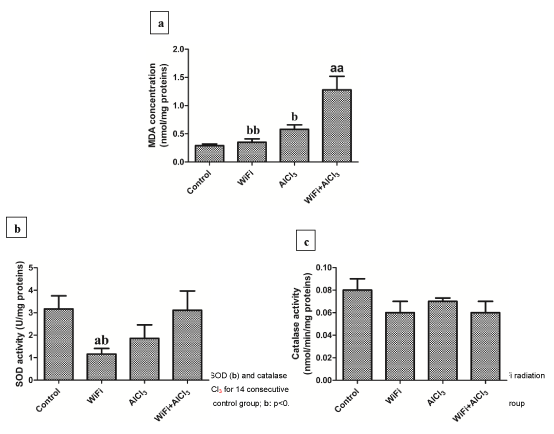
Oxidative stress markers assessment in brain: The lipid peroxidation was determined by Malondialdehyde (MDA) measurement according to the double heating method of Draper and Hadley [47]. The activity of Superoxide Dismutase (SOD) and Catalase (CAT) was assayed according to Misra and Fridovich [48] and Aebi [49] respectively. Protein concentration was determined by the method of Bradford [50].
Cerebral oligoelements and metals contents measurements
A part of each brain sample was incinerated at 50°C in the oven until getting a residue of constant weight which was then cooled to room temperature. Concentrate nitric acid was added to the residue (in the rate of 3ml /100mg of dry weight and then 1ml for each extra 100mg) for sample recovery. Samples were then heated at 120°C in a thermoblock until mineralization. After cooling the resulting residue, its volume was increased to 10 ml with double distilled water. Aluminum (Al), iron (Fe), zinc (Zn), calcium (Ca), lead (Pb) and cadmium (Cd) concentrations were measured by inductively coupled plasma-atomic emission spectroscopy.
Statistical analysis
Statistical analysis of the obtained data were performed by Statview® software using analysis of variance (ANOVA) for comparison between groups, followed by a Fisher’s Protected Least Significant Difference (PLSD) post-hoc test for multiple comparisons between all groups. Values for p<0.05 and p<0.001 were considered statistically significant. Data are given as mean±standard error of the mean (SEM). Histograms were done by GraphPad Prism 5®software.
Results
Open field test
Emotional behavior: WiFi radiation and aluminum alone and associated induced anxiety by reducing (p<0.05) center entries and time compared to control group (Table 1).
Locomotor beahavior: Aluminum affected locomotor abilities by decreasing (p<0.05) the locomotion time and the number of crossed peripherals parts of the arena (Table 1).
Objects exploration: Aluminum alone and associated with WiFi radiation decreased (p<0.05) the number and the time of objects exploration compared to control group. Aluminum’s effect on objects exploration time was even highly significant (p<0.001). Aluminum diminished (p<0.05) the number and the time of objects exploration compared to WiFi group (Table 1).
Oxidative stress in brain: The co-exposure to WiFi radiation and aluminum increased significantly cerebral MDA level compared to control (p<0.001), WiFi (p<0.001) and aluminum (p<0.05) groups. WiFi exposure reduced SOD activity compared to control group (p<0.05).WiFi and aluminum co-treatment, however, increased (p<0.05) SOD activity compared to WiFi group reaching a comparable level as control one’s. We noticed no significant (p>0.05) difference in CAT activity in all experimental groups (Figure 1).
Oligoelements and metals contents in brain
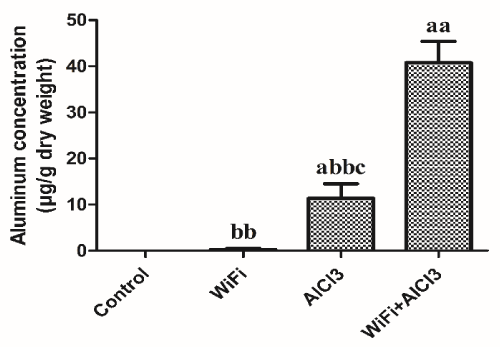
Following aluminum treatment, rats displayed a significant increase (p<0.05) in cerebral Al content compared to control and WiFi groups (Figure 2). The co-exposure to WiFi and aluminum induced a significant increase in cerebral Al (p<0.001), Fe (p<0.001) and Cd (p<0.05) contents compared to control, WiFi and aluminum groups. Besides, WiFi and aluminum association increased (p<0.05) cerebral Pb content compared to WiFi and Al groups. We noticed no significant effect (p>0.05) of the treatment on cerebral Zn and Ca levels (Table 2).
Discussion
Our results showed that a 14 day exposure to WiFi radiation (2h/day) induced anxiety. For a similar treatment period, aluminum adminiration (at 200mg/Kg/day) impaired emotional, locomotor and exploratory behavior.The latter was more altered by aluminum than by WiFi radations. WiFi radiation associated with aluminum induced emotional and exploratory behavior deficits. At biochemical level, WiFi and aluminum co-exposure induced cerebral oxidative stress by increasing MDA level compared to control, WiFi and aluminum groups. WiFi exposure reduced SOD activity compared to control group. As for oligoelements levels and metals accumulation in the brain, Al administration increased Al content compared to control and WiFi groups. The co-exposure to WiFi radiation and Al increased Al, Fe and Cd contents compared to control, WiFi and Al groups and Pb content compared to WiFi and Al groups.
Behaviorally, we found that WiFi radiation (2h/day for 14 days) induced anxiety. Aluminum (200mg/Kg/day for 14 days) affected emotional, locomotor and exploratory behavior. The co-exposure to WiFi radiation and aluminum altered emotional and exploratory behavior. Exploratory behavior impairments were more important due to aluminum than WiFi radiation.
Previous studies proved hazardous impacts of radiofrequency radiation on behavior. WiFi exposure for 2h/day along 20 days induced an anxiety like behavior without impairing spatial learning and memory abilities in male rats [44]. Chronic exposure to 2.45GHz WiFi signal for 12h/day during 30 days impaired the discrimination between the novel and familiar objects in unimodal and multimodal object recognition tasks in male rats [51]. Long term exposure to 2.5GHz WiFi radiation (4, 6 and 8 weeks) increased anxiety and affected locomotor function in male rats [52]. The exposure to 2.45 GHz WiFi radiation for 2h/day along 40 days and for 4h/day during 45 days elicited learning and memory deficits in male rats [53] and memory decline with anxiety in female rats [54] respectively. The exposure to 900 MHz radiofrequency radiation disrupted dendritic arborization pattern in basolateral amygdala leading to place preference alteration in adolescent male rats [55]. Radiofrequencies cause at least 13 neuropsychiatric effects including depression in humans [56].
However, according to asystematic review dealing with the effects of radiofrequencies exposure, mostly released by mobile phones, on cognitive behavior of laboratory animals, among 62 studies having been published since 1993, 21 studies reported significant impairments, 20 studies reported no significant effects, and 4 studies reported beneficial impacts [16]. Short-term radiofrequency exposure from new generation of mobile phones (Universal Mobile Telecommunications System (UMTS) and Long-Term Evolution (LTE)) reduced EEG alpha power with no effects on cognitive performance in healthy young-adult university students [57]. Emotional and behavioral problems in 5-year-old children were induced by radiofrequency radiation emitted by mobile phone base stations and indoor sources (contributing very little to radiofrequencies exposure) rather than mobile phone radifrequencies (leading to an exposure peak in the head) [58]. Long-term exposure (2h/day for 8 months) to 1950MHz radiorequencies exerted beneficial effects on Alzheimer’s disease by rescuing hyperactivity-like and anxiolytic behaviors, improving cognitive deficits and increasing glucose metabolism in hippocampus and amygdala regions of the brain in Alzheimer’s model mice [28].
Our results are in line with several previous investigations proving behavioral impairments of aluminum exposure. Indeed, AlCl3 treatment at 50 mg/Kg for 15 days and 28 days resulted in spatial learning deficit in mice [59] and rats [43], respectively. Daily oral administration of AlCl3 (100mg/Kg) for 15 days induced rat model of Alzheimer’s disease by trigging anxiety, frontal-dependent motor deficits and cognitive decline [37]. AlCl3 oral administration (100mg/Kg) for 42 days induced anxiety, spatial memory performance deficits and motor dysfunction, signs of Alzheimer’s disease-like pathology in rats [38,39]. Memory impairments were also associated with chronic AlCl3 exposure at 40mg/Kg and 175mg/kg for 60 days in mice [60] and rats [61] respectively, and at 150 mg/kg for 90 days in rats [62]. Chronic aluminum exposure at 12 and 72mg/Kg for 270 and 360 days resulted in learning, memory and exploratory behavior deficits in a dose and time dependent manner in rats [63]. Occupational aluminum exposure for a long time could be associated with an increased risk of cognitive impairments in aluminum workers [64,65]. Moreover, chronic alaluminum (0,3%) exposure in drinking water during 4 months since the intra-uterine age led to locomotor impairments in rats [66].
As for brain oxidative stress, WiFi radiation and aluminum co-exposure increased cerebral MDA level compared to control, WiFi and Al groups.WiFi radiation alone reduced SOD activity compared to control and co-exposed WiFi and Al groups. According to several previous studies, WiFi radiation altered behavior through inducing brain oxidative stress especially at brain level. Indeed, WiFi is an important threat to human health causing oxidative stress [20]. For instance, repeated 2.45GHz WiFi exposure for 2h/day during 20 days increased cerebral MDA level and CAT activity in male Wistar rats [44]. The exposure to 2.45GHz radiation for 4h/day during 45 days elicited the depletion of brain antioxidant enzyme systems (SOD and CAT) and reduced glutathione levels leading to memory decline and anxiety behavior in female Sprague Dawley rats [54]. Long term exposure (2h/day for 6 months) to 900, 1800, and 2100 MHz mobile phone radiofrequencies increased lipid peroxidation and oxidative deoxyribonucleic acid (DNA) damage in the frontal lobe of the brain in male rats [67]. Continuous exposure to 2.45GHz radiofrequencies (24h/day for two months and a half) affected oxidative defense system in male Wistar rats by decreasing total antioxidant capacity of plasma and the activities of several antioxidant enzymes, including CAT, glutathione peroxidase (GSH-Px) and SOD, and increasing glutathione S-transferase (GST) activity [68].
Regarding aluminum effects on oxidative equilibrum, AlCl3 treatment 50mg/kg for 15 days increased the level of oxidative stress markers such as MDA, Advanced Oxidation of Protein Products (AOPP) and Nitric Oxide (NO) in various brain regions (prefrontal cortex, striatum, parietal cortex, hippocampus, hypothalamus and cerebellum) in mice [59]. Daily oral administration of AlCl3 100 mg/Kg for 15 days induced lipid peroxidation and SOD activity depletion in the prefrontal cortex and the hippocampus in rats [37]. Oral intake of AlCl3 100mg/Kg for 42 days caused oxidative stress in rat brain tissues leading to Alzheimer disease like symptoms [38,39,69]. AlCl3 exposure at a dose of 17mg/Kg and 84mg/Kg for 4 weeks reduced brain total antioxidant status (decrease in GSH level and inhibition of GPx and SOD activities) with a subsequent increase in lipid peroxidationin male rats [70,71]. AlCl3 treatment 175 mg/Kg for 60 days induced oxidative stress in the hippocampus and the frontal cortex in rats [61]. AlCl3 administration 10mg/Kg and 150mg /Kg for 3 months increased lipid peroxidation and decreased GSH, CAT, and SOD activities in the brain [72] and mainly in the hippocampus in rats [62]. AlCl3 exposure 10mg/Kg from day 6 to 64 of age induced hippocampal and cortical oxidative stress by reducing catalase actvity and increasing lipid peroxidation in adult male rats [40]. Moreover, in vitro investigations showed that AlCl3 (50μM) exposure caused reactive oxygen species generation in primary hippocampal neuronal cells [73] and hippocampal synaptosomes of rats [74]. AlCl3 (500mg/L) added to the deionized drinking water starting from day 6 of gestation until just after weaning or until the age of 70 days postnatal life reduced tissue contents of vitamin C, GSH, total proteinsand the activities of Na+/K+-ATPase and SOD and increased cerebral lipid peroxidation and NO level in rats [75].
The assessment of oligoelements and metals levels in the brain showed that aluminum treatment increased Al content compared to control and WiFi groups. The co-exposure to WiFi and aluminum increased Al, Fe and Cd levels compared to control, WiFi and Al groups. Futhermore, WiFi and Al co-exposure increased Pb level compared to WiFi and Al groups. There was no significant effect of WiFi radiation and aluminum neither alone nor associated on cerebral Zn and Ca levels. In line with our deleterious effects of Al, a Camelford resident died following late-onset epilepsy development due to aluminum consumption in potable water. The case report showed aluminum-loaded glial cells and neuronal debris, aluminum accumulations in the occipital lobe and the hippocampus and abnormal calcifications in the occipital lobe [36]. Sub-chronic AlCl3 administration at a dose of 2mg/Kg/day for 1 and 3 months induced dose-dependent Al accumulationin the brain and the blood of male rats. Surprisingly, the strong positive correlation between Al content in the brain and the blood for 1 month exposure disappeared by the third month probably due to neural barrier adaptation to Al exposure or the saturation of brain Al transport [76]. AlCl3 exposure 50 μM caused Al accumulation in primary hippocampal neuronal cells of rats [73]. Aluminum measurements in brain tissues from 12 donors diagnosed with familial Alzheimer’s disease revealed extremely high aluminum concentrations which proves aluminum’s role in Alzheimer’s disease development [77]. Besides, AlCl3 administration10mg/Kg for 3 months decreased seric Zn, Copper (Cu), Magnesium (Mg) and increased seric Fe in rats [72]. Mobile phone radiofrequencies exposure induced Fe and Al accumulations in the stratum granulosum of rabbit’s cerebellum [78].
Conclusion
The present experiment demonstrated that exposure to WiFi radiation and aluminum alone and especially when combined altered emotional, locomotor and/or exploratory behavior through oxidative stress induction, oligoelements homeostasis disruption and metals accumulation in the brain. Therefore, the public should be aware and limit its exposure as much as possible to WiFi radiation and aluminum.
Financial support of the Tunisian Ministry of Higher Education and Scientific Research is gratefully acknowledged. Financial disclosures: none declared.
- Russell CL (2018) 5 G wireless telecommunications expansion: Public health and environmental implications. Environ Res 165: 484-495. Link: http://bit.ly/2NH3MB8
- Kostoff RN, Heroux P, Aschner M, Tsatsakis A (2020) Adverse health effects of 5G mobile networking technology under real-life conditions. Toxicol Lett 323: 35-40. Link: http://bit.ly/3qU9GgO
- Simko M, Mattsson MO (2019) 5G Wireless Communication and Health Effects-A Pragmatic Review Based on Available Studies Regarding 6 to 100 GHz. Int J Environ Res Public Health 16: 3406. Link: http://bit.ly/2NG3B9p
- Lahham A, Ayyad H (2019) Personal Exposure to Radiofrequency Electromagnetic Fields Among Palestinian Adults. Health Phys 117: 396-402. Link: http://bit.ly/2Mp4UJh
- Massardier-Pilonchery A, Nerriere E, Croidieu S, Ndagijimana F, Gaudaire F, et al. (2019) Assessment of Personal Occupational Exposure to Radiofrequency Electromagnetic Fields in Libraries and Media Libraries, Using Calibrated On-Body Exposimeters. Int J Environ Res Public Health 16: 2087. Link:http://bit.ly/2NwRDiq
- Lopez-Furelos A, Salas-Sanchez AA, Ares-Pena FJ, Leiro-Vidal JM, Lopez-Martin E (2018) Exposure to radiation from single or combined radio frequencies provokes macrophage dysfunction in the RAW 264.7 cell line. Int J Radiat Biol 94: 607-618. Link: http://bit.ly/3pXm8Lf
- Marshall TG, Heil TJR (2017) Electrosmog and autoimmune disease. Immunol Res 65: 129-135. Link: http://bit.ly/3uraTOZ
- Yu H, Zhang J, Ji Q, Yu K, Wang P, et al. (2019) Melatonin alleviates aluminium chloride-induced immunotoxicity by inhibiting oxidative stress and apoptosis associated with the activation of Nrf2 signaling pathway. Ecotoxicol Environ Saf 173: 131-141. Link: http://bit.ly/3r5Wp4E
- Jaffar FHF, Osman K, Ismail NH, Chin KY, Ibrahim SF (2019) Adverse Effects of Wi-Fi Radiation on Male Reproductive System: A Systematic Review. Tohoku J Exp Med 248: 169-179. Link: http://bit.ly/3bB7Gna
- Singh R, Nath R, Mathur AK, Sharma RS (2018) Effect of radiofrequency radiation on reproductive health. Indian J Med Res 148: S92-S99. Link: http://bit.ly/37NND3N
- Yu G, Tang Z, Chen H, Chen Z, Wang L, et al. (2019) Long-term exposure to 4G smartphone radiofrequency electromagnetic radiation diminished male reproductive potential by directly disrupting Spock3-MMP2-BTB axis in the testes of adult rats. Sci Total Environ 698: 133860. Link: http://bit.ly/3klWU8l
- Saili L, Hanini A, Smirani C, Azzouz I, Azzouz A, et al. (2015) Effects of acute exposure to WIFI signals (2.45GHz) on heart variability and blood pressure in Albinos rabbit. Environ Toxicol Pharmacol 40: 600-605. Link: http://bit.ly/3pV7yEd
- Shahabi S, Hassanzadeh Taji I, Hoseinnezhaddarzi M, Mousavi F, Shirchi S, et al. (2018) Exposure to cell phone radiofrequency changes corticotrophin hormone levels and histology of the brain and adrenal glands in male Wistar rat. Iran J Basic Med Sci 21: 1269-1274. Link: http://bit.ly/3klfLQO
- Kim JH, Lee JK, Kim HG, Kim KB, Kim HR (2019) Possible Effects of Radiofrequency Electromagnetic Field Exposure on Central Nerve System. Biomol Ther (Seoul) 27: 265-275. Link: http://bit.ly/3pT4ubB
- Zhi WJ, Wang LF, Hu XJ (2017) Recent advances in the effects of microwave radiation on brains. Mil Med Res 4: 29. Link: http://bit.ly/3srXpRa
- Sienkiewicz Z, van Rongen E (2019) Can Low-Level Exposure to Radiofrequency Fields Effect Cognitive Behaviour in Laboratory Animals? A Systematic Review of the Literature Related to Spatial Learning and Place Memory. Int J Environ Res Public Health 16: 1607. Link: http://bit.ly/3dHhted
- Wallace J, Selmaoui B (2019) Effect of mobile phone radiofrequency signal on the alpha rhythm of human waking EEG: A review. Environ Res 175: 274-286. Link: http://bit.ly/3qXjqH5
- Narayanan SN, Jetti R, Kesari KK, Kumar RS, Nayak SB, et al. (2019) Radiofrequency electromagnetic radiation-induced behavioral changes and their possible basis. Environ Sci Pollut Res Int 26: 30693-30710. Link: http://bit.ly/3uGzRtM
- Fragopoulou AF, Polyzos A, Papadopoulou MD, Sansone A, Manta AK, et al. (2018) Hippocampal lipidome and transcriptome profile alterations triggered by acute exposure of mice to GSM 1800 MHz mobile phone radiation: An exploratory study. Brain Behav 8: e01001. Link: http://bit.ly/2MmgKUi
- Pall ML (2018) Wi-Fi is an important threat to human health. Environ Res 164: 405-416. Link: http://bit.ly/3aUxAmV
- Johansson O, Redmayne M (2016) Exacerbation of demyelinating syndrome after exposure to wireless modem with public hotspot. Electromagn Biol Med 35: 393-397. Link: http://bit.ly/2O3wpIz
- Baan R, Grosse Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, et al. (2011) Carcinogenicity of radiofrequency electromagnetic fields. Lancet Oncol 12: 624-626. Link: https://bit.ly/3slhWGM
- Roosli M, Lagorio S, Schoemaker MJ, Schuz J, Feychting M (2019) Brain and Salivary Gland Tumors and Mobile Phone Use: Evaluating the Evidence from Various Epidemiological Study Designs. Annu Rev Public Health 40: 221-238. Link: http://bit.ly/3slhWGM
- Sharma S, Wu SY, Jimenez H, Xing F, Zhu D, et al. (2019) Ca(2+) and CACNA1H mediate targeted suppression of breast cancer brain metastasis by AM RF EMF. EBioMedicine 44: 194-208. Link: http://bit.ly/3aPKUZI
- Banaceur S, Banasr S, Sakly M, Abdelmelek H (2013) Whole body exposure to 2.4 GHz WIFI signals: effects on cognitive impairment in adult triple transgenic mouse models of Alzheimer's disease (3xTg-AD). Behav Brain Res 240: 197-201. Link: http://bit.ly/3aX3rDz
- Jeong YJ, Kang GY, Kwon JH, Choi HD, Pack JK, et al. (2015) 1950 MHz Electromagnetic Fields Ameliorate Abeta Pathology in Alzheimer's Disease Mice. Curr Alzheimer Res 12: 481-492. Link: http://bit.ly/3srOlfa
- Mortazavi S, Shojaei-Fard M, Haghani M, Shokrpour N, Mortazavi S (2013) Exposure to mobile phone radiation opens new horizons in Alzheimer's disease treatment. J Biomed Phys Eng 3: 109-112. Link: http://bit.ly/3qVnZBE
- Son Y, Kim JS, Jeong YJ, Jeong YK, Kwon JH, et al. (2018) Long-term RF exposure on behavior and cerebral glucose metabolism in 5xFAD mice. Neurosci Lett 666: 64-69. Link: http://bit.ly/3bHaPSm
- Weisser K, Goen T, Oduro JD, Wangorsch G, Hanschmann KO, et al. (2019) Aluminium in plasma and tissues after intramuscular injection of adjuvanted human vaccines in rats. Archives of Toxicology 93: 2787-2796. Link: https://bit.ly/3aUbl0q
- Weisser K, Goen T, Oduro JD, Wangorsch G, Hanschmann KO, et al. (2019) Aluminium from adjuvanted subcutaneous allergen immunotherapeutics in rats is mainly detected in bone. Allergy 75: 215-217. Link: http://bit.ly/3knPhOK
- Glynn A, Lignell S (2019) Increased urinary excretion of aluminium after ingestion of the food additive sodium aluminium phosphate (SALP) - a study on healthy volunteers. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 36: 1236-1243. Link: https://bit.ly/2NZTHzj
- Ma J, Jiang G, Zheng W, Zhang M (2019) A longitudinal assessment of aluminum contents in foodstuffs and aluminum intake of residents in Tianjin metropolis. Food Science & Nutrition 7: 997-1003. Link: https://bit.ly/2NtUJUl
- Exley C, Mold MJ (2015) The binding, transport and fate of aluminium in biological cells. J Trace Elem Med Biol 30: 90-95. Link: https://bit.ly/2ZS29D6
- Lukiw WJ, Kruck TPA, Percy ME, Pogue AI, et al. (2019) Aluminum in neurological disease - a 36 year multicenter study. J Alzheimers Dis Parkinsonism 8: 457. Link: https://bit.ly/2NTW2M1
- Russ TC, Killin LOJ, Hannah J, Batty GD, Deary IJ, et al. (2019) Aluminium and fluoride in drinking water in relation to later dementia risk. Br J Psychiatry 16: 29-34. Link: https://bit.ly/3dLstXK
- Mold M, Cottle J, Exley C (2019) Aluminium in Brain Tissue in Epilepsy: A Case Report from Camelford. Int J Environ Res Public Health 16: 2129. Link: https://bit.ly/3bIXut4
- Olajide OJ, Yawson EO, Gbadamosi IT, Arogundade TT, Lambe E, et al. (2017) Ascorbic acid ameliorates behavioural deficits and neuropathological alterations in rat model of Alzheimer's disease. Environ Toxicol Pharmacol 50: 200-211. Link: https://bit.ly/3dJkBpV
- Ahmad Rather M, Justin-Thenmozhi A, Manivasagam T, Saravanababu C, Guillemin GJ, et al. (2019) Asiatic Acid Attenuated Aluminum Chloride-Induced Tau Pathology, Oxidative Stress and Apoptosis Via AKT/GSK-3beta Signaling Pathway in Wistar Rats. Neurotox Res 35: 955-968. Link: https://bit.ly/3knBis9
- Auti ST, Kulkarni YA (2019) Neuroprotective Effect of Cardamom Oil Against Aluminum Induced Neurotoxicity in Rats. Front Neurol 10: 399. Link: https://bit.ly/2Mudy9q
- Rijal S, Changdar N, Kinra M, Kumar A, Nampoothiri M, et al. (2019) Neuromodulatory potential of phenylpropanoids; para-methoxycinnamic acid and ethyl-p-methoxycinnamate on aluminum-induced memory deficit in rats. Toxicol Mech Methods 29: 334-343. Link: https://bit.ly/3q1qmlr
- Hao YX, Li MQ, Zhang JS, Zhang QL, Jiao X, et al. (2019) Aluminum-induced "mixed" cell death in mice cerebral tissue and potential intervention. Neurotox Res 37: 835-846. Link: https://bit.ly/3utus97
- Dalla Torre G, Mujika JI, Lachowicz JI, Ramos MJ, Lopez X (2019) The interaction of aluminum with catecholamine-based neurotransmitters: can the formation of these species be considered a potential risk factor for neurodegenerative diseases? Dalton Trans 48: 6003-6018. Link: https://bit.ly/2NZTpIJ
- Chavali VD, Agarwal M, Vyas VK, Saxena B (2020) Neuroprotective Effects of Ethyl Pyruvate against Aluminum Chloride-Induced Alzheimer's Disease in Rats via Inhibiting Toll-Like Receptor 4. J Mol Neurosci 70: 836-850. Link: https://bit.ly/3dJktqr
- Othman H, Ammari M, Sakly M, Abdelmelek H (2017) Effects of repeated restraint stress and WiFi signal exposure on behavior and oxidative stress in rats. Metab Brain Dis 32: 1459-1469. Link: https://bit.ly/3srsiF8
- Omran GA (2019) Hematological and immunological impairment following in-utero and postnatal exposure to aluminum sulfate in female offspring of albino rats. Immunopharmacol Immunotoxicol 41: 40-47. Link: https://bit.ly/3pQkyuJ
- Othman H, Ammari M, Sakly M, Abdelmelek H (2017) Effects of prenatal exposure to WIFI signal (2.45GHz) on postnatal development and behavior in rat: Influence of maternal restraint. Behav Brain Res 326: 291-302. Link: https://bit.ly/2ZS1ode
- Draper HH, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 186: 421-431. Link: https://bit.ly/3aRGuS0
- Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247: 3170-3175. Link: https://bit.ly/37IaTjG
- Aebi H (1984) Catalase in vitro. Methods Enzymol 105: 121-126. Link: https://bit.ly/2Pd70wP
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254. Link: https://bit.ly/2ZOkgtN
- Hassanshahi A, Shafeie SA, Fatemi I, Hassanshahi E, Allahtavakoli M, et al. (2017) The effect of Wi-Fi electromagnetic waves in unimodal and multimodal object recognition tasks in male rats. Neurol Sci 38: 1069-1076. Link: https://bit.ly/3pRo7Rp
- Obajuluwa AO, Akinyemi AJ, Afolabi OB, Adekoya K, Sanya JO, et al. (2017) Exposure to radio-frequency electromagnetic waves alters acetylcholinesterase gene expression, exploratory and motor coordination-linked behaviour in male rats. Toxicology Reports 4: 530-534. Link: https://bit.ly/2ZMBRCf
- Karimi N, Bayat M, Haghani M, Saadi HF, Ghazipour GR (2018) 2.45 GHz microwave radiation impairs learning, memory, and hippocampal synaptic plasticity in the rat. Toxicol Ind Health 34: 873-883. Link: https://bit.ly/3stjgrw
- Varghese R, Majumdar A, Kumar G, Shukla A (2018) Rats exposed to 2.45GHz of non-ionizing radiation exhibit behavioral changes with increased brain expression of apoptotic caspase 3. Pathophysiology 25: 19-30. Link: https://bit.ly/3aPYM62
- Narayanan SN, Mohapatra N, John PKN, Kumar RS, Nayak SB, et al. (2018) Radiofrequency electromagnetic radiation exposure effects on amygdala morphology, place preference behavior and brain caspase-3 activity in rats. Environ Toxicol Pharmacol 58: 220-229. Link: https://bit.ly/2NC2SGd
- Pall ML (2016) Microwave frequency electromagnetic fields (EMFs) produce widespread neuropsychiatric effects including depression. J Chem Neuroanat 75: 43-51. Link: https://bit.ly/3snadbt
- Vecsei Z, Knakker B, Juhasz P, Thuroczy G, Trunk A, et al. (2018) Short-term radiofrequency exposure from new generation mobile phones reduces EEG alpha power with no effects on cognitive performance. Scientific Reports 8: 18010. Link: https://go.nature.com/2O35BIo
- Guxens M, Vermeulen R, Steenkamer I, Beekhuizen J, Vrijkotte TGM, et al. (2019) Radiofrequency electromagnetic fields, screen time, and emotional and behavioural problems in 5-year-old children. Int J Hyg Environ Health 222: 188-194. Link: https://bit.ly/3ktMnrJ
- Al-Amin MM, Chowdury MIA, Saifullah ARM, Alam MN, Jain P, et al. (2019) Levocarnitine Improves AlCl3-Induced Spatial Working Memory Impairment in Swiss albino Mice. Front Neurosci 13: 278. Link: https://bit.ly/3qXqxzs
- Saba K, Rajnala N, Veeraiah P, Tiwari V, Rana RK, et al. (2017) Energetics of Excitatory and Inhibitory Neurotransmission in Aluminum Chloride Model of Alzheimer's Disease: Reversal of Behavioral and Metabolic Deficits by Rasa Sindoor. Front Mol Neurosci 10: 323. Link: https://bit.ly/3sr9ndL
- Nampoothiri M, Kumar N, Venkata Ramalingayya G, Gopalan Kutty N, Krishnadas N, et al. (2017) Effect of insulin on spatial memory in aluminum chloride-induced dementia in rats. Neuroreport 28: 540-544. Link: https://bit.ly/2NCGHQe
- Cao Z, Wang F, Xiu C, Zhang J, Li Y (2017) Hypericum perforatum extract attenuates behavioral, biochemical, and neurochemical abnormalities in Aluminum chloride-induced Alzheimer's disease rats. Biomed Pharmacother 91: 931-937. Link: https://bit.ly/2ZMBgjZ
- Wang F, Kang P, Li Z, Niu Q (2019) Role of MLL in the modification of H3K4me3 in aluminium-induced cognitive dysfunction. Chemosphere 232: 121-129. Link: https://bit.ly/3upkl5c
- Meng H, Wang S, Guo J, Zhao Y, Zhang S, et al. (2019) Cognitive impairment of workers in a large-scale aluminium factory in China: a cross-sectional study. BMJ open 9: e027154. Link: https://bit.ly/3kld0iq
- Qiu HY, Ren P, Li R, Zhang QL, Lu XT, et al. (2016) Association between H3K4me3/BDNF and the cognitive function of workers occupationally exposed to aluminum. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 34: 900-904. Link: https://bit.ly/3qSWU2d
- Laabbar W, Elgot A, Elhiba O, Gamrani H (2019) Curcumin prevents the midbrain dopaminergic innervations and locomotor performance deficiencies resulting from chronic aluminum exposure in rat. J Chem Neuroanat 100: 101654. Link: https://bit.ly/2ZOjKfl
- Alkis ME, Bilgin HM, Akpolat V, Dasdag S, Yegin K, et al. (2019) Effect of 900-, 1800-, and 2100-MHz radiofrequency radiation on DNA and oxidative stress in brain. Electromagn Biol Med 38: 32-47. Link: https://bit.ly/3aOvrsJ
- Kamali K, Taravati A, Sayyadi S, Gharib FZ, Maftoon H (2018) Evidence of oxidative stress after continuous exposure to Wi-Fi radiation in rat model. Environ Sci Pollut Res Int 25: 35396-35403. Link: https://bit.ly/37JtH2c
- Zakaria MMH, Hajipour B, Estakhri R, Saleh BM (2017) Anti-oxidative effect of resveratrol on aluminum induced toxicity in rat cerebral tissue. Bratisl Lek Listy 118: 269-272. Link: https://bit.ly/3upk8Ps
- Said MM, Rabo MM (2017) Neuroprotective effects of eugenol against aluminiuminduced toxicity in the rat brain. Arh Hig Rada Toksikol 68: 27-37. Link: https://bit.ly/3utweXT
- Wahby MM, Mohammed DS, Newairy AA, Abdou HM, Zaky A (2017) Aluminum-induced molecular neurodegeneration: The protective role of genistein and chickpea extract. Food Chem Toxicol 107: 57-67. Link: https://bit.ly/3aPtZ9B
- Mohamed NE, Abd El-Moneim AE (2017) Ginkgo biloba extract alleviates oxidative stress and some neurotransmitters changes induced by aluminum chloride in rats. Nutrition 35: 93-99. Link: https://bit.ly/3dLlVbG
- Wang X, Fan X, Yuan S, Jiao W, Liu B, et al. (2017) Chlorogenic acid protects against aluminium-induced cytotoxicity through chelation and antioxidant actions in primary hippocampal neuronal cells. Food Funct 8: 2924-2934. Link: https://bit.ly/3srr9gO
- Kar F, Hacioglu C, Uslu S, Kanbak G (2019) Curcumin Acts as Post-protective Effects on Rat Hippocampal Synaptosomes in a Neuronal Model of Aluminum-Induced Toxicity. Neurochem Res 44: 2020-2029. Link: https://bit.ly/3dHe52Z
- Kinawy AA (2019) Synergistic oxidative impact of aluminum chloride and sodium fluoride exposure during early stages of brain development in the rat. Environ Sci Pollut Res Int 26: 10951-10960. Link: https://bit.ly/3srU9Fq
- Drobyshev EJ, Solovyev ND, Gorokhovskiy BM, Kashuro VA (2018) Accumulation Patterns of Sub-chronic Aluminum Toxicity Model After Gastrointestinal Administration in Rats. Biol Trace Elem Res 185: 384-394. Link: https://bit.ly/37I9WYE
- Mirza A, King A, Troakes C, Exley C (2017) Aluminium in brain tissue in familial Alzheimer's disease. J Trace Elem Med Biol 40: 30-36. Link: https://bit.ly/2ZQSflw
- Kopani M, Filova B, Sevcik P, Kosnac D, Misek J, et al. (2017) Iron deposition in rabbit cerebellum after exposure to generated and mobile GSM electromagnetic fields. Bratisl Lek Listy 118: 575-579. Link: http://bit.ly/37O3XSh
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
